Giant cell tumor of bone: Difference between revisions
No edit summary |
Farima Kahe (talk | contribs) No edit summary |
||
| (16 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
'''For patient information, click [[Giant cell tumor of bone (patient information)|here]]''' | '''For patient information, click [[Giant cell tumor of bone (patient information)|here]]''' | ||
{{CMG}};{{AE}} {{Rohan}} | {{CMG}}; {{AE}} {{Rohan}} | ||
{{SK}} Osteoclastoma; Giant cell myeloma; Giant cell tumor; Giant cell tumor of the bone | {{SK}} Osteoclastoma; Giant cell myeloma; Giant cell tumor; Giant cell tumor of the bone | ||
==Overview== | ==Overview== | ||
Giant cell tumor of bone is a relatively uncommon tumor of the bone. It is characterized by the presence of multinucleated giant cells (osteoclast-like cells). Giant cell tumor of bone accounts for 4-5% of primary bone tumors and 18.2% of benign bone tumors. | Giant cell tumor of bone is a relatively uncommon tumor of the bone. It is characterized by the presence of [[multinucleated]] giant cells ([[osteoclast]]-like cells). Giant cell tumor of bone accounts for 4-5% of primary bone tumors and 18.2% of benign bone tumors. Giant cell tumor of bone almost invariably occur when the growth plate has closed and are therefore typically observed in early adulthood, with 80% of cases reported between the ages of 20 and 50, with a peak [[incidence]] between 20 and 30. Giant cell tumor of bone typically occur as single lesions. They usually prefers the [[epiphyses]] of long bones. Although any bone can be affected, the most common sites are distal [[femur]], proximal [[tibia]], and distal [[radius]]. The progression to giant cell tumor of bone usually involves the over-expression in RANK/RANKL signalling pathway with resultant over-proliferation of [[osteoclasts]]. On gross pathology, [[hemorrhage]], presence of co-existent [[aneurysmal bone cyst]], and [[fibrosis]] are characteristic findings of giant cell tumor of bone. On microscopic histopathological analysis, prominent and diffuse osteoclastic giant cells and [[mononuclear cells]] with frequent mitotic figures are characteristic findings of giant cell tumor of bone. Symptoms of giant cell tumor of bone include localized pain, localized swelling, and decreased range of motion. Physical examination findings will depend on the location of the giant cell tumor. Common physical examination findings of giant cell tumor are localized [[swelling]] and [[tenderness]] at the site of the tumor. Giant cell tumor of bone must be differentiated from [[aneurysmal bone cyst]], [[chondroblastoma]], simple bone cyst, [[osteoblastoma]],giant cell rich [[osteosarcoma]], and [[brown tumor]] of hyperparathyroidism. X-ray may be helpful in the diagnosis of giant cell tumor of bone. Common complications of giant cell tumor include [[malignant]] transformation, recurrence, and metastasis. Findings on x-ray suggestive of giant cell tumor include metaepiphyseal location of mass and grow to the articular surface of the involved bone with narrow zone of transition. Surgery is the mainstay of treatment for giant cell tumor. | ||
==Historical Perspective== | ==Historical Perspective== | ||
*In 1818, Cooper and Travers first described giant cell tumor (GCT) of bone.<ref name="pmid18322700">{{cite journal| author=Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G et al.| title=Giant cell tumor of bone: treatment and outcome of 214 cases. | journal=J Cancer Res Clin Oncol | year= 2008 | volume= 134 | issue= 9 | pages= 969-78 | pmid=18322700 | doi=10.1007/s00432-008-0370-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18322700 }} </ref> | *In 1818, Cooper and Travers first described giant cell tumor (GCT) of [[bone]].<ref name="pmid18322700">{{cite journal| author=Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G et al.| title=Giant cell tumor of bone: treatment and outcome of 214 cases. | journal=J Cancer Res Clin Oncol | year= 2008 | volume= 134 | issue= 9 | pages= 969-78 | pmid=18322700 | doi=10.1007/s00432-008-0370-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18322700 }} </ref> | ||
*In 1845, Par H. Lebert described the first microscopic observations of multinucleated giant cells and fusiform cells as 'tumeur fiblastique'.<ref name="pmid7004712">{{cite journal| author=McCarthy EF| title=Giant-cell tumor of bone: an historical perspective. | journal=Clin Orthop Relat Res | year= 1980 | volume= | issue= 153 | pages= 14-25 | pmid=7004712 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7004712 }} </ref> | *In 1845, Par H. Lebert described the first [[microscopic]] observations of [[multinucleated]] [[giant cells]] and fusiform cells as 'tumeur fiblastique'.<ref name="pmid7004712">{{cite journal| author=McCarthy EF| title=Giant-cell tumor of bone: an historical perspective. | journal=Clin Orthop Relat Res | year= 1980 | volume= | issue= 153 | pages= 14-25 | pmid=7004712 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7004712 }} </ref> | ||
*In 1854, Sir James Paget provided the first description of the giant cell tumor in English literature.<ref name="pmid19970672">{{cite journal| author=Jaffe HL, Lichtenstein L| title=Benign Chondroblastoma of Bone: A Reinterpretation of the So-Called Calcifying or Chondromatous Giant Cell Tumor. | journal=Am J Pathol | year= 1942 | volume= 18 | issue= 6 | pages= 969-91 | pmid=19970672 | doi= | pmc=2032980 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19970672 }} </ref> | *In 1854, Sir James Paget provided the first description of the giant cell tumor in English literature.<ref name="pmid19970672">{{cite journal| author=Jaffe HL, Lichtenstein L| title=Benign Chondroblastoma of Bone: A Reinterpretation of the So-Called Calcifying or Chondromatous Giant Cell Tumor. | journal=Am J Pathol | year= 1942 | volume= 18 | issue= 6 | pages= 969-91 | pmid=19970672 | doi= | pmc=2032980 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19970672 }} </ref> | ||
*In early 1900, Bloodgood a surgeon at Johns Hopkins University, was credited with coining the term "giant-cell tumor" in his publication on radiographic features, conservative treatment, and use of bone grafts.<ref name="pmid17862876">{{cite journal| author=Bloodgood JC| title=II. The Conservative Treatment of Giant-Cell Sarcoma, with the Study of Bone Transplantation. | journal=Ann Surg | year= 1912 | volume= 56 | issue= 2 | pages= 210-39 | pmid=17862876 | doi= | pmc=1407379 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17862876 }} </ref> | *In early 1900, Bloodgood a surgeon at Johns Hopkins University, was credited with coining the term "giant-cell tumor" in his publication on radiographic features, conservative treatment, and use of bone [[grafts]].<ref name="pmid17862876">{{cite journal| author=Bloodgood JC| title=II. The Conservative Treatment of Giant-Cell Sarcoma, with the Study of Bone Transplantation. | journal=Ann Surg | year= 1912 | volume= 56 | issue= 2 | pages= 210-39 | pmid=17862876 | doi= | pmc=1407379 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17862876 }} </ref> | ||
*In 1940, Jaffe and Lichtenstein described the clinical-radiographic-histologic identity of giant cell tumor.<ref name="pmid15595466">{{cite journal| author=Icihikawa K, Tanino R| title=Soft tissue giant cell tumor of low malignant potential. | journal=Tokai J Exp Clin Med | year= 2004 | volume= 29 | issue= 3 | pages= 91-5 | pmid=15595466 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15595466 }} </ref><ref name="pmid21089702">{{cite journal| author=Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC et al.| title=The efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro study. | journal=J Bone Joint Surg Br | year= 2010 | volume= 92 | issue= 10 | pages= 1475-9 | pmid=21089702 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id= | *In 1940, Jaffe and Lichtenstein described the clinical-radiographic-histologic identity of giant cell tumor.<ref name="pmid15595466">{{cite journal| author=Icihikawa K, Tanino R| title=Soft tissue giant cell tumor of low malignant potential. | journal=Tokai J Exp Clin Med | year= 2004 | volume= 29 | issue= 3 | pages= 91-5 | pmid=15595466 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15595466 }} </ref><ref name="pmid21089702">{{cite journal| author=Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC et al.| title=The efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro study. | journal=J Bone Joint Surg Br | year= 2010 | volume= 92 | issue= 10 | pages= 1475-9 | pmid=21089702 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgidbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21089702 }} </ref> | ||
==Classification== | |||
*Giant cell tumor (GCT) can be classified based on imaging findings and on mechanism of origin. | |||
===Mechanism of Origin=== | |||
Based on mechanism of origin, [[malignant]] giant cell tumor (MGCT) can be classified into: | |||
===Primary Malignant Giant Cell Tumor=== | |||
*When MGCT arises de novo, it is called primary MGCT. | |||
*Metastatizes to [[lung]] in 2-5%. | |||
===Secondary Malignant Giant Cell Tumor=== | |||
*It occurs following [[radiation]] or multiple resections of giant cell tumor. | |||
===Enneking (MSTS) Staging System=== | |||
*The Enneking surgical staging system (also known as the MSTS system) for benign [[Musculoskeletal system|musculoskeletal]] [[Tumor|tumors]] based on [[radiographic]] characteristics of the [[tumor]] host margin.<ref name="pmid20333492">{{cite journal| author=Jawad MU, Scully SP| title=In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system. | journal=Clin Orthop Relat Res | year= 2010 | volume= 468 | issue= 7 | pages= 2000-2 | pmid=20333492 | doi=10.1007/s11999-010-1315-7 | pmc=2882012 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20333492 }} </ref> | |||
*It is widely accepted and routinely used [[classification]]. | |||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 1000px" align="center" | |||
| valign="top" | | |||
|- | |||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Stages}} | |||
! style="background: #4479BA; width: 300px;" | {{fontcolor|#FFF|Description}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |1 | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Latent: Well demarcated borders | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |2 | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Active: Indistinct borders | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold; text-align:center;" |3 | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Aggressive: Indistinct borders | |||
|} | |||
==Pathophysiology== | ==Pathophysiology== | ||
*The exact [[pathogenesis]] of giant cell tumor (GCT) is not fully understood.<ref>{{cite book | last = Peabody | first = Terrance | title = Orthopaedic oncology : primary and metastatic tumors of the skeletal system | publisher = Springer | location = Cham | year = 2014 | isbn = 9783319073224 }}</ref> | *The exact [[pathogenesis]] of giant cell tumor (GCT) is not fully understood.<ref>{{cite book | last = Peabody | first = Terrance | title = Orthopaedic oncology : primary and metastatic tumors of the skeletal system | publisher = Springer | location = Cham | year = 2014 | isbn = 9783319073224 }}</ref><ref name="pmid12579271">{{cite journal |author=Gamberi G, Serra M, Ragazzini P, Magagnoli G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci P, Benassi MS |title=Identification of markers of possible prognostic value in 57 giant cell tumors of bone |journal=[[Oncology Reports]] |volume=10 |issue=2 |pages=351–6 |year=2003 |pmid=12579271 |doi= |url=http://www.spandidos-publications.com/or/10/2/351 |accessdate=2012-01-18}}</ref> | ||
*Various theories have been proposed concerning the [[pathogenesis]] of giant cell tumor: | *Various theories have been proposed concerning the [[pathogenesis]] of giant cell tumor: | ||
**Over-expression in RANK/RANKL signalling pathway with resultant over-proliferation of osteoclasts.<ref name="pmid15607894">{{cite journal| author=Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA et al.| title=Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. | journal=J Orthop Res | year= 2005 | volume= 23 | issue= 1 | pages= 203-9 | pmid=15607894 | doi=10.1016/j.orthres.2004.06.018 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15607894 }} </ref> | **Over-expression in [[RANK]]/[[RANKL]] signalling pathway with resultant over-proliferation of [[osteoclasts]].<ref name="pmid15607894">{{cite journal| author=Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA et al.| title=Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. | journal=J Orthop Res | year= 2005 | volume= 23 | issue= 1 | pages= 203-9 | pmid=15607894 | doi=10.1016/j.orthres.2004.06.018 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15607894 }} </ref> | ||
**The stromal cells are believed to be the major neoplastic and proliferative component of giant cell tumor. | **The [[Stromal cell|stromal cells]] are believed to be the major [[neoplastic]] and proliferative component of giant cell tumor. | ||
**The stromal cells often make osteoids, thus have the potential to differentiate into osteoblastic cells. | **The [[Stromal cell|stromal cells]] often make [[Osteoid|osteoids]], thus have the potential to differentiate into [[Osteoblast|osteoblastic cells]]. | ||
**Stromal cells in GCT secrete various chemokines including monocyte chemoattractant protein (MCP)-1 and SDF-1, which attract blood monocytes and stimulate their migration into tumor tissues. | **[[Monocyte|Monocytes]] derived from [[CD14]] and [[CD34]] positive precursor cells, which also express the chemokine receptor [[CXCR4]]. | ||
*Giant cell tumor of bone typically occur in the epiphyses of long bones. <ref name="ShrivastavaNawghare2008">{{cite journal|last1=Shrivastava|first1=Sandeep|last2=Nawghare|first2=Shishir P|last3=Kolwadkar|first3=Yogesh|last4=Singh|first4=Pradeep|title=Giant cell tumour in the diaphysis of radius – a report|journal=Cases Journal|volume=1|issue=1|year=2008|pages=106|issn=1757-1626|doi=10.1186/1757-1626-1-106}}</ref> | **[[Stromal cell|Stromal cells]] in GCT secrete various chemokines including [[monocyte]] chemoattractant protein ([[MCP-1|MCP]])-1 and [[Stromal cell-derived factor-1|SDF-1]], which attract blood [[monocytes]] and stimulate their migration into tumor tissues. | ||
*The bones often involved by epiphyseal GCT are distal femur, proximal tibia, and distal radius.<ref name="pmid1728946">{{cite journal| author=Bridge JA, Neff JR, Mouron BJ| title=Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. | journal=Cancer Genet Cytogenet | year= 1992 | volume= 58 | issue= 1 | pages= 2-13 | pmid=1728946 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1728946 }} </ref> | **The over-expression of [[interleukin 6]] (IL6) in GCT has been seen as a further [[autocrine]]/[[paracrine]] factor connected with the formation of multinucleated giant cell.<ref name="pmid14998856">{{cite journal| author=Gamberi G, Benassi MS, Ragazzini P, Pazzaglia L, Ponticelli F, Ferrari C et al.| title=Proteases and interleukin-6 gene analysis in 92 giant cell tumors of bone. | journal=Ann Oncol | year= 2004 | volume= 15 | issue= 3 | pages= 498-503 | pmid=14998856 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14998856 }} </ref> | ||
*Giant cell tumor of bone typically occur in the [[Epiphysis|epiphyses]] of [[Long bone|long bones]]. <ref name="ShrivastavaNawghare2008">{{cite journal|last1=Shrivastava|first1=Sandeep|last2=Nawghare|first2=Shishir P|last3=Kolwadkar|first3=Yogesh|last4=Singh|first4=Pradeep|title=Giant cell tumour in the diaphysis of radius – a report|journal=Cases Journal|volume=1|issue=1|year=2008|pages=106|issn=1757-1626|doi=10.1186/1757-1626-1-106}}</ref> | |||
*The bones often involved by epiphyseal GCT are distal [[femur]], proximal [[tibia]], and distal [[radius]].<ref name="pmid1728946">{{cite journal| author=Bridge JA, Neff JR, Mouron BJ| title=Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. | journal=Cancer Genet Cytogenet | year= 1992 | volume= 58 | issue= 1 | pages= 2-13 | pmid=1728946 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1728946 }} </ref> | |||
===Genetics=== | ===Genetics=== | ||
*Mutation in the histone 3.3 gene H3F3A (p.Gly34)is seen in approximately 92% of giant cell tumours of bone.<ref name="pmid28505000">{{cite journal| author=Amary F, Berisha F, Ye H, Gupta M, Gutteridge A, Baumhoer D et al.| title=H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone. | journal=Am J Surg Pathol | year= 2017 | volume= 41 | issue= 8 | pages= 1059-1068 | pmid=28505000 | doi=10.1097/PAS.0000000000000859 | pmc=5510691 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28505000 }} </ref><ref name="pmid26457357">{{cite journal| author=Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV| title=Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. | journal=Am J Surg Pathol | year= 2015 | volume= 39 | issue= 11 | pages= 1576-83 | pmid=26457357 | doi=10.1097/PAS.0000000000000512 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26457357 }} </ref><ref name="pmid8640728">{{cite journal| author=McComb EN, Johansson SL, Neff JR, Nelson M, Bridge JA| title=Chromosomal anomalies exclusive of telomeric associations in giant cell tumor of bone. | journal=Cancer Genet Cytogenet | year= 1996 | volume= 88 | issue= 2 | pages= 163-6 | pmid=8640728 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8640728 }} </ref> | *Mutation in the [[histone]] 3.3 gene [[H3F3A]] (p.Gly34)is seen in approximately 92% of giant cell tumours of bone.<ref name="pmid28505000">{{cite journal| author=Amary F, Berisha F, Ye H, Gupta M, Gutteridge A, Baumhoer D et al.| title=H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone. | journal=Am J Surg Pathol | year= 2017 | volume= 41 | issue= 8 | pages= 1059-1068 | pmid=28505000 | doi=10.1097/PAS.0000000000000859 | pmc=5510691 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28505000 }} </ref><ref name="pmid26457357">{{cite journal| author=Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV| title=Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. | journal=Am J Surg Pathol | year= 2015 | volume= 39 | issue= 11 | pages= 1576-83 | pmid=26457357 | doi=10.1097/PAS.0000000000000512 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26457357 }} </ref><ref name="pmid8640728">{{cite journal| author=McComb EN, Johansson SL, Neff JR, Nelson M, Bridge JA| title=Chromosomal anomalies exclusive of telomeric associations in giant cell tumor of bone. | journal=Cancer Genet Cytogenet | year= 1996 | volume= 88 | issue= 2 | pages= 163-6 | pmid=8640728 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8640728 }} </ref> | ||
*A subset of the mutations can be easily detected using a G34W mutation specific antibody. | *A subset of the [[mutations]] can be easily detected using a G34W [[mutation]] specific [[antibody]]. | ||
==Causes== | ==Causes== | ||
| Line 35: | Line 70: | ||
==Differentiating Giant cell tumor of bone from other Diseases== | ==Differentiating Giant cell tumor of bone from other Diseases== | ||
Giant cell tumor of bone must be differentiated from: | Giant cell tumor of bone must be differentiated from:<ref name="pmid10095427">{{cite journal| author=Salzer-Kuntschik M| title=[Differential diagnosis of giant cell tumor of bone]. | journal=Verh Dtsch Ges Pathol | year= 1998 | volume= 82 | issue= | pages= 154-9 | pmid=10095427 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10095427 }} </ref> | ||
*[[ | *[[Brown tumor|Brown tumor of hyperparathyroidism]] | ||
**It can look like GCT on radiographs except it occurs as multiple lesions and associated with serum [[calcium]] level abnormalities. | |||
*[[Chondroblastoma]] | *[[Chondroblastoma]] | ||
* | **It is [[epiphyseal]] in location | ||
**It may also demonstrate [[aneurysmal bone cyst]] formation | |||
*[[ | **It has extensive surrounding [[soft tissue]] and marrow [[edema]] | ||
*[[ | **[[Chondroblastoma]] have sclerotic margin and central [[calcification]] of [[Chondroid chordoma|chondroid]] matrix "ring and arcs" pattern | ||
* | *Giant cell rich [[osteosarcoma]] | ||
*[[ | **It occurs in [[metaphysis]] of the bone | ||
* | **It has bimodal age distribution; affecting adolescents and elderly. | ||
*Chordoma | |||
**It mimics GCT in [[sacrum]] | |||
* | **It occurs in midline | ||
*Giant cell | |||
* | |||
* | |||
* | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
*In the United States and Europe, giant cell tumors(GCT) represent approximately 5% of all primary bone tumors and 21% of all benign bone tumors.<ref name="pmid12579271">{{cite journal |author=Gamberi G, Serra M, Ragazzini P, Magagnoli G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci P, Benassi MS |title=Identification of markers of possible prognostic value in 57 giant cell tumors of bone |journal=[[Oncology Reports]] |volume=10 |issue=2 |pages=351–6 |year=2003 |pmid=12579271 |doi= |url=http://www.spandidos-publications.com/or/10/2/351 |accessdate=2012-01-18}}</ref> | *In the United States and Europe, giant cell tumors(GCT) represent approximately 5% of all primary [[bone tumors]] and 21% of all [[benign]] [[bone tumors]].<ref name="pmid12579271">{{cite journal |author=Gamberi G, Serra M, Ragazzini P, Magagnoli G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci P, Benassi MS |title=Identification of markers of possible prognostic value in 57 giant cell tumors of bone |journal=[[Oncology Reports]] |volume=10 |issue=2 |pages=351–6 |year=2003 |pmid=12579271 |doi= |url=http://www.spandidos-publications.com/or/10/2/351 |accessdate=2012-01-18}}</ref> | ||
*Giant cell tumors occur most commonly in the third decade of life; less than 5% of GCTs occur in patients who are skeletally immature.<ref name="pmid3805057">{{cite journal| author=Campanacci M, Baldini N, Boriani S, Sudanese A| title=Giant-cell tumor of bone. | journal=J Bone Joint Surg Am | year= 1987 | volume= 69 | issue= 1 | pages= 106-14 | pmid=3805057 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3805057 }} </ref> | *Giant cell tumors occur most commonly in the third decade of life; less than 5% of GCTs occur in patients who are skeletally immature.<ref name="pmid3805057">{{cite journal| author=Campanacci M, Baldini N, Boriani S, Sudanese A| title=Giant-cell tumor of bone. | journal=J Bone Joint Surg Am | year= 1987 | volume= 69 | issue= 1 | pages= 106-14 | pmid=3805057 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3805057 }} </ref> | ||
*GCTs usually occur in patients older than 19 years. <ref name="pmid1609086">{{cite journal| author=Kransdorf MJ, Sweet DE, Buetow PC, Giudici MA, Moser RP| title=Giant cell tumor in skeletally immature patients. | journal=Radiology | year= 1992 | volume= 184 | issue= 1 | pages= 233-7 | pmid=1609086 | doi=10.1148/radiology.184.1.1609086 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1609086 }} </ref> | *GCTs usually occur in patients older than 19 years. <ref name="pmid1609086">{{cite journal| author=Kransdorf MJ, Sweet DE, Buetow PC, Giudici MA, Moser RP| title=Giant cell tumor in skeletally immature patients. | journal=Radiology | year= 1992 | volume= 184 | issue= 1 | pages= 233-7 | pmid=1609086 | doi=10.1148/radiology.184.1.1609086 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1609086 }} </ref> | ||
| Line 62: | Line 92: | ||
*There is no racial predilection to giant cell tumor. | *There is no racial predilection to giant cell tumor. | ||
==Risk Factors== | |||
There are no established risk factors for giant cell tumor. | |||
== | ==Screening== | ||
There is insufficient evidence to recommend routine screening for giant cell tumor. | |||
=== | ==Natural History, Complications, and Prognosis== | ||
*The [[prognosis]] of giant cell tumor is generally | *If left untreated, few patients with giant cell tumor may progress to develop [[lung]] [[metastasis]].<ref name="MuheremuNiu2014">{{cite journal|last1=Muheremu|first1=Aikeremujiang|last2=Niu|first2=Xiaohui|title=Pulmonary metastasis of giant cell tumor of bones|journal=World Journal of Surgical Oncology|volume=12|issue=1|year=2014|pages=261|issn=1477-7819|doi=10.1186/1477-7819-12-261}}</ref> | ||
*Common complications of giant cell tumor include: | |||
**[[Malignant transformation]] | |||
***[[Malignant transformation]] is far more common in men (M:F of ~3:1) | |||
***Sarcomatous transformation is observed, especially in [[radiotherapy]] treated inoperable tumors. | |||
**Secondary [[Aneurysmal bone cyst]] (ABC) | |||
***To differentiate from primary ABC based on enhancing soft-tissue component in GCT; which is not present in primary ABC. | |||
**Recurrence | |||
***Local recurrence rate of giant cell tumor of bone is about 10 to 40%. | |||
***Lucency is seen at bone-cement interface | |||
***It is diagnosed with [[Biopsy|CT guided biopsy]] | |||
**Pathologic [[fracture]] | |||
**[[Infection|Postoperative infection]] | |||
***There is an increased risk with en bloc resection followed by endoprosthesis. | |||
*The [[prognosis]] of giant cell tumor is generally good, despite of recurrences and [[pulmonary metastases]]. | |||
*The [[mortality rate]] due to giant cell tumour is about 4%. | |||
==Diagnosis== | ==Diagnosis== | ||
===Diagnostic Study of Choice=== | |||
{| align="right" | |||
| | |||
[[File:Giant-cell-tumour-histology.jpg|300px|thumb| Histological appearance: Giant Cell Tumor. [https://radiopaedia.org/cases/giant-cell-tumour-histology?lang=us Source: Case courtesy of Dr Alexandra Stanislavsky, Radiopaedia.org, rID: 14823]]] | |||
|} | |||
*Biopsy is the diagnostic study of choice for the diagnosis of giant cell tumor.<ref name="pmid7497439">{{cite journal| author=Molenaar WM, van den Berg E, Dolfin AC, Zorgdrager H, Hoekstra HJ| title=Cytogenetics of fine needle aspiration biopsies of sarcomas. | journal=Cancer Genet Cytogenet | year= 1995 | volume= 84 | issue= 1 | pages= 27-31 | pmid=7497439 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7497439 }} </ref> | |||
*Gross pathological findings include: | |||
**Giant cell tumors are variable in appearance, depending on amount of [[hemorrhage]], presence of co-existent [[aneurysmal bone cyst]], and degree of presence [[fibrosis]]. | |||
*Histopathological findings include: | |||
**Presence of numerous [[Cathepsin K|Cathepsin-K]] producing, [[CD33]] +, [[CD14]] - [[multinucleated]] [[osteoclast]]-like giant cells and plump spindle-shaped [[Stromal cell|stromal cells]] that represent the main proliferating cell population. | |||
**The spindle-shaped [[mononuclear cells]] represents the neoplastic population and are characterized at the [[Cytogenetics|cytogenetic]] level by telomeric associations and a peculiar telomere-protecting capping mechanism. | |||
*Areas of regressive change such as [[necrosis]] or [[fibrosis]] as well as extensive [[Bleeding|hemorrhage]] are frequently present. | |||
*Frequent mitotic figures in the [[mononuclear cells]] may be seen, especially in pregnant women or those on the [[oral contraceptive pill]], due to increased hormone levels. | |||
===History and Symptoms=== | ===History and Symptoms=== | ||
* | Symptoms of giant cell tumor include: | ||
* | *Localized bone [[pain]] | ||
*Localized [[swelling]] | |||
*Decreased [[range of motion]] of the affected joint | |||
*[[Limp]] | |||
{| align="right" | |||
| | |||
[[File:Giant-cell-tumour-femur xray.jpg|200px|thumb| X-Ray of Femur showing giant cell tumor. [https://radiopaedia.org/cases/giant-cell-tumour-femur?lang=us Source: Case courtesy of Radswiki, Radiopaedia.org, rID: 11453]]] | |||
|} | |||
===Physical Examination=== | ===Physical Examination=== | ||
*Patients with giant cell tumor usually appears well. | |||
*Common physical examination findings of giant cell tumor include:<ref>{{cite book | last = Peabody | first = Terrance | title = Orthopaedic oncology : primary and metastatic tumors of the skeletal system | publisher = Springer | location = Cham | year = 2014 | isbn = 9783319073224 }}</ref> | |||
**[[Tenderness]] to [[palpation]] | |||
**Soft tissue [[swelling]] | |||
**Decreased [[range of motion]] | |||
**[[Muscle atrophy]] | |||
**Joint effusion | |||
**Involvement of adjacent structures such as peripheral [[nerves]] or [[veins]] | |||
===Laboratory Findings=== | |||
There are no diagnostic laboratory findings associated with giant cell tumor. | |||
===Electrocardiogram=== | |||
There are no [[ECG]] findings associated with giant cell tumor. | |||
{| align="right" | |||
| | |||
[[File:CT giant cell tumor.gif|200px|thumb| CT scan of Femur showing giant cell tumor. [https://radiopaedia.org/cases/giant-cell-tumour-femur?lang=us Source: Case courtesy of Radswiki, Radiopaedia.org, rID: 11453]]] | |||
|} | |||
===X Ray=== | ===X Ray=== | ||
X-ray | *Three views of affected [[bone]] or [[joint]] are recommended. | ||
* | *X-ray findings include:<ref name="pmid11553835">{{cite journal |author=Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ |title=From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation |journal=[[Radiographics : a Review Publication of the Radiological Society of North America, Inc]] |volume=21 |issue=5 |pages=1283–309 |year=2001 |pmid=11553835 |doi= |url=http://radiographics.rsnajnls.org/cgi/pmidlookup?view=long&pmid=11553835 |accessdate=2012-01-18}}</ref> | ||
*Narrow zone of transition | *[[Eccentric Lesion|Eccentric]] [[Metaphysis|metaphyseal]] location and grow to the [[articular surface]] of the involved bone | ||
*No surrounding sclerosis | *Narrow zone of transition with a broader zone of transition is observed in more aggressive giant cell tumors | ||
*Overlying cortex is thinned, expanded or deficient | *No surrounding [[sclerosis]] | ||
*Periosteal reaction | *Overlying [[cortex]] is thinned, expanded or deficient | ||
*[[Periosteal reaction]] usually not seen | |||
*No matrix [[calcification]]/mineralisation | *No matrix [[calcification]]/mineralisation | ||
'''Chest X-Ray''' | |||
*Chest [[Radiography|radiograph]] should be done to look for benign [[pulmonary]] [[metastasis]] which may occur with giant cell tumor. | |||
===Echocardiography or Ultrasound=== | |||
There are no [[echocardiography]]/[[ultrasound]] findings associated with giant cell tumor. | |||
===CT scan=== | |||
CT findings for giant cell tumor include: | |||
*Absence of bone and intralesional [[mineralization]]. | |||
{| align="right" | |||
| | |||
[[File:MRI giant-cell-tumour-femur.jpg|200px|thumb| MRI of Femur showing giant cell tumor. [https://radiopaedia.org/cases/giant-cell-tumour-femur?lang=us Source: Case courtesy of Radswiki, Radiopaedia.org, rID: 11453]]] | |||
|} | |||
===MRI=== | ===MRI=== | ||
*MRI is performed to delineate the extent of the [[neoplasm]]. | |||
*MRI findings of giant cell tumor of bone include: | |||
**T1: Low to intermediate solid component and Low signal periphery | |||
**T2: Intermediate to high signal | |||
**MRI may also reveal an effusion of the [[joint]]. | |||
===Other Imaging Findings=== | |||
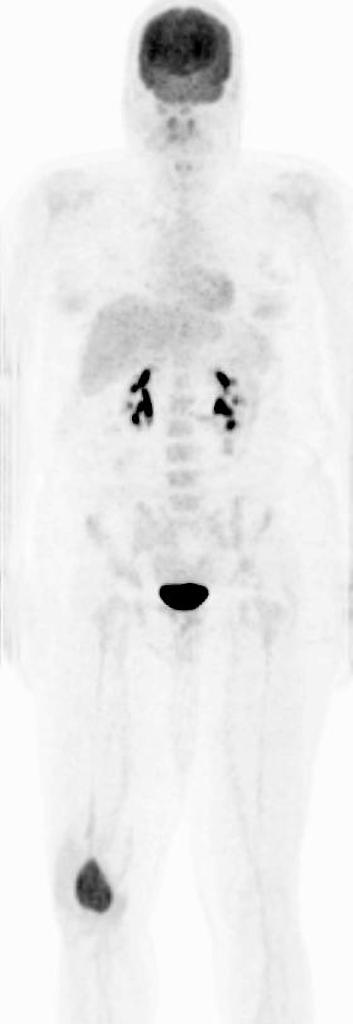
===Bone Scan=== | |||
*Increased uptake is observed around the lesion of giant cell tumor, especially around the periphery, with a central photopenic region (doughnut sign). | |||
*Increased blood pool activity is also observed. | |||
===Other Diagnostic Studies=== | |||
There are no other diagnostic studies associated with giant cell tumor. | |||
=== | |||
* | |||
*Increased blood pool activity is also observed | |||
==Treatment== | ==Treatment== | ||
===Medical Therapy=== | |||
*[[Denosumab]] | |||
**It is recommended for the treatment of unresectable GCT of bone (GCTB) in adults and skeletally mature adolescents.<ref name="pmid20149736">{{cite journal| author=Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY et al.| title=Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. | journal=Lancet Oncol | year= 2010 | volume= 11 | issue= 3 | pages= 275-80 | pmid=20149736 | doi=10.1016/S1470-2045(10)70010-3 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20149736 }} </ref> | |||
**It is a [[monoclonal antibody]] against [[RANKL|RANK-ligand]]. | |||
**It decreases the size of the bone defect in giant cell tumor. | |||
**It shows dramatic [[sclerosis]] and reconstitution of [[cortical bone]] after treatment. | |||
*[[Bisphosphonates]] | |||
**They are [[osteoclast]] inhibitors which may decrease the size of the defect in giant cell tumors.<ref name="pmid18322700">{{cite journal| author=Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G et al.| title=Giant cell tumor of bone: treatment and outcome of 214 cases. | journal=J Cancer Res Clin Oncol | year= 2008 | volume= 134 | issue= 9 | pages= 969-78 | pmid=18322700 | doi=10.1007/s00432-008-0370-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18322700 }} </ref><ref name="pmid17962092">{{cite journal| author=Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF| title=Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case-control study. | journal=Bone | year= 2008 | volume= 42 | issue= 1 | pages= 68-73 | pmid=17962092 | doi=10.1016/j.bone.2007.08.038 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17962092 }} </ref> | |||
{| align="right" | |||
| | |||
[[File:Bone scan giant-cell-tumour-femur.jpg|200px|thumb| Bone scan showing giant cell tumor. [https://radiopaedia.org/cases/giant-cell-tumour-femur?lang=us Source: Case courtesy of Radswiki, Radiopaedia.org, rID: 11453]]] | |||
|} | |||
===Surgery=== | ===Surgery=== | ||
* | Surgery is the mainstay of treatment for giant cell tumor.<ref name="PuriAgarwal2007">{{cite journal|last1=Puri|first1=Ajay|last2=Agarwal|first2=Manish|title=Treatment of giant cell tumor of bone: Current concepts|journal=Indian Journal of Orthopaedics|volume=41|issue=2|year=2007|pages=101|issn=0019-5413|doi=10.4103/0019-5413.32039}}</ref><ref name="pmid3006962">{{cite journal| author=Goldring SR, Schiller AL, Mankin HJ, Dayer JM, Krane SM| title=Characterization of cells from human giant cell tumors of bone. | journal=Clin Orthop Relat Res | year= 1986 | volume= | issue= 204 | pages= 59-75 | pmid=3006962 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3006962 }} </ref> | ||
* | |||
* | ===Extensive Curettage and Reconstruction with adjuvant treatment=== | ||
* | |||
'''Indications''' | |||
*Lesions amenable to currettage<ref name="pmid21089702">{{cite journal| author=Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC et al.| title=The efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro study. | journal=J Bone Joint Surg Br | year= 2010 | volume= 92 | issue= 10 | pages= 1475-9 | pmid=21089702 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21089702 }} </ref><ref name="pmid3006962">{{cite journal| author=Goldring SR, Schiller AL, Mankin HJ, Dayer JM, Krane SM| title=Characterization of cells from human giant cell tumors of bone. | journal=Clin Orthop Relat Res | year= 1986 | volume= | issue= 204 | pages= 59-75 | pmid=3006962 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3006962 }} </ref> | |||
*Hand lesion treatment is controversial. | |||
*If no cortical breakthrough, then treat with curettage and cementing. | |||
*If significant cortical breakthrough, then intercalary resection with free fibular graft. | |||
'''Technique''' | |||
*The challenge of treatment is to remove lesion while preserving joint and providing support to subchondral joint. | |||
*Extensive exterioration which is removal of a large [[Cortical bone|cortical]] window over the lesion is required. | |||
*Lesion can be filled with [[bone cement]] or [[autograft]]/[[allograft]] bone. | |||
*Synthetic grafts such as [[polymethylmethacrylate]] (PMMA) have improved patient recovery and tumor removal due to the graft's [[exothermic reaction]] that causes thermal [[necrosis]] of cells and innate inflammatory reaction.<ref name="pmid16736146">{{cite journal| author=Oh JH, Yoon PW, Lee SH, Cho HS, Kim WS, Kim HS| title=Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant. | journal=Int Orthop | year= 2006 | volume= 30 | issue= 6 | pages= 490-4 | pmid=16736146 | doi=10.1007/s00264-006-0154-3 | pmc=3172752 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16736146 }} </ref> | |||
'''Chemical Adjuvants Used''' | |||
*[[Liquid nitrogen]] | |||
*[[Alcohols]] | |||
*[[Phenol]] | |||
*[[Hydrogen peroxide]] | |||
*[[Zinc chloride]] | |||
*[[Argon|Argon Laser]] | |||
'''Outcomes''' | |||
*10-30% recurrence with [[curettage]] alone verses 3% with [[adjuvant treatment]]. | |||
===Amputation=== | |||
'''Indications''' | |||
*[[Hand]] lesions with [[Cortical bone|cortical]] breakthrough which are not amendable to intercalary resection.<ref name="pmid22662295">{{cite journal| author=Kim Y, Nizami S, Goto H, Lee FY| title=Modern interpretation of giant cell tumor of bone: predominantly osteoclastogenic stromal tumor. | journal=Clin Orthop Surg | year= 2012 | volume= 4 | issue= 2 | pages= 107-16 | pmid=22662295 | doi=10.4055/cios.2012.4.2.107 | pmc=3360182 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22662295 }} </ref> | |||
===Radiation Therapy=== | |||
{| align="right" | |||
| | |||
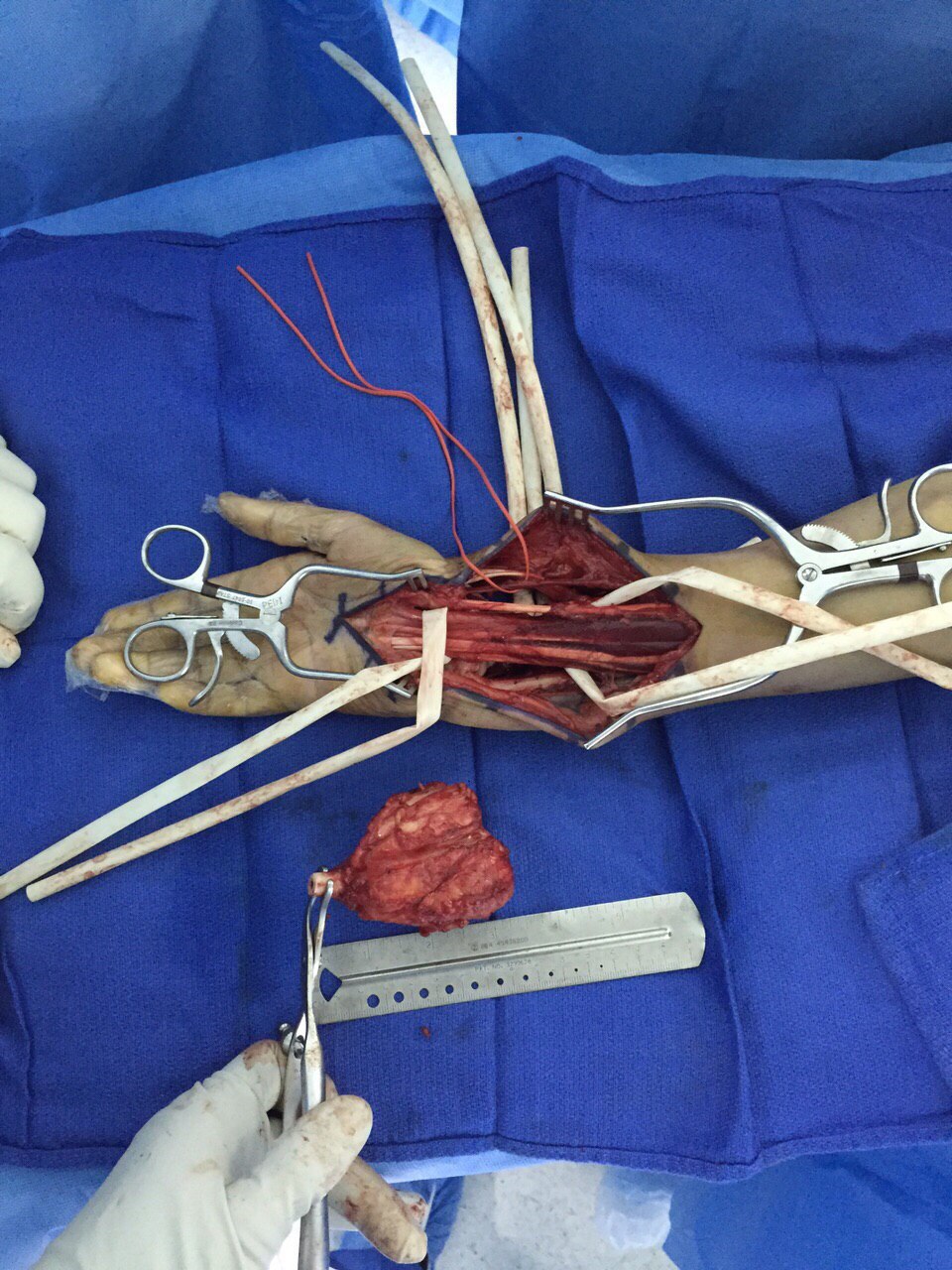
[[File:Giant cell tumor intra-op.JPG|300px|thumb|Excision of giant cell tumor of distal radius. Source: Case courtesy by: [[User:Rohan Bhimani|Dr. Rohan A. Bhimani]]]] | |||
|} | |||
'''Indications''' | |||
*Reserved for cases in which surgical treatment is not feasible.<ref name="pmid10192357">{{cite journal| author=Nair MK, Jyothirmayi R| title=Radiation therapy in the treatment of giant cell tumor of bone. | journal=Int J Radiat Oncol Biol Phys | year= 1999 | volume= 43 | issue= 5 | pages= 1065-9 | pmid=10192357 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10192357 }} </ref> | |||
*[[Tumors]] are in locations not amenable to operative treatment. | |||
*Patients in whom a potential for significant [[morbidity]] from tumor [[relapse]] or subsequent [[surgery]] exists. | |||
'''Technique''' | |||
*Megavoltage radiation is used. | |||
*Dose: 35 to 70 Gy<ref name="pmid8491687">{{cite journal| author=Bennett CJ, Marcus RB, Million RR, Enneking WF| title=Radiation therapy for giant cell tumor of bone. | journal=Int J Radiat Oncol Biol Phys | year= 1993 | volume= 26 | issue= 2 | pages= 299-304 | pmid=8491687 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8491687 }} </ref> | |||
===Primary Prevention=== | |||
There are no established measures for the [[primary prevention]] of giant cell tumor. | |||
===Secondary Prevention=== | |||
There are no established measures for the [[secondary prevention]] of giant cell tumor. | |||
<br><br><br><br><br><br> | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Latest revision as of 21:28, 24 January 2019
For patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rohan A. Bhimani, M.B.B.S., D.N.B., M.Ch.[2]
Synonyms and keywords: Osteoclastoma; Giant cell myeloma; Giant cell tumor; Giant cell tumor of the bone
Overview
Giant cell tumor of bone is a relatively uncommon tumor of the bone. It is characterized by the presence of multinucleated giant cells (osteoclast-like cells). Giant cell tumor of bone accounts for 4-5% of primary bone tumors and 18.2% of benign bone tumors. Giant cell tumor of bone almost invariably occur when the growth plate has closed and are therefore typically observed in early adulthood, with 80% of cases reported between the ages of 20 and 50, with a peak incidence between 20 and 30. Giant cell tumor of bone typically occur as single lesions. They usually prefers the epiphyses of long bones. Although any bone can be affected, the most common sites are distal femur, proximal tibia, and distal radius. The progression to giant cell tumor of bone usually involves the over-expression in RANK/RANKL signalling pathway with resultant over-proliferation of osteoclasts. On gross pathology, hemorrhage, presence of co-existent aneurysmal bone cyst, and fibrosis are characteristic findings of giant cell tumor of bone. On microscopic histopathological analysis, prominent and diffuse osteoclastic giant cells and mononuclear cells with frequent mitotic figures are characteristic findings of giant cell tumor of bone. Symptoms of giant cell tumor of bone include localized pain, localized swelling, and decreased range of motion. Physical examination findings will depend on the location of the giant cell tumor. Common physical examination findings of giant cell tumor are localized swelling and tenderness at the site of the tumor. Giant cell tumor of bone must be differentiated from aneurysmal bone cyst, chondroblastoma, simple bone cyst, osteoblastoma,giant cell rich osteosarcoma, and brown tumor of hyperparathyroidism. X-ray may be helpful in the diagnosis of giant cell tumor of bone. Common complications of giant cell tumor include malignant transformation, recurrence, and metastasis. Findings on x-ray suggestive of giant cell tumor include metaepiphyseal location of mass and grow to the articular surface of the involved bone with narrow zone of transition. Surgery is the mainstay of treatment for giant cell tumor.
Historical Perspective
- In 1818, Cooper and Travers first described giant cell tumor (GCT) of bone.[1]
- In 1845, Par H. Lebert described the first microscopic observations of multinucleated giant cells and fusiform cells as 'tumeur fiblastique'.[2]
- In 1854, Sir James Paget provided the first description of the giant cell tumor in English literature.[3]
- In early 1900, Bloodgood a surgeon at Johns Hopkins University, was credited with coining the term "giant-cell tumor" in his publication on radiographic features, conservative treatment, and use of bone grafts.[4]
- In 1940, Jaffe and Lichtenstein described the clinical-radiographic-histologic identity of giant cell tumor.[5][6]
Classification
- Giant cell tumor (GCT) can be classified based on imaging findings and on mechanism of origin.
Mechanism of Origin
Based on mechanism of origin, malignant giant cell tumor (MGCT) can be classified into:
Primary Malignant Giant Cell Tumor
- When MGCT arises de novo, it is called primary MGCT.
- Metastatizes to lung in 2-5%.
Secondary Malignant Giant Cell Tumor
- It occurs following radiation or multiple resections of giant cell tumor.
Enneking (MSTS) Staging System
- The Enneking surgical staging system (also known as the MSTS system) for benign musculoskeletal tumors based on radiographic characteristics of the tumor host margin.[7]
- It is widely accepted and routinely used classification.
| Stages | Description |
|---|---|
| 1 | Latent: Well demarcated borders |
| 2 | Active: Indistinct borders |
| 3 | Aggressive: Indistinct borders |
Pathophysiology
- The exact pathogenesis of giant cell tumor (GCT) is not fully understood.[8][9]
- Various theories have been proposed concerning the pathogenesis of giant cell tumor:
- Over-expression in RANK/RANKL signalling pathway with resultant over-proliferation of osteoclasts.[10]
- The stromal cells are believed to be the major neoplastic and proliferative component of giant cell tumor.
- The stromal cells often make osteoids, thus have the potential to differentiate into osteoblastic cells.
- Monocytes derived from CD14 and CD34 positive precursor cells, which also express the chemokine receptor CXCR4.
- Stromal cells in GCT secrete various chemokines including monocyte chemoattractant protein (MCP)-1 and SDF-1, which attract blood monocytes and stimulate their migration into tumor tissues.
- The over-expression of interleukin 6 (IL6) in GCT has been seen as a further autocrine/paracrine factor connected with the formation of multinucleated giant cell.[11]
- Giant cell tumor of bone typically occur in the epiphyses of long bones. [12]
- The bones often involved by epiphyseal GCT are distal femur, proximal tibia, and distal radius.[13]
Genetics
- Mutation in the histone 3.3 gene H3F3A (p.Gly34)is seen in approximately 92% of giant cell tumours of bone.[14][15][16]
- A subset of the mutations can be easily detected using a G34W mutation specific antibody.
Causes
There are no established causes for giant cell tumor of the bone.[17]
Differentiating Giant cell tumor of bone from other Diseases
Giant cell tumor of bone must be differentiated from:[18]
- Brown tumor of hyperparathyroidism
- It can look like GCT on radiographs except it occurs as multiple lesions and associated with serum calcium level abnormalities.
- Chondroblastoma
- It is epiphyseal in location
- It may also demonstrate aneurysmal bone cyst formation
- It has extensive surrounding soft tissue and marrow edema
- Chondroblastoma have sclerotic margin and central calcification of chondroid matrix "ring and arcs" pattern
- Giant cell rich osteosarcoma
- It occurs in metaphysis of the bone
- It has bimodal age distribution; affecting adolescents and elderly.
- Chordoma
- It mimics GCT in sacrum
- It occurs in midline
Epidemiology and Demographics
- In the United States and Europe, giant cell tumors(GCT) represent approximately 5% of all primary bone tumors and 21% of all benign bone tumors.[9]
- Giant cell tumors occur most commonly in the third decade of life; less than 5% of GCTs occur in patients who are skeletally immature.[19]
- GCTs usually occur in patients older than 19 years. [20]
- Women are more commonly affected than men, with a 1.5:1 ratio.[21]
- There is no racial predilection to giant cell tumor.
Risk Factors
There are no established risk factors for giant cell tumor.
Screening
There is insufficient evidence to recommend routine screening for giant cell tumor.
Natural History, Complications, and Prognosis
- If left untreated, few patients with giant cell tumor may progress to develop lung metastasis.[22]
- Common complications of giant cell tumor include:
- Malignant transformation
- Malignant transformation is far more common in men (M:F of ~3:1)
- Sarcomatous transformation is observed, especially in radiotherapy treated inoperable tumors.
- Secondary Aneurysmal bone cyst (ABC)
- To differentiate from primary ABC based on enhancing soft-tissue component in GCT; which is not present in primary ABC.
- Recurrence
- Local recurrence rate of giant cell tumor of bone is about 10 to 40%.
- Lucency is seen at bone-cement interface
- It is diagnosed with CT guided biopsy
- Pathologic fracture
- Postoperative infection
- There is an increased risk with en bloc resection followed by endoprosthesis.
- Malignant transformation
- The prognosis of giant cell tumor is generally good, despite of recurrences and pulmonary metastases.
- The mortality rate due to giant cell tumour is about 4%.
Diagnosis
Diagnostic Study of Choice
 |
- Biopsy is the diagnostic study of choice for the diagnosis of giant cell tumor.[23]
- Gross pathological findings include:
- Giant cell tumors are variable in appearance, depending on amount of hemorrhage, presence of co-existent aneurysmal bone cyst, and degree of presence fibrosis.
- Histopathological findings include:
- Presence of numerous Cathepsin-K producing, CD33 +, CD14 - multinucleated osteoclast-like giant cells and plump spindle-shaped stromal cells that represent the main proliferating cell population.
- The spindle-shaped mononuclear cells represents the neoplastic population and are characterized at the cytogenetic level by telomeric associations and a peculiar telomere-protecting capping mechanism.
- Areas of regressive change such as necrosis or fibrosis as well as extensive hemorrhage are frequently present.
- Frequent mitotic figures in the mononuclear cells may be seen, especially in pregnant women or those on the oral contraceptive pill, due to increased hormone levels.
History and Symptoms
Symptoms of giant cell tumor include:
- Localized bone pain
- Localized swelling
- Decreased range of motion of the affected joint
- Limp
 |
Physical Examination
- Patients with giant cell tumor usually appears well.
- Common physical examination findings of giant cell tumor include:[24]
- Tenderness to palpation
- Soft tissue swelling
- Decreased range of motion
- Muscle atrophy
- Joint effusion
- Involvement of adjacent structures such as peripheral nerves or veins
Laboratory Findings
There are no diagnostic laboratory findings associated with giant cell tumor.
Electrocardiogram
There are no ECG findings associated with giant cell tumor.
 |
X Ray
- Three views of affected bone or joint are recommended.
- X-ray findings include:[25]
- Eccentric metaphyseal location and grow to the articular surface of the involved bone
- Narrow zone of transition with a broader zone of transition is observed in more aggressive giant cell tumors
- No surrounding sclerosis
- Overlying cortex is thinned, expanded or deficient
- Periosteal reaction usually not seen
- No matrix calcification/mineralisation
Chest X-Ray
- Chest radiograph should be done to look for benign pulmonary metastasis which may occur with giant cell tumor.
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with giant cell tumor.
CT scan
CT findings for giant cell tumor include:
- Absence of bone and intralesional mineralization.
 |
MRI
- MRI is performed to delineate the extent of the neoplasm.
- MRI findings of giant cell tumor of bone include:
- T1: Low to intermediate solid component and Low signal periphery
- T2: Intermediate to high signal
- MRI may also reveal an effusion of the joint.
Other Imaging Findings
Bone Scan
- Increased uptake is observed around the lesion of giant cell tumor, especially around the periphery, with a central photopenic region (doughnut sign).
- Increased blood pool activity is also observed.
Other Diagnostic Studies
There are no other diagnostic studies associated with giant cell tumor.
Treatment
Medical Therapy
- Denosumab
- It is recommended for the treatment of unresectable GCT of bone (GCTB) in adults and skeletally mature adolescents.[26]
- It is a monoclonal antibody against RANK-ligand.
- It decreases the size of the bone defect in giant cell tumor.
- It shows dramatic sclerosis and reconstitution of cortical bone after treatment.
- Bisphosphonates
- They are osteoclast inhibitors which may decrease the size of the defect in giant cell tumors.[1][27]
 |
Surgery
Surgery is the mainstay of treatment for giant cell tumor.[28][29]
Extensive Curettage and Reconstruction with adjuvant treatment
Indications
- Lesions amenable to currettage[6][29]
- Hand lesion treatment is controversial.
- If no cortical breakthrough, then treat with curettage and cementing.
- If significant cortical breakthrough, then intercalary resection with free fibular graft.
Technique
- The challenge of treatment is to remove lesion while preserving joint and providing support to subchondral joint.
- Extensive exterioration which is removal of a large cortical window over the lesion is required.
- Lesion can be filled with bone cement or autograft/allograft bone.
- Synthetic grafts such as polymethylmethacrylate (PMMA) have improved patient recovery and tumor removal due to the graft's exothermic reaction that causes thermal necrosis of cells and innate inflammatory reaction.[30]
Chemical Adjuvants Used
Outcomes
- 10-30% recurrence with curettage alone verses 3% with adjuvant treatment.
Amputation
Indications
Radiation Therapy
 |
Indications
- Reserved for cases in which surgical treatment is not feasible.[32]
- Tumors are in locations not amenable to operative treatment.
- Patients in whom a potential for significant morbidity from tumor relapse or subsequent surgery exists.
Technique
- Megavoltage radiation is used.
- Dose: 35 to 70 Gy[33]
Primary Prevention
There are no established measures for the primary prevention of giant cell tumor.
Secondary Prevention
There are no established measures for the secondary prevention of giant cell tumor.
References
- ↑ 1.0 1.1 Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G; et al. (2008). "Giant cell tumor of bone: treatment and outcome of 214 cases". J Cancer Res Clin Oncol. 134 (9): 969–78. doi:10.1007/s00432-008-0370-x. PMID 18322700.
- ↑ McCarthy EF (1980). "Giant-cell tumor of bone: an historical perspective". Clin Orthop Relat Res (153): 14–25. PMID 7004712.
- ↑ Jaffe HL, Lichtenstein L (1942). "Benign Chondroblastoma of Bone: A Reinterpretation of the So-Called Calcifying or Chondromatous Giant Cell Tumor". Am J Pathol. 18 (6): 969–91. PMC 2032980. PMID 19970672.
- ↑ Bloodgood JC (1912). "II. The Conservative Treatment of Giant-Cell Sarcoma, with the Study of Bone Transplantation". Ann Surg. 56 (2): 210–39. PMC 1407379. PMID 17862876.
- ↑ Icihikawa K, Tanino R (2004). "Soft tissue giant cell tumor of low malignant potential". Tokai J Exp Clin Med. 29 (3): 91–5. PMID 15595466.
- ↑ 6.0 6.1 Gortzak Y, Kandel R, Deheshi B, Werier J, Turcotte RE, Ferguson PC; et al. (2010). "The efficacy of chemical adjuvants on giant-cell tumour of bone. An in vitro study". J Bone Joint Surg Br. 92 (10): 1475–9. PMID 21089702.
- ↑ Jawad MU, Scully SP (2010). "In brief: classifications in brief: enneking classification: benign and malignant tumors of the musculoskeletal system". Clin Orthop Relat Res. 468 (7): 2000–2. doi:10.1007/s11999-010-1315-7. PMC 2882012. PMID 20333492.
- ↑ Peabody, Terrance (2014). Orthopaedic oncology : primary and metastatic tumors of the skeletal system. Cham: Springer. ISBN 9783319073224.
- ↑ 9.0 9.1 Gamberi G, Serra M, Ragazzini P, Magagnoli G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci P, Benassi MS (2003). "Identification of markers of possible prognostic value in 57 giant cell tumors of bone". Oncology Reports. 10 (2): 351–6. PMID 12579271. Retrieved 2012-01-18.
- ↑ Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA; et al. (2005). "Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone". J Orthop Res. 23 (1): 203–9. doi:10.1016/j.orthres.2004.06.018. PMID 15607894.
- ↑ Gamberi G, Benassi MS, Ragazzini P, Pazzaglia L, Ponticelli F, Ferrari C; et al. (2004). "Proteases and interleukin-6 gene analysis in 92 giant cell tumors of bone". Ann Oncol. 15 (3): 498–503. PMID 14998856.
- ↑ Shrivastava, Sandeep; Nawghare, Shishir P; Kolwadkar, Yogesh; Singh, Pradeep (2008). "Giant cell tumour in the diaphysis of radius – a report". Cases Journal. 1 (1): 106. doi:10.1186/1757-1626-1-106. ISSN 1757-1626.
- ↑ Bridge JA, Neff JR, Mouron BJ (1992). "Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature". Cancer Genet Cytogenet. 58 (1): 2–13. PMID 1728946.
- ↑ Amary F, Berisha F, Ye H, Gupta M, Gutteridge A, Baumhoer D; et al. (2017). "H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone". Am J Surg Pathol. 41 (8): 1059–1068. doi:10.1097/PAS.0000000000000859. PMC 5510691. PMID 28505000.
- ↑ Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV (2015). "Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma". Am J Surg Pathol. 39 (11): 1576–83. doi:10.1097/PAS.0000000000000512. PMID 26457357.
- ↑ McComb EN, Johansson SL, Neff JR, Nelson M, Bridge JA (1996). "Chromosomal anomalies exclusive of telomeric associations in giant cell tumor of bone". Cancer Genet Cytogenet. 88 (2): 163–6. PMID 8640728.
- ↑ Peabody, Terrance (2014). Orthopaedic oncology : primary and metastatic tumors of the skeletal system. Cham: Springer. ISBN 9783319073224.
- ↑ Salzer-Kuntschik M (1998). "[Differential diagnosis of giant cell tumor of bone]". Verh Dtsch Ges Pathol. 82: 154–9. PMID 10095427.
- ↑ Campanacci M, Baldini N, Boriani S, Sudanese A (1987). "Giant-cell tumor of bone". J Bone Joint Surg Am. 69 (1): 106–14. PMID 3805057.
- ↑ Kransdorf MJ, Sweet DE, Buetow PC, Giudici MA, Moser RP (1992). "Giant cell tumor in skeletally immature patients". Radiology. 184 (1): 233–7. doi:10.1148/radiology.184.1.1609086. PMID 1609086.
- ↑ Picci P, Manfrini M, Zucchi V, Gherlinzoni F, Rock M, Bertoni F; et al. (1983). "Giant-cell tumor of bone in skeletally immature patients". J Bone Joint Surg Am. 65 (4): 486–90. PMID 6833323.
- ↑ Muheremu, Aikeremujiang; Niu, Xiaohui (2014). "Pulmonary metastasis of giant cell tumor of bones". World Journal of Surgical Oncology. 12 (1): 261. doi:10.1186/1477-7819-12-261. ISSN 1477-7819.
- ↑ Molenaar WM, van den Berg E, Dolfin AC, Zorgdrager H, Hoekstra HJ (1995). "Cytogenetics of fine needle aspiration biopsies of sarcomas". Cancer Genet Cytogenet. 84 (1): 27–31. PMID 7497439.
- ↑ Peabody, Terrance (2014). Orthopaedic oncology : primary and metastatic tumors of the skeletal system. Cham: Springer. ISBN 9783319073224.
- ↑ Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ (2001). "From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation". Radiographics : a Review Publication of the Radiological Society of North America, Inc. 21 (5): 1283–309. PMID 11553835. Retrieved 2012-01-18.
- ↑ Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY; et al. (2010). "Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study". Lancet Oncol. 11 (3): 275–80. doi:10.1016/S1470-2045(10)70010-3. PMID 20149736.
- ↑ Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF (2008). "Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case-control study". Bone. 42 (1): 68–73. doi:10.1016/j.bone.2007.08.038. PMID 17962092.
- ↑ Puri, Ajay; Agarwal, Manish (2007). "Treatment of giant cell tumor of bone: Current concepts". Indian Journal of Orthopaedics. 41 (2): 101. doi:10.4103/0019-5413.32039. ISSN 0019-5413.
- ↑ 29.0 29.1 Goldring SR, Schiller AL, Mankin HJ, Dayer JM, Krane SM (1986). "Characterization of cells from human giant cell tumors of bone". Clin Orthop Relat Res (204): 59–75. PMID 3006962.
- ↑ Oh JH, Yoon PW, Lee SH, Cho HS, Kim WS, Kim HS (2006). "Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant". Int Orthop. 30 (6): 490–4. doi:10.1007/s00264-006-0154-3. PMC 3172752. PMID 16736146.
- ↑ Kim Y, Nizami S, Goto H, Lee FY (2012). "Modern interpretation of giant cell tumor of bone: predominantly osteoclastogenic stromal tumor". Clin Orthop Surg. 4 (2): 107–16. doi:10.4055/cios.2012.4.2.107. PMC 3360182. PMID 22662295.
- ↑ Nair MK, Jyothirmayi R (1999). "Radiation therapy in the treatment of giant cell tumor of bone". Int J Radiat Oncol Biol Phys. 43 (5): 1065–9. PMID 10192357.
- ↑ Bennett CJ, Marcus RB, Million RR, Enneking WF (1993). "Radiation therapy for giant cell tumor of bone". Int J Radiat Oncol Biol Phys. 26 (2): 299–304. PMID 8491687.