De Quervain's thyroiditis pathophysiology: Difference between revisions
No edit summary |
|||
| Line 9: | Line 9: | ||
* Secretion of [[thyroid hormones]] follows upper control from the [[hypothalamus]] and the [[pituitary]]. [[Thyrotropin-releasing hormone|Thyroid releasing hormone (TRH)]] acts on [[thyrotropes]] releasing cells in the [[pituitary]] causing them to release [[Thyroid-stimulating hormone|thyroid stimulating hormone (TSH)]]. | * Secretion of [[thyroid hormones]] follows upper control from the [[hypothalamus]] and the [[pituitary]]. [[Thyrotropin-releasing hormone|Thyroid releasing hormone (TRH)]] acts on [[thyrotropes]] releasing cells in the [[pituitary]] causing them to release [[Thyroid-stimulating hormone|thyroid stimulating hormone (TSH)]]. | ||
* [[TSH]] acts on [[thyroid gland]] by binding to specific membrane receptors and activating an [[intracellular]] pathway involving [[cAMP]] that ends in the formation and secretion of [[thyroid hormones]]. | * [[TSH]] acts on [[thyroid gland]] by binding to specific membrane receptors and activating an [[intracellular]] pathway involving [[cAMP]] that ends in the formation and secretion of [[thyroid hormones]]. | ||
* [[Iodine]] is essential for the synthesis of [[thyroid hormones]]. [[Iodide]] is | * [[Iodine]] is essential for the synthesis of [[thyroid hormones]]. [[Iodide]] is uptaken through a special Na/I transporter found in the membrane of [[thyroid]] follicular cell. After the [[iodide]] uptake, it goes through a series of organic reactions ending in the formation of the two forms of [[thyroid hormones]]: [[T3]] and [[T4]]. [[T3]] and [[T4]] remain stored in the [[thyroglobulin]] of the follicles and are released in response to further stimulation by [[TSH]] to the [[Thyroid follicle|thyroid follicles]]. | ||
* While [[T3]] is 3 to 5 times more potent than [[T4]], it represents only one-fourth of the total hormone secretion. [[T3]] is thought to be the biologically active form of the hormone. Most of the circulating [[T3]] is due to peripheral conversion of [[T4]] in the liver and peripheral tissues while only a small percentage is secreted directly from the [[thyroid gland]] itself. | * While [[T3]] is 3 to 5 times more potent than [[T4]], it represents only one-fourth of the total hormone secretion. [[T3]] is thought to be the biologically active form of the hormone. Most of the circulating [[T3]] is due to peripheral conversion of [[T4]] in the liver and peripheral tissues while only a small percentage is secreted directly from the [[thyroid gland]] itself. | ||
* [[T3]] and [[T4]] act on nuclear receptors ([[DNA]] binding [[proteins]]) and cause the regulate the [[transcription]] of many [[proteins]] to regulate the [[metabolic rate]] of the body. | * [[T3]] and [[T4]] act on nuclear receptors ([[DNA]] binding [[proteins]]) and cause the regulate the [[transcription]] of many [[proteins]] to regulate the [[metabolic rate]] of the body. | ||
| Line 19: | Line 19: | ||
*De Quervain's thyroiditis is associated with HLAB35. It is postulated that the [[antigen]] triggers the activation of [[HLA]] B35 positive [[inflammatory cells]] which in turn activates the [[cytotoxic T-lymphocytes]]. | *De Quervain's thyroiditis is associated with HLAB35. It is postulated that the [[antigen]] triggers the activation of [[HLA]] B35 positive [[inflammatory cells]] which in turn activates the [[cytotoxic T-lymphocytes]]. | ||
*[[Cytotoxic T cell]] recognition of viral and cell [[antigens]] presented in a complex leads to the [[thyroid follicular cell]] damage. | *[[Cytotoxic T cell]] recognition of viral and cell [[antigens]] presented in a complex leads to the [[thyroid follicular cell]] damage. | ||
*The [[autoimmune]] process leads to | *The [[autoimmune]] process leads to [[inflammatory cells]] infiltration of the gland. The changes may lead to [[granulomatous]] or non-granulomatous lesions. | ||
**'''Granulomatous lesion''' | **'''Granulomatous lesion''' | ||
***Granulomatous lesion comprises of colloid, small lymphocytes, neutrophils, macrophages with or without epithelioid features, and multinucleated giant cells of foreign body type. In the granulomatous lesion, the giant cells are usually CD68+, thyroglobulin– and cytokeratin–. Small lymphocytes in the granulomas are CD3+, CD8+, CD45RO+ cytotoxic T-cells. Numerous plasmacytoid monocytes were also closely associated with the granulomas. | ***[[Granulomatous|Granulomatous lesion]] comprises of colloid, small [[lymphocytes]], neutrophils, macrophages with or without epithelioid features, and multinucleated giant cells of foreign body type. In the granulomatous lesion, the giant cells are usually CD68+, thyroglobulin– and cytokeratin–. Small lymphocytes in the granulomas are CD3+, CD8+, CD45RO+ cytotoxic T-cells. Numerous plasmacytoid monocytes were also closely associated with the granulomas. | ||
**'''Non-granulomatous lesion''' | **'''Non-granulomatous lesion''' | ||
***Follicles in the non-granulomatous lesion are infiltrated by CD8+ T-lymphocytes, plasmacytoid monocytes, and histiocytes, resulting in disrupted basement membrane and rupture of the follicles. | ***Follicles in the non-granulomatous lesion are infiltrated by CD8+ T-lymphocytes, plasmacytoid monocytes, and histiocytes, resulting in disrupted basement membrane and rupture of the follicles. | ||
Revision as of 16:02, 3 October 2017
|
De Quervain's thyroiditis Microchapters |
|
Differentiating De Quervain's thyroiditis from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Furqan M M. M.B.B.S[2]
Overview
The exact pathogenesis of de Quervain's thyroiditis is unclear. It is proposed that cytotoxic T cell recognition of complex viral and cell antigens presentation leads to the thyroid follicular cell damage which is responsible for the pathogenesis of de Quervain's thyroiditis. De Quervain's thyroiditis is usually preceded by a viral prodrome and also have a genetic predisposition. HLA B35 and HLA B15/62 are associated with de Quervain's thyroiditis.
Pathophysiology
The control, synthesis, and release of the thyroid hormone is usually controlled by hypothalamus and pituitary gland.[1][2]
- Thyroid hormones (T3 and T4) are regulating basal metabolic rate, influence oxygen consumption by tissues. They are crucial for normal development of the brain and growth of the body.
- Secretion of thyroid hormones follows upper control from the hypothalamus and the pituitary. Thyroid releasing hormone (TRH) acts on thyrotropes releasing cells in the pituitary causing them to release thyroid stimulating hormone (TSH).
- TSH acts on thyroid gland by binding to specific membrane receptors and activating an intracellular pathway involving cAMP that ends in the formation and secretion of thyroid hormones.
- Iodine is essential for the synthesis of thyroid hormones. Iodide is uptaken through a special Na/I transporter found in the membrane of thyroid follicular cell. After the iodide uptake, it goes through a series of organic reactions ending in the formation of the two forms of thyroid hormones: T3 and T4. T3 and T4 remain stored in the thyroglobulin of the follicles and are released in response to further stimulation by TSH to the thyroid follicles.
- While T3 is 3 to 5 times more potent than T4, it represents only one-fourth of the total hormone secretion. T3 is thought to be the biologically active form of the hormone. Most of the circulating T3 is due to peripheral conversion of T4 in the liver and peripheral tissues while only a small percentage is secreted directly from the thyroid gland itself.
- T3 and T4 act on nuclear receptors (DNA binding proteins) and cause the regulate the transcription of many proteins to regulate the metabolic rate of the body.
- The higher regulation of thyroxine secretion follows the negative feedback role, meaning that high levels of T3 and T4 will suppress TRH and TSH secretion and vice versa (Low levels of thyroxine will stimulate TRH and TSH secretion). This is useful in diagnosing the cause of hyperthyroidism.
- TSH will be low in primary hyperthyroidism where the gland is the source of the excess hormones. In secondary hyperthyroidism, TSH will be high as the pituitary or the hypothalamus are the sources of the disease.
Pathogenesis
The exact pathogenesis of de Quervain's thyroiditis is unclear, but autoimmunity mechanism is proposed.[3][4][5]
- De Quervain's thyroiditis is usually preceded by a viral prodrome. Various viral infections are associated with the de Quervain's thyroiditis including mumps, adenovirus, Epstein–Barr virus, coxsackievirus, cytomegalovirus, influenza, echovirus, and enterovirus.
- De Quervain's thyroiditis is associated with HLAB35. It is postulated that the antigen triggers the activation of HLA B35 positive inflammatory cells which in turn activates the cytotoxic T-lymphocytes.
- Cytotoxic T cell recognition of viral and cell antigens presented in a complex leads to the thyroid follicular cell damage.
- The autoimmune process leads to inflammatory cells infiltration of the gland. The changes may lead to granulomatous or non-granulomatous lesions.
- Granulomatous lesion
- Granulomatous lesion comprises of colloid, small lymphocytes, neutrophils, macrophages with or without epithelioid features, and multinucleated giant cells of foreign body type. In the granulomatous lesion, the giant cells are usually CD68+, thyroglobulin– and cytokeratin–. Small lymphocytes in the granulomas are CD3+, CD8+, CD45RO+ cytotoxic T-cells. Numerous plasmacytoid monocytes were also closely associated with the granulomas.
- Non-granulomatous lesion
- Follicles in the non-granulomatous lesion are infiltrated by CD8+ T-lymphocytes, plasmacytoid monocytes, and histiocytes, resulting in disrupted basement membrane and rupture of the follicles.
- Granulomatous lesion
Genetics
- De Quervain's thyroiditis is associated with:[6][7]
- The human leukocyte antigen (HLA) B35
- HLA B15/62 (in rare cases)
Associated conditions
The following conditions may be associated with De Quervain's thyroiditis:[8]
- Rheumatoid arthritis
- Sjogren syndrome
- Ulcerative colitis
- Urticaria
- Thyroid malignancy
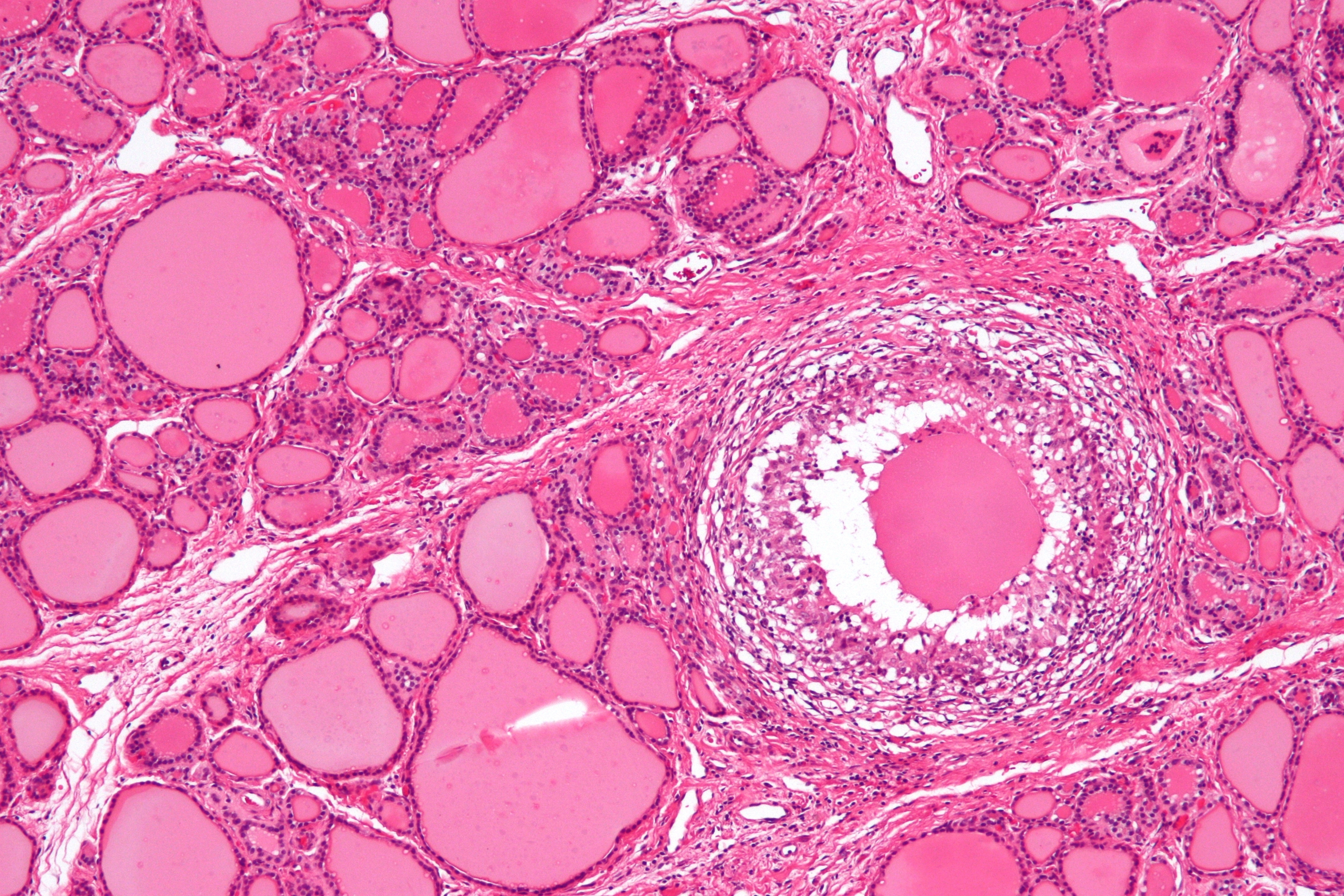
Gross Pathology
On gross pathology, subacute thyroiditis frequently resembles thyroid malignancy. Subacute thyroiditis usually has the following features:[9]
- Firm to dense consistency
- Pale white color
- Poorly defined margins
- Involvement of adjacent normal thyroid
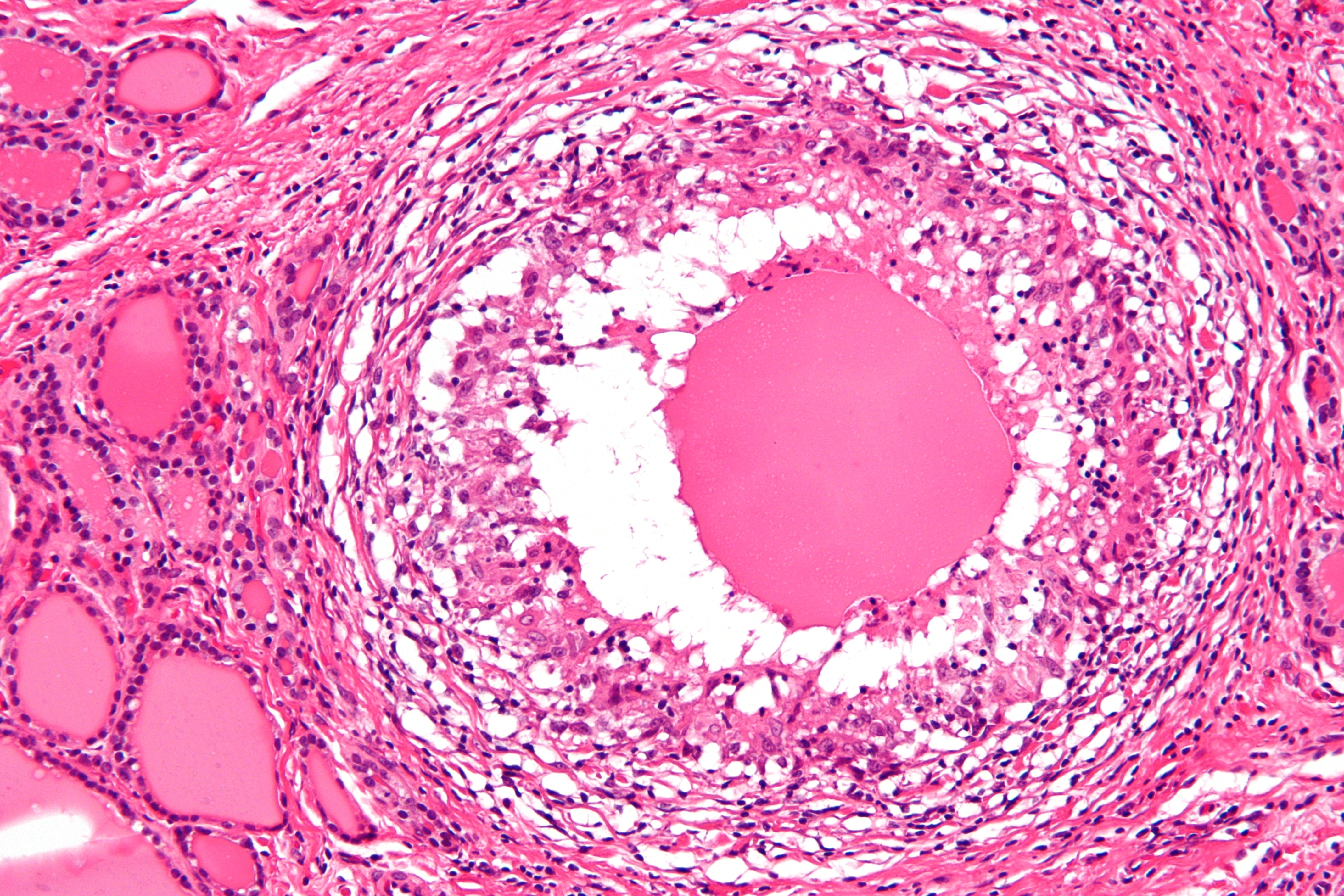
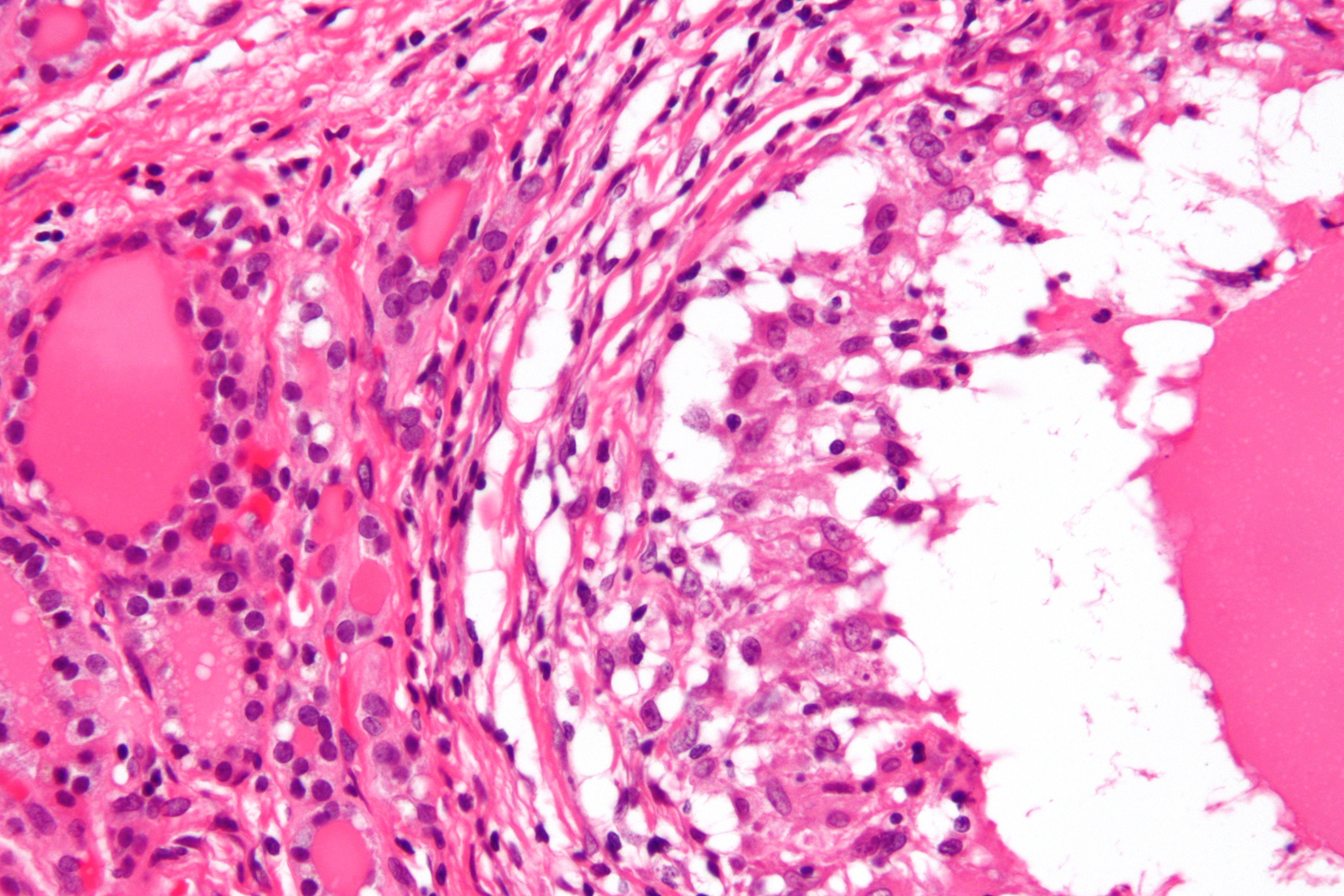
Microscopic pathology
The primary pathology of de Quervain's thyroiditis is:[3][9]
- Infiltration with polymorphonuclear leukocytes initially
- Predominance of lymphocytes and macrophages in advanced form
- Destruction of the follicular epithelium
- Parenchymal destruction and scar tissue
- Loss of the follicular integrity
Gallery
-
De Quervain's thyroiditis (By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=18491382)
-
De Quervain's thyroiditis (By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=18491421)
-
De Quervain's thyroiditis (By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=18491421)
References
- ↑ De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, Rousset B, Dupuy C, Miot F, Dumont J. "Thyroid Hormone Synthesis And Secretion". PMID 25905405.
- ↑ Kirsten D (2000). "The thyroid gland: physiology and pathophysiology". Neonatal Netw. 19 (8): 11–26. doi:10.1891/0730-0832.19.8.11. PMID 11949270.
- ↑ 3.0 3.1 Kojima M, Nakamura S, Oyama T, Sugihara S, Sakata N, Masawa N (2002). "Cellular composition of subacute thyroiditis. an immunohistochemical study of six cases". Pathol. Res. Pract. 198 (12): 833–7. doi:10.1078/0344-0338-00344. PMID 12608662.
- ↑ Erdem N, Erdogan M, Ozbek M, Karadeniz M, Cetinkalp S, Ozgen AG, Saygili F, Yilmaz C, Tuzun M, Kabalak T (2007). "Demographic and clinical features of patients with subacute thyroiditis: results of 169 patients from a single university center in Turkey". J. Endocrinol. Invest. 30 (7): 546–50. PMID 17848836.
- ↑ Desailloud R, Hober D (2009). "Viruses and thyroiditis: an update". Virol. J. 6: 5. doi:10.1186/1743-422X-6-5. PMC 2654877. PMID 19138419.
- ↑ Nyulassy S, Hnilica P, Buc M, Guman M, Hirschová V, Stefanovic J (1977). "Subacute (de Quervain's) thyroiditis: association with HLA-Bw35 antigen and abnormalities of the complement system, immunoglobulins and other serum proteins". J. Clin. Endocrinol. Metab. 45 (2): 270–4. doi:10.1210/jcem-45-2-270. PMID 885992.
- ↑ de Bruin TW, Riekhoff FP, de Boer JJ (1990). "An outbreak of thyrotoxicosis due to atypical subacute thyroiditis". J. Clin. Endocrinol. Metab. 70 (2): 396–402. doi:10.1210/jcem-70-2-396. PMID 2298855.
- ↑ Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ (2003). "Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study". J. Clin. Endocrinol. Metab. 88 (5): 2100–5. doi:10.1210/jc.2002-021799. PMID 12727961.
- ↑ 9.0 9.1 Shrestha RT, Hennessey J. Acute and Subacute, and Riedel’s Thyroiditis.