Calcitriol (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Calcitriol (injection) is a fat-soluble vitamin that is FDA approved for the treatment of hypocalcemia in patients undergoing chronic renal dialysis.. Common adverse reactions include weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, metallic taste, anorexia, abdominal pain, and epigastric discomfort.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Calcijex (calcitriol injection) is indicated in the management of hypocalcemia in patients undergoing chronic renal dialysis. It has been shown to significantly reduce elevated parathyroid hormone levels. Reduction of PTH has been shown to result in an improvement in renal osteodystrophy.
Dosage
- The optimal dose of Calcijex (calcitriol injection) must be carefully determined for each patient.
- The effectiveness of Calcijex therapy is predicated on the assumption that each patient is receiving an adequate and appropriate daily intake of calcium. The RDA for calcium in adults is 800 mg. To ensure that each patient receives an adequate daily intake of calcium, the physician should either prescribe a calcium supplement or instruct the patient in proper dietary measures.
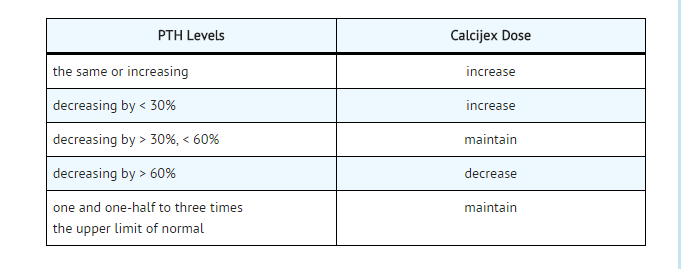
- The recommended initial dose of Calcijex, depending on the severity of the hypocalcemia and/or secondary hyperparathyroidism, is 1 mcg (0.02 mcg/kg) to 2 mcg administered intravenously three times weekly, approximately every other day. Doses as small as 0.5 mcg and as large as 4 mcg three times weekly have been used as an initial dose. If a satisfactory response is not observed, the dose may be increased by 0.5 to 1 mcg at two to four week intervals. During this titration period, serum calcium and phosphorus levels should be obtained at least twice weekly. If hypercalcemia or a serum calcium times phosphate product greater than 70 is noted, the drug should be immediately discontinued until these parameters are appropriate. Then, the Calcijex dose should be reinitiated at a lower dose. Doses may need to be reduced as the PTH levels decrease in response to the therapy. Thus, incremental dosing must be individualized and commensurate with PTH, serum calcium and phosphorus levels. The following is a suggested approach in dose titration:
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Discard unused portion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitriol (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitriol (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Calcitriol (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Calcitriol (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Calcitriol (injection) in pediatric patients.
Contraindications
- Calcijex (calcitriol injection) should not be given to patients with hypercalcemia or evidence of vitamin D toxicity.
- Calcijex (calcitriol injection) is contraindicated in patients with previous hypersensitivity to calcitriol or any of its excipients.
Warnings
- Since calcitriol is the most potent metabolite of vitamin D available, prescription-based doses of vitamin D and its derivatives should be withheld or used with caution during treatment to avoid the risk of hypercalcemia.
- A non-aluminum phosphate-binding compound should be used to control serum phosphorus levels in patients undergoing dialysis.
- Overdosage of any form of vitamin D is dangerous. Progressive hypercalcemia due to overdosage of vitamin D and its metabolites may be so severe as to require emergency attention. Chronic hypercalcemia can lead to generalized vascular calcification, nephrocalcinosis, and other soft-tissue calcification. The serum calcium times phosphate (Ca x P) product should not be allowed to exceed 70 mg2/dL2. Radiographic evaluation of suspect anatomical regions may be useful in the early detection of this condition.
Adverse Reactions
Clinical Trials Experience
- Adverse effects of Calcijex (calcitriol injection) are, in general, similar to those encountered with excessive vitamin D intake. The early and late signs and symptoms of vitamin D intoxication associated with hypercalcemia include:
Early
- Weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, metallic taste, anorexia, abdominal pain, and epigastric discomfort.
Late
- Polyuria, polydipsia, anorexia, weight loss, nocturia, conjunctivitis (calcific), pancreatitis, photophobia, rhinorrhea, pruritus, hyperthermia, decreased libido, elevated BUN, albuminuria, hypercholesterolemia, elevated SGOT and SGPT, ectopic calcification, hypertension, cardiac arrhythmias, nephrocalcinosis, sensory disturbance, dehydration, apathy, and rarely, overt psychosis.
- Occasional mild pain on injection has been observed.
Postmarketing Experience
- Rare cases of hypersensitivity reactions have been reported, including anaphylaxis.
Drug Interactions
- Concomitant use of magnesium-containing preparations should be used with caution or avoided since such use may lead to the development of hypermagnesemia.
- Corticosteroids with glucocorticoid activity may counteract the bone and mineral metabolism effects of vitamin D analogues.
- Cytochrome P450 enzyme-inducing anticonvulsants such as carbamazepine, phenobarbital, and phenytoin may reduce the effects of vitamin D because they increase vitamin D catabolism.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects
Pregnancy Category C
- Calcitriol has been found to be teratogenic in rabbits when given orally at doses of 0.08 and 0.3 mcg/kg. All 15 fetuses in 3 litters at these doses showed external and skeletal abnormalities. However, none of the other 23 litters (156 fetuses) showed external and skeletal abnormalities compared with controls. Teratogenicity studies in rats at doses up to 0.45 mcg/kg orally showed no evidence of teratogenic potential. There are no adequate and well-controlled studies in pregnant women. Calcijex should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
- In the rabbit, oral dosages of 0.3 mcg/kg/day administered on days 7 to 18 of gestation resulted in 19% maternal mortality, a decrease in mean fetal body weight and a reduced number of newborns surviving to 24 hours. A study of the effects on orally administered calcitriol on peri-and postnatal development in rats resulted in hypercalcemia in the offspring of dams given calcitriol at doses of 0.08 or 0.3 mcg/kg/day, hypercalcemia and hypophosphatemia in dams given calcitriol at a dose of 0.08 or 0.3 mcg/kg/day and increased serum urea nitrogen in dams given calcitriol at a dose of 0.3 mcg/kg/day. In another study in rats, maternal weight gain was slightly reduced at an oral dose of 0.3 mcg/kg/day administered on days 7 to 15 of gestation.
- The offspring of a woman administered oral calcitriol at 17 to 36 mcg/day during pregnancy manifested mild hypercalcemia in the first 2 days of life which returned to normal at day 3.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Calcitriol (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Calcitriol (injection) during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from calcitriol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Calcijex were examined in a 12-week randomized, double-blind, placebo-controlled study of 35 pediatric patients, aged 13-18 years, with end-stage renal disease on hemodialysis. Sixty-six percent of the patients were male, 57% were African-American, and nearly all had received some form of vitamin D therapy prior to the study. The initial dose of Calcijex was 0.5 mcg, 1.0 mcg, or 1.5 mcg, 3 times per week, based on baseline iPTH level of less than 500 pg/mL, 500-1000 pg/mL, or greater than 1000 pg/mL, respectively. The dose of Calcijex was adjusted in 0.25 mcg increments based on the levels of serum iPTH, calcium, and Ca x P. The mean baseline levels of iPTH were 769 pg/mL for the 16 Calcijex-treated patients and 897 pg/mL for the 19 placebo-treated subjects. The mean weekly dose of Calcijex ranged from 1.0 mcg to 1.4 mcg. In the primary efficacy analysis, 7 of 16 (44%) subjects in the Calcijex group had 2 consecutive 30% decreases from baseline iPTH compared with 3 of 19 (16%) patients in the placebo group (95% CI for the difference between groups -6%, 62%). One Calcijex-treated patient experienced transient hypercalcemia (> 11.0 mg/dL), while 6 of 16 (38%) Calcijex-treated patients vs. 2 of 19 (11%) placebo-treated patients experienced Ca x P > 75.
Geriatic Use
- Clinical studies of Calcijex did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosage range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Calcitriol (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Calcitriol (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Calcitriol (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Calcitriol (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Calcitriol (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Calcitriol (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Calcitriol (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Calcitriol (injection) in the drug label.
Overdosage
- Administration of Calcijex (calcitriol injection) to patients in excess of their requirements can cause hypercalcemia, hypercalciuria and hyperphosphatemia. High intake of calcium and phosphate concomitant with Calcijex may lead to similar abnormalities.
Treatment of Hypercalcemia and Overdosage in Patients on Hemodialysis
- General treatment of hypercalcemia (greater than 1 mg/dL above the upper limit of normal range) consists of immediate discontinuation of Calcijex therapy, institution of a low calcium diet and withdrawal of calcium supplements. Serum calcium levels should be determined daily until normocalcemia ensues. Hypercalcemia usually resolves in two to seven days. When serum calcium levels have returned to within normal limits, Calcijex therapy may be reinstituted at a dose 0.5 mcg less than prior therapy. Serum calcium levels should be obtained at least twice weekly after all dosage changes.
- Persistent or markedly elevated serum calcium levels may be corrected by dialysis against a calcium-free dialysate.
Treatment of Accidental Overdosage of Calcitriol Injection
- The treatment of acute accidental overdosage of Calcijex should consist of general supportive measures. Serial serum electrolyte determinations (especially calcium), rate of urinary calcium excretion and assessment of electrocardiographic abnormalities due to hypercalcemia should be obtained. Such monitoring is critical in patients receiving digitalis. Discontinuation of supplemental calcium and low calcium diet are also indicated in accidental overdosage. Due to the relatively short duration of the pharmacological action of calcitriol, further measures are probably unnecessary. Should, however, persistent and markedly elevated serum calcium levels occur, there are a variety of therapeutic alternatives which may be considered, depending on the patients' underlying condition. These include the use of drugs such as phosphates and corticosteroids as well as measures to induce an appropriate forced diuresis. The use of peritoneal dialysis against a calcium-free dialysate has also been reported.
Pharmacology
Mechanism of Action
There is limited information regarding Mechanism of Action of Calcitriol (injection) in the drug label.
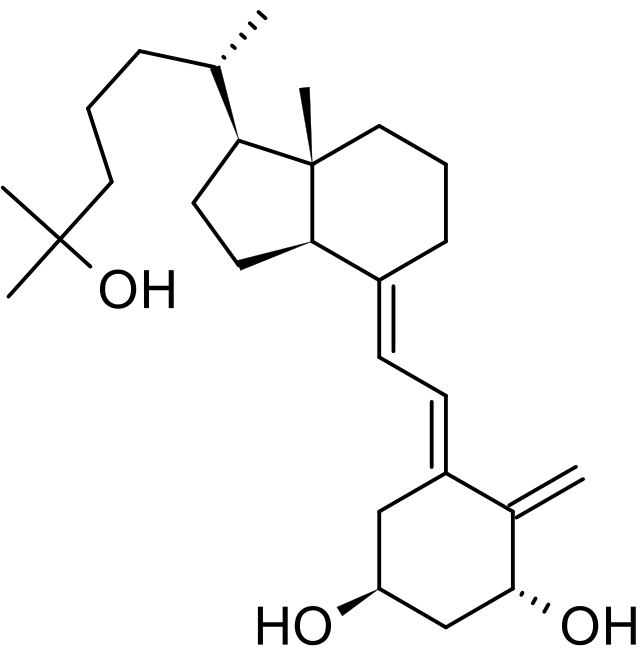
Structure
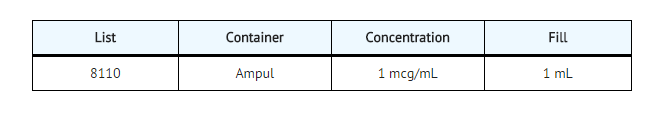
- Calcijex (calcitriol injection) is synthetically manufactured calcitriol and is available as a sterile, isotonic, clear, colorless to yellow, aqueous solution for intravenous injection. Calcijex is available in 1 mL ampuls. Each 1 mL contains calcitriol, 1 mcg; Polysorbate 20, 4 mg; sodium ascorbate 2.5 mg added. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. pH is 6.5 (5.9 to 7.0). Contains no more than 1 mcg/mL of aluminum.
- Calcitriol is a crystalline compound which occurs naturally in humans. It is soluble in organic solvents but relatively insoluble in water.
- Calcitriol is chemically designated (5Z,7E)-9, 10-secocholesta-5,7,10(19)-triene-1α,3β,25-triol and has the following structural formula:
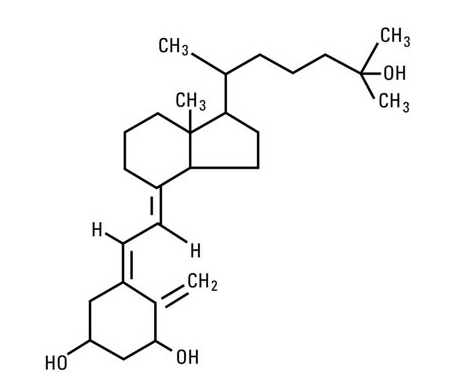
- The other names frequently used for calcitriol are 1α,25-dihydroxycholecalciferol, 1α,25-dihydroxyvitamin D3, 1,25-DHCC, 1,25(OH)2D3 and 1,25-diOHC.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Calcitriol (injection) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Calcitriol (injection) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Calcitriol (injection) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Calcitriol (injection) in the drug label.
How Supplied
- Calcijex (calcitriol injection) is supplied as follows:
Storage
- Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F).
Images
Drug Images
{{#ask: Page Name::Calcitriol (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
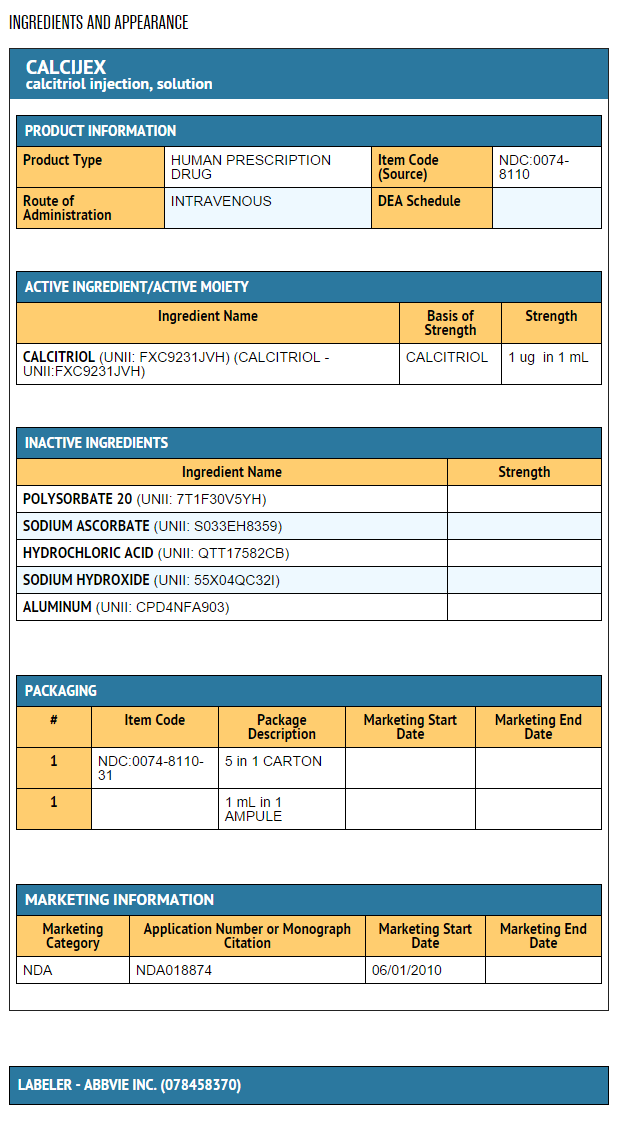
{{#ask: Label Page::Calcitriol (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for the Patient
- The patient and his or her parents should be informed about adherence to instructions about diet and calcium supplementation and avoidance of the use of unapproved non-prescription drugs, including magnesium-containing antacids. Patients should also be carefully informed about the symptoms of hypercalcemia.
Precautions with Alcohol
- Alcohol-Calcitriol (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CALCIJEX®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "calcitriol injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Calcitriol (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Calcitriol (injection) |Label Name=Calcitriol (injection)11.png
}}
{{#subobject:
|Label Page=Calcitriol (injection) |Label Name=Calcitriol (injection)11.png
}}