Bourbon virus infection pathophysiology: Difference between revisions
No edit summary |
m (Bot: Removing from Primary care) |
||
| (22 intermediate revisions by 5 users not shown) | |||
| Line 4: | Line 4: | ||
{{CMG}}; {{AE}} {{HK}} | {{CMG}}; {{AE}} {{HK}} | ||
==Overview== | ==Overview== | ||
Bourbon virus is a [[Negative-sense|negative sense]] segmented [[RNA viruses|RNA virus]] belonging to the genus [[Thogotovirus]], family [[Orthomyxoviridae|Orthomyxovirida]]. It is transmitted by insects and replicates in both [[Arthropod|arthropods]] and [[vertebrate]] hosts. The [[Negative-sense ssRNA virus|negative sense]] [[RNA viruses|RNA virus]] replicates within the [[Cell nucleus|nuclei]] of the host cells. [[Thogotovirus|Thogoto]] virus Infection induces a sustained [[type 1 interferon]] response in the host until the [[Adaptive immune system|adaptive immunity]] takes effect. Microscopically, bourbon viruses are 80-120nm in diameter with a [[genome]] size of approximately 10Kb. | |||
==Pathophysiology== | ==Pathophysiology== | ||
Bourbon virus is a negative sense segmented RNA virus | Bourbon virus is a [[Negative-sense|negative sense]] [[RNA viruses|segmented RNA virus]] which belongs to the genus [[Thogotovirus]], family [[Orthomyxoviridae|Orthomyxovirida]]. | ||
===Transmission=== | ===Transmission=== | ||
* | *Bourbon virus is transmitted mainly by ticks, although other [[arthropods]] may also be involved in transmission. | ||
*The virus is able to replicate in vertebrate and tick cells. | *The [[virus]] is able to replicate in [[vertebrate]] and tick cells. | ||
===Adherence=== | ===Adherence=== | ||
*Virus attaches to the N-acetylneuraminic acid component found in host cell membrane (sialic acid receptors).<ref name="urlReceptor-Mediated Endocytosis and the Sorting of Internalized Proteins - Molecular Cell Biology - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK21639/ |title=Receptor-Mediated Endocytosis and the Sorting of Internalized Proteins - Molecular Cell Biology - NCBI Bookshelf |format= |work= |accessdate=}}</ref> | *[[Virus]] attaches to the [[N-Acetylneuraminic acid|N-acetylneuraminic acid]] component found in host [[cell membrane]] ([[Sialic acid|sialic acid receptors]]).<ref name="urlReceptor-Mediated Endocytosis and the Sorting of Internalized Proteins - Molecular Cell Biology - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK21639/ |title=Receptor-Mediated Endocytosis and the Sorting of Internalized Proteins - Molecular Cell Biology - NCBI Bookshelf |format= |work= |accessdate=}}</ref> | ||
===Endocytosis=== | ===Endocytosis=== | ||
*The virus gets endocytosed by clathrins into the host cell. | *The [[virus]] gets [[Endocytosis|endocytosed]] by [[Clathrin|clathrins]] into the host cell. | ||
*Endosome acidification induces fusion of virus membrane with the vesicle membrane. | *[[Endosome]] acidification induces fusion of virus membrane with the vesicle membrane. | ||
===Virology and replication=== | ===Virology and replication=== | ||
*Thogoto viruses | *[[Thogotovirus|Thogoto viruses]] differ from [[influenza virus]] in having capped viral [[Messenger RNA|mRNA]] without host messenger-derived heterogeneous sequences at the 5' end of the [[genome]].<ref name="urlwww.ncbi.nlm.nih.gov">{{cite web |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC192294/pdf/718347.pdf |title=www.ncbi.nlm.nih.gov |format= |work= |accessdate=}}</ref><ref name="pmid8971034">{{cite journal |vauthors=Albo C, Martín J, Portela A |title=The 5' ends of Thogoto virus (Orthomyxoviridae) mRNAs are homogeneous in both length and sequence |journal=J. Virol. |volume=70 |issue=12 |pages=9013–7 |year=1996 |pmid=8971034 |pmc=191002 |doi= |url=}}</ref> | ||
*Five larger [[RNA|RNA segments]] each encode only one gene product, the sixth segment encodes two [[Matrix protein|matrix proteins]], M and ML.<ref name="urlMicrobiology Society Journals | Functional comparison of the two gene products of Thogoto virus segment 6">{{cite web |url=http://jgv.microbiologyresearch.org/content/journal/jgv/10.1099/vir.0.80300-0#tab2 |title=Microbiology Society Journals | Functional comparison of the two gene products of Thogoto virus segment 6 |format= |work= |accessdate=}}</ref> | |||
*Viral RNA polymerases (PA, PB1 and PB2) transcribe one mRNA from each | *Viral [[RNA polymerase|RNA polymerases]] (PA, PB1 and PB2) transcribe one [[Messenger RNA|mRNA]] from each [[genome]] segment. | ||
*Splicing of segment 6 mRNA gives rise to mRNA coding for the matrix protein M1. | *Splicing of segment 6 [[Messenger RNA|mRNA]] gives rise to [[Messenger RNA|mRNA]] coding for the [[matrix protein]] M1.<ref name="urlMicrobiology Society Journals | Functional comparison of the two gene products of Thogoto virus segment 6">{{cite web |url=http://jgv.microbiologyresearch.org/content/journal/jgv/10.1099/vir.0.80300-0#tab2 |title=Microbiology Society Journals | Functional comparison of the two gene products of Thogoto virus segment 6 |format= |work= |accessdate=}}</ref> | ||
*Transcription of genomic segments by the viral polymerase produces mRNAs that are capped and polyadenylated by the viral polymerase. | *[[Transcription (genetics)|Transcription]] of [[genomic]] segments by the viral polymerase produces [[Messenger RNA|mRNAs]] that are capped and [[Polyadenylation|polyadenylated]] by the viral polymerase. | ||
*The M1 protein is involved in export of genome from the nucleus. | *The M1 protein is involved in export of genome from the nucleus. | ||
*Assembly of the virus takes place in the cytoplasm from where new virions are released to infect other cells. | *Assembly of the [[virus]] takes place in the [[cytoplasm]] from where new virions are released to infect other cells. | ||
===Host response=== | ===Host response=== | ||
*Thogoto | *[[Thogotovirus|Thogoto virus]] Infection induces a sustained [[type 1 interferon]] response in the host until the [[Adaptive immune system|adaptive immunity]] takes effect.<ref name="pmid20861272">{{cite journal |vauthors=Kochs G, Bauer S, Vogt C, Frenz T, Tschopp J, Kalinke U, Waibler Z |title=Thogoto virus infection induces sustained type I interferon responses that depend on RIG-I-like helicase signaling of conventional dendritic cells |journal=J. Virol. |volume=84 |issue=23 |pages=12344–50 |year=2010 |pmid=20861272 |pmc=2976394 |doi=10.1128/JVI.00931-10 |url=}}</ref> | ||
*IFN expression is mediated by specialized plasmacytoid dendritic cells (pDC). | *[[Interferon|IFN]] expression is mediated by specialized [[Dendritic cells|plasmacytoid dendritic cells]] (pDC). | ||
*The interferon-induced dynamin-like MxA protein is involved in host antiviral activity against thogoto viruses.<ref name="pmid24448803">{{cite journal |vauthors=Patzina C, Haller O, Kochs G |title=Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus |journal=J. Biol. Chem. |volume=289 |issue=9 |pages=6020–7 |year=2014 |pmid=24448803 |pmc=3937669 |doi=10.1074/jbc.M113.543892 |url=}}</ref><ref name="pmid21166595">{{cite journal |vauthors=Haller O, Kochs G |title=Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity |journal=J. Interferon Cytokine Res. |volume=31 |issue=1 |pages=79–87 |year=2011 |pmid=21166595 |doi=10.1089/jir.2010.0076 |url=}}</ref><ref name="pmid7745744">{{cite journal |vauthors=Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O |title=Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus |journal=J. Virol. |volume=69 |issue=6 |pages=3904–9 |year=1995 |pmid=7745744 |pmc=189115 |doi= |url=}}</ref> | *The [[interferon]]-induced [[Dynamin|dynamin-like]] MxA protein is involved in host antiviral activity against [[Thogotovirus|thogoto viruses]].<ref name="pmid24448803">{{cite journal |vauthors=Patzina C, Haller O, Kochs G |title=Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus |journal=J. Biol. Chem. |volume=289 |issue=9 |pages=6020–7 |year=2014 |pmid=24448803 |pmc=3937669 |doi=10.1074/jbc.M113.543892 |url=}}</ref><ref name="pmid21166595">{{cite journal |vauthors=Haller O, Kochs G |title=Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity |journal=J. Interferon Cytokine Res. |volume=31 |issue=1 |pages=79–87 |year=2011 |pmid=21166595 |doi=10.1089/jir.2010.0076 |url=}}</ref><ref name="pmid7745744">{{cite journal |vauthors=Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O |title=Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus |journal=J. Virol. |volume=69 |issue=6 |pages=3904–9 |year=1995 |pmid=7745744 |pmc=189115 |doi= |url=}}</ref><ref name="pmid8992952">{{cite journal |vauthors=Pringle CR |title=Virus taxonomy 1996 - a bulletin from the Xth International Congress of Virology in Jerusalem |journal=Arch. Virol. |volume=141 |issue=11 |pages=2251–6 |year=1996 |pmid=8992952 |doi= |url=}}</ref> | ||
*MxA recognizes the nucleocapsids of invading viruses, causing an early block of the viral replication cycle.<ref name="pmid24448803">{{cite journal |vauthors=Patzina C, Haller O, Kochs G |title=Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus |journal=J. Biol. Chem. |volume=289 |issue=9 |pages=6020–7 |year=2014 |pmid=24448803 |pmc=3937669 |doi=10.1074/jbc.M113.543892 |url=}}</ref><ref name="pmid1548781">{{cite journal |vauthors=Pavlovic J, Haller O, Staeheli P |title=Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle |journal=J. Virol. |volume=66 |issue=4 |pages=2564–9 |year=1992 |pmid=1548781 |pmc=289059 |doi= |url=}}</ref> | *MxA recognizes the [[Nucleocapsid|nucleocapsids]] of invading [[Virus|viruses]], causing an early block of the viral replication cycle.<ref name="pmid24448803">{{cite journal |vauthors=Patzina C, Haller O, Kochs G |title=Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus |journal=J. Biol. Chem. |volume=289 |issue=9 |pages=6020–7 |year=2014 |pmid=24448803 |pmc=3937669 |doi=10.1074/jbc.M113.543892 |url=}}</ref><ref name="pmid1548781">{{cite journal |vauthors=Pavlovic J, Haller O, Staeheli P |title=Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle |journal=J. Virol. |volume=66 |issue=4 |pages=2564–9 |year=1992 |pmid=1548781 |pmc=289059 |doi= |url=}}</ref> | ||
*[[Cytokines]] involved in the pathogenesis of bourbon virus infection are [[Interleukin 1|interleukin-1]], [[Interleukin 6|interleukin-6]], [[Interleukin 10|interleukin-10]], [[Macrophage inflammatory protein|macrophage inflammatory protein 1]], monocyte chemoattractant protein (MCP)–1, and [[interferon]] (IFN).<ref name="urlThe Interferon Antagonist ML Protein of Thogoto Virus Targets General Transcription Factor IIB">{{cite web |url=http://jvi.asm.org/content/82/22/11446.full |title=The Interferon Antagonist ML Protein of Thogoto Virus Targets General Transcription Factor IIB |format= |work= |accessdate=}}</ref> | |||
==Genetics== | |||
[[Image:Rna_new.png|400px|align right|Negative stranded RNA virus genome replication]] | |||
==Associated conditions== | |||
Few rare conditions associated with bourbon virus infection are: <ref name="pmid25899080">{{cite journal |vauthors=Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, Hunt DC, Staples JE |title=Novel thogotovirus associated with febrile illness and death, United States, 2014 |journal=Emerging Infect. Dis. |volume=21 |issue=5 |pages=760–4 |year=2015 |pmid=25899080 |pmc=4412252 |doi=10.3201/eid2105.150150 |url=}}</ref> | |||
*[[Neuromyelitis optica]] | |||
*[[Meningitis]] | |||
*[[Hemophagocytic lymphohistiocytosis]] (HLH) | |||
==Gross Pathology== | |||
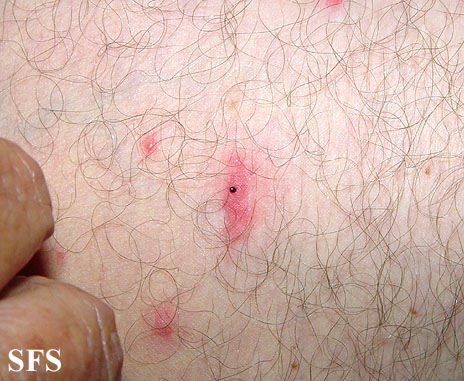
Rash may be the first sign of infection. | |||
*The rash of Bourbon virus infection is usually circular and located at the site of the tick bite. | |||
[[Image:Tick bite03.jpg|300px|align right|Rash after tick bite]] | |||
==Microscopic Pathology== | |||
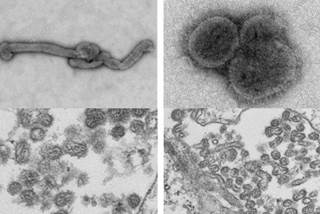
*[[Thogotovirus|Thogoto viruses]] are spherical, enveloped [[RNA viruses|single stranded RNA viruses]] with a segmented [[genome]]. | |||
*Virions are 80-120nm in diameter with a [[genome]] size of approximately 10Kb. | |||
*The 6-7 segments of [[genome]] encodes for 7-9 proteins with each segment size ranging from a low of 0.9Kb to 2.3Kb. | |||
[[Image:Thogotovirus.jpg|350px|align right|Bourbon virus]] | |||
==References== | ==References== | ||
| Line 35: | Line 56: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:Disease]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Pulmonology]] | |||
[[Category:Dermatology]] | |||
[[Category:Emergency medicine]] | |||
[[Category:Infectious disease]] | |||
Latest revision as of 20:40, 29 July 2020
|
Bourbon virus infection Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Bourbon virus infection pathophysiology On the Web |
|
American Roentgen Ray Society Images of Bourbon virus infection pathophysiology |
|
Risk calculators and risk factors for Bourbon virus infection pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Overview
Bourbon virus is a negative sense segmented RNA virus belonging to the genus Thogotovirus, family Orthomyxovirida. It is transmitted by insects and replicates in both arthropods and vertebrate hosts. The negative sense RNA virus replicates within the nuclei of the host cells. Thogoto virus Infection induces a sustained type 1 interferon response in the host until the adaptive immunity takes effect. Microscopically, bourbon viruses are 80-120nm in diameter with a genome size of approximately 10Kb.
Pathophysiology
Bourbon virus is a negative sense segmented RNA virus which belongs to the genus Thogotovirus, family Orthomyxovirida.
Transmission
- Bourbon virus is transmitted mainly by ticks, although other arthropods may also be involved in transmission.
- The virus is able to replicate in vertebrate and tick cells.
Adherence
- Virus attaches to the N-acetylneuraminic acid component found in host cell membrane (sialic acid receptors).[1]
Endocytosis
- The virus gets endocytosed by clathrins into the host cell.
- Endosome acidification induces fusion of virus membrane with the vesicle membrane.
Virology and replication
- Thogoto viruses differ from influenza virus in having capped viral mRNA without host messenger-derived heterogeneous sequences at the 5' end of the genome.[2][3]
- Five larger RNA segments each encode only one gene product, the sixth segment encodes two matrix proteins, M and ML.[4]
- Viral RNA polymerases (PA, PB1 and PB2) transcribe one mRNA from each genome segment.
- Splicing of segment 6 mRNA gives rise to mRNA coding for the matrix protein M1.[4]
- Transcription of genomic segments by the viral polymerase produces mRNAs that are capped and polyadenylated by the viral polymerase.
- The M1 protein is involved in export of genome from the nucleus.
- Assembly of the virus takes place in the cytoplasm from where new virions are released to infect other cells.
Host response
- Thogoto virus Infection induces a sustained type 1 interferon response in the host until the adaptive immunity takes effect.[5]
- IFN expression is mediated by specialized plasmacytoid dendritic cells (pDC).
- The interferon-induced dynamin-like MxA protein is involved in host antiviral activity against thogoto viruses.[6][7][8][9]
- MxA recognizes the nucleocapsids of invading viruses, causing an early block of the viral replication cycle.[6][10]
- Cytokines involved in the pathogenesis of bourbon virus infection are interleukin-1, interleukin-6, interleukin-10, macrophage inflammatory protein 1, monocyte chemoattractant protein (MCP)–1, and interferon (IFN).[11]
Genetics
Associated conditions
Few rare conditions associated with bourbon virus infection are: [12]
Gross Pathology
Rash may be the first sign of infection.
- The rash of Bourbon virus infection is usually circular and located at the site of the tick bite.
Microscopic Pathology
- Thogoto viruses are spherical, enveloped single stranded RNA viruses with a segmented genome.
- Virions are 80-120nm in diameter with a genome size of approximately 10Kb.
- The 6-7 segments of genome encodes for 7-9 proteins with each segment size ranging from a low of 0.9Kb to 2.3Kb.
References
- ↑ "Receptor-Mediated Endocytosis and the Sorting of Internalized Proteins - Molecular Cell Biology - NCBI Bookshelf".
- ↑ "www.ncbi.nlm.nih.gov" (PDF).
- ↑ Albo C, Martín J, Portela A (1996). "The 5' ends of Thogoto virus (Orthomyxoviridae) mRNAs are homogeneous in both length and sequence". J. Virol. 70 (12): 9013–7. PMC 191002. PMID 8971034.
- ↑ 4.0 4.1 "Microbiology Society Journals | Functional comparison of the two gene products of Thogoto virus segment 6".
- ↑ Kochs G, Bauer S, Vogt C, Frenz T, Tschopp J, Kalinke U, Waibler Z (2010). "Thogoto virus infection induces sustained type I interferon responses that depend on RIG-I-like helicase signaling of conventional dendritic cells". J. Virol. 84 (23): 12344–50. doi:10.1128/JVI.00931-10. PMC 2976394. PMID 20861272.
- ↑ 6.0 6.1 Patzina C, Haller O, Kochs G (2014). "Structural requirements for the antiviral activity of the human MxA protein against Thogoto and influenza A virus". J. Biol. Chem. 289 (9): 6020–7. doi:10.1074/jbc.M113.543892. PMC 3937669. PMID 24448803.
- ↑ Haller O, Kochs G (2011). "Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity". J. Interferon Cytokine Res. 31 (1): 79–87. doi:10.1089/jir.2010.0076. PMID 21166595.
- ↑ Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O (1995). "Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus". J. Virol. 69 (6): 3904–9. PMC 189115. PMID 7745744.
- ↑ Pringle CR (1996). "Virus taxonomy 1996 - a bulletin from the Xth International Congress of Virology in Jerusalem". Arch. Virol. 141 (11): 2251–6. PMID 8992952.
- ↑ Pavlovic J, Haller O, Staeheli P (1992). "Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle". J. Virol. 66 (4): 2564–9. PMC 289059. PMID 1548781.
- ↑ "The Interferon Antagonist ML Protein of Thogoto Virus Targets General Transcription Factor IIB".
- ↑ Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, Hunt DC, Staples JE (2015). "Novel thogotovirus associated with febrile illness and death, United States, 2014". Emerging Infect. Dis. 21 (5): 760–4. doi:10.3201/eid2105.150150. PMC 4412252. PMID 25899080.