Anileridine
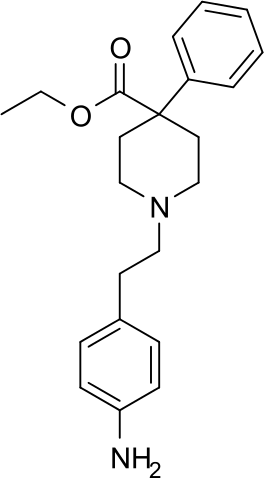 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Routes of administration | Tablets, injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | > 95% |
| Metabolism | Hepatic |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C22H28N2O2 |
| Molar mass | 352.47 g/mol |
| 3D model (JSmol) | |
| Melting point | 83 °C (181.4 °F) |
| |
| |
| | |
|
WikiDoc Resources for Anileridine |
|
Articles |
|---|
|
Most recent articles on Anileridine Most cited articles on Anileridine |
|
Media |
|
Powerpoint slides on Anileridine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Anileridine at Clinical Trials.gov Clinical Trials on Anileridine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Anileridine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Anileridine Discussion groups on Anileridine Patient Handouts on Anileridine Directions to Hospitals Treating Anileridine Risk calculators and risk factors for Anileridine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Anileridine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Anileridine (trade name: Leritine) is a synthetic analgesic drug and is a member of the piperidine class of analgesic agents developed by Merck & Co. in the 1950s. It differs from pethidine (meperidine) in that the N-methyl group of meperidine is replaced by an N-aminophenethyl group, which increases its analgesic activity.
Anileridine is no longer manufactured in the US or Canada.[1]
Administration
Pharmacokinetics
Anileridine usually takes effect within 15 minutes of either oral or intravenous administration, and lasts 2–3 hours.[3] It is mostly metabolized by the liver.
References
- ↑ "Discontinued Prescription Drug Products". Canadian Pharmacists' Association. Retrieved 28 July 2008.
- ↑ "Pharmaceutical Information - LERITINE". RxMed. Retrieved 16 June 2010.
- ↑ "Anileridine Consumer Information". MedicineNet. Retrieved 28 July 2008.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Piperidines
- Synthetic opioids
- Mu-opioid agonists
- Analgesics