Reperfusion injury overview
Editors-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editors-In-Chief: Shivam Singla, M.D [2]
Overview
Reperfusion injury, also known as ischemia-reperfusion injury (IRI) or re-oxygenation injury, is the tissue damage which results from the restoration of blood supply to the tissue after a period of ischemia, anoxia or hypoxia from different pathologies. During the period of absence of blood to the tissues a condition is created in which the resulting reperfusion will result in inflammation and oxidative damage through the involvement of various mechanisms mainly involving oxidation, free radical formation and complement activation which ultimately leads to cell death, rather than restoration of normal function.
Various intracellular or extracellular changes during ischemia leads to increased intracellular calcium and ATP depletion that will ultimately land up in the cell death if the ongoing process does not stopped. Reperfusion forms reactive oxygen species . This leads to Increased mitochondrial pore permeability, complement activation & cytochrome release, inflammation and edema formation, Neutrophil platelet adhesion and thrombosis leading to progressive tissue death. In Heart reperfusion injury is attributed to oxidative stress which in turn leads to arrhythmias, Infarction and Myocardial stunning. In case of trauma the resulting restoration of blood flow to the tissue after prolonged ischemia aggravates tissue damage by either directly causing additional injury or by unmasking the injury sustained during the ischemic period. Reperfusion injury can occur in any organ of body mainly seen in the heart, intestine, kidney, lung, and muscle, and is due to microvascular damage
Pathophysiology
Mainly divided into 2 phases
1) Ischemic phase
2) Reperfusion Phase
Ischemic Phase
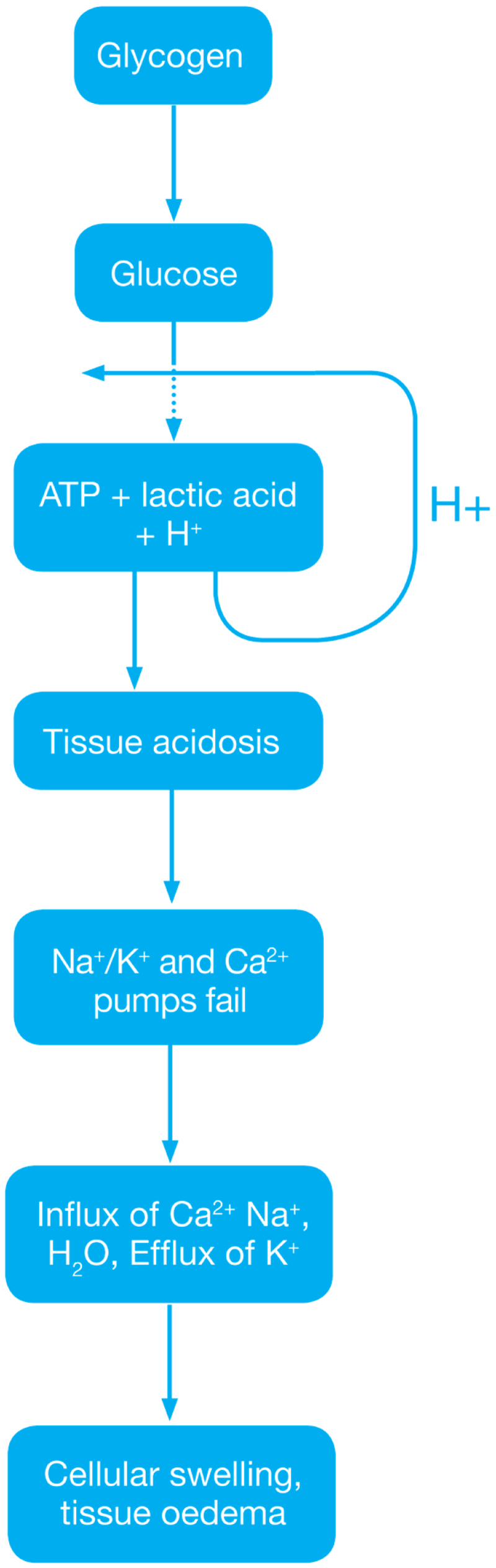
During this phase mainly the dysregulation of metabolic pathways occurs and in the reperfusion phase there will be generation of free radicals.
- Ischemia when the blood supply to the tissues decreases with respect to the demand required to function properly. This results in deficiency in oxygen, glucose and various other substrates required for cellular metabolism. As previously dais the derangement or dysregulation of metabolic function begins in this phase. Due to less oxygen supply cellular metabolism shifts to anaerobic glycolysis causing the glycogen to breakdown resulting in the production of 2 ATP and a lactic acid. This decrease in tissue PH starts further inhibits the ATP generation by negative feed back mechanism. ATP gets broken down into ADP, AMP and IMP. This finally gets converted to adenosine, inosine, hypoxanthine and xanthine.
- Lack of ATP at the cellular level causes impairment in the function of ionic pumps - Na+/K+ and Ca2+ pumps. As a result cytosolic sodium rises which in turn withdraws water to maintain the osmotic equilibrium consequently resulting in the cellular swelling. To maintain ionic balance potassium ion escape from the cell. Calcium is released from the mitochondria to the cytoplasm and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent cytosolic proteases. These converts the enzyme xanthine dehydrogenase to xanthine oxidase. Phospholipases activated during ischemia promotes membrane degradation and increases level of free fatty acids
- Ischemia also induces expression of a large number of genes and transcription factors, which play a major role in the damage to the tissues.
- Transcription factors
- Activating protein-1 (AP-1)
- Hypoxia-inducible factor-1 (HIF-1) which in turn activates transcription of VEGF, Erythropoietin and Glucose transporter-1
- Nuclear factor-kappa b (NF-kb)
- Activation of NF-kb occurs during both the ischemic and reperfusion phases
- Transcription factors
Reperfusion Phase
Reactive oxygen species
The ROS play major role in the tissue damage related to ischemia reperfusion injury. Once the ischemic tissue is reperfused the molecular oxygen catalyzes the conversion of hypoxanthine to uric acid and liberating the superoxide anion (O2-). This superoxide gets further converted to (H2O2) and the hydroxyl radical (OH•). This OH ion causes the peroxidation lipids in the cell membranes resulting in the production and release of proinflammatory eicosanoids and ultimately cell death.

During the Ischemia reperfusion injury ROS also activate endothelial cells, which further produces numerous adhesion molecules
- E-selectin
- VCAM-1 (vascular cell adhesion molecule-1)
- ICAM-1 (intercellular adhesion molecule-1)
- EMLMl Am -1 ( endothelial-leukocyte adhesion molecule)
- PAi-1 (plasminogen activator inhibitor-1 ), and
- interleukin-8 (il-8)
Eicosanoids
ROS causes lipid peroxidation of cell membranes resulting in release of
- Arachidonic acid (substrate for prostaglandins)
- Prostaglandins usually have a vasodilatory effect hat provides protective effect during Ischemia reperfusion injury. But they have short life so their fast depletion leads to vasoconstriction ultimately leading to reduced blood flow and exacerbation of ischemia.
- Thromboxane
- Plasma thromboxane A2 level rises within minutes after reperfusion, resulting in vasoconstriction and platelet aggregation. This usually coincide with rapid rise in pulmonary artery pressure and a subsequent increase in pulmonary microvascular permeability.
- Leukotrienes
- Leukotrienes are also synthesized from arachidonic acid. Leukotrienes acts directly in the endothelial cells and smooth muscle and indirectly on the neutrophils. The leukotrienes C4, D4, and E4 alters the endothelial cytoskeleton, resulting in increased vascular permeability and smooth muscle contraction, and finally leading to vasoconstriction.
Risk Factors
Risk factors for reperfusion injury include
- Hypertension with left ventricular hypertrophy,
- Congestive heart failure,
- Increased age,
- Diabetes, and
- Hyperlipidemia
Natural History, Complications and Prognosis
Reperfusion injury may be responsible for about 50% of the total infarct size after an acute myocardial infarction as well as myocardial stunning, congestive heart failure and reperfusion arrhythmias such as ventricular arrhythmias.
Medical Therapy
Various proposed medical managements studied are:
- Therapeutic hypothermia
- It has been shown in rats that neurons sometimes die completely 24 hours after the blood flow returns. Some claim that this delayed reaction is the result of the multiple inflammatory immune responses that occur during reperfusion. Such inflammatory reactions cause intracranial pressure, a pressure that leads to cell damage and cell death in some cases. Hypothermia has been shown to help reduce intracranial pressure and thus decrease the adverse effects of inflammatory immune responses during reperfusion. Besides that, reperfusion also increases free radical development. Hypothermia has also been shown to decrease the patient's development of deadly free radicals during reperfusion.
- Hydrogen sulfide treatment
- There are several preliminary studies in mice that seem to show that treatment with hydrogen sulfide ( H2S) could have a protective effect against reperfusion injury.
- Cyclosporin
- In addition to its well-known immunosuppressive capabilities, the one-time administration of cyclosporine at the time of percutaneous coronary intervention (PCI) has been found to deliver a 40 percent reduction in infarct size in a small group proof of concept study of human patients with reperfusion injury published in The New England Journal of Medicine in 2008.
- Cyclosporine has been confirmed in studies to inhibit the actions of cyclophilin D, a protein which is induced by excessive intracellular calcium flow to interact with other pore components and help open the MPT pore. Inhibiting cyclophilin D has been shown to prevent the opening of the MPT pore and protect the mitochondria and cellular energy production from excessive calcium inflows.
- Reperfusion leads to biochemical imbalances within the cell that lead to cell death and increased infarct size. More specifically, calcium overload and excessive production of reactive oxygen species in the first few minutes after reperfusion set off a cascade of biochemical changes that result in the opening of the so-called mitochondrial permeability transition pore (MPT pore) in the mitochondrial membrane of cardiac cells.
- The opening of the MPT pore leads to the inrush of water into the mitochondria, resulting in mitochondrial dysfunction and collapse. Upon collapse, the calcium is then released to overwhelm the next mitochondria in a cascading series of events that cause mitochondrial energy production supporting the cell to be reduced or stopped completely. The cessation of energy production results in cellular death. Protecting mitochondria is a viable cardio protective strategy.
- Cyclosporine is currently in a phase II/III (adaptive) clinical study in Europe to determine its ability to ameliorate neuronal cellular damage in traumatic brain injury.
- TRO40303
- TRO40303 is a new cardio protective compound that was shown to inhibit the MPT pore and reduce infarct size after ischemia-reperfusion.
- Stem cell therapy
- Recent investigations suggest a possible beneficial effect of mesenchymal stem cells on heart and kidney reperfusion injury
- Superoxide dismutase
- Superoxide dismutase is an important antioxidant enzyme that transforms superoxide anions to water and hydrogen peroxide. Recent work has demonstrated important therapeutic effects on pre-clinical models of reperfusion damage following an ischemic stroke .
- Metformin
- A series of 2009 studies published in the Journal of Cardiovascular Pharmacology indicate that metformin may prevent injury to cardiac reperfusion by inhibiting Mitochondrial Complex I and opening up MPT pore and in rats.
- Cannabinoids
- A research published in 2012 shows that the synthetic analog of phytocannabinoid tetrahydrocannabivarin (THCV), 8-Tetrahydrocannabivarin (THCV) and its 11-OH-8-THCV metabolite prevents hepatic ischemia / reperfusion injury by minimizing oxidative stress and inflammatory reactions through cannabinoid CB2 receptors, thereby lowering tissue damage and protective effects of inflammation. Pretreatment with a CB2 receptor antagonist, whereas a CB1 antagonist appeared to strengthen it, attenuated the defensive effects of somewhere else.
- An earlier study published in 2011 found that cannabidiol (CBD) also protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signals and oxidative and nitrative stress response, resulting in cell death and tissue damage, but is independent of classic CB1 and CB2 receptors.