Lupus nephritis pathophysiology
| https://https://www.youtube.com/watch?v=HwzNQ4Oav00&t=4s |350}} |
|
Lupus nephritis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lupus nephritis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Lupus nephritis pathophysiology |
|
Risk calculators and risk factors for Lupus nephritis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2], Raviteja Guddeti, M.B.B.S. [3]
Overview
Pathophysiology
Systemic lupus erythematosus (SLE, or lupus) is an autoimmune disease. This means there is a problem with the body's immune system.
Normally, the immune system helps protect the body from harmful substances. But in patients with an autoimmune disease, the immune system cannot tell the difference between harmful substances and healthy ones. As a result, the immune system attacks otherwise healthy cells and tissue.
Overview
The exact pathogenesis of [disease name] is not fully understood.
OR
It is thought that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
OR
[Pathogen name] is usually transmitted via the [transmission route] route to the human host.
OR
Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
OR
[Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
OR
The progression to [disease name] usually involves the [molecular pathway].
OR
The pathophysiology of [disease/malignancy] depends on the histological subtype.
Pathophysiology
Pathogenesis
Immune system, genetic, and environmental factors are considered in the pathogenesis of SLE.
- Immune system [1]:
- Plasma cells and B lymphocytes:
Numbers of plasma cells(PC) are high in the kidney medulla of patients with severe lupus nephritis( LN). PCs and B cells produce auto antibodies. Increased number and activation of B cells may cause worsening proteinuria and severe damage.
B cells in LN patients have more MicroRNAs (miRNAs) which modulate gene expression [2]. Over expression of the miR-30a could lower the level of Lyn (type of protein tyrosine kinases), and lower level of Lyn may cause deposition of immune complexes in the kidney [3][4].
- Macrophages:
Increase expression of Sialoadhesin (Sn), a macrophage-restricted adhesion molecule may play a role in causing sever LN [5].
- Inflammatory cytokines:
Tumor necrosis factor (TNF) is a cytokine (cell signaling protein) that play role in inflammation process. One of the sub types of TNF is TNF-like weak inducer of apoptosis (TWEAK) which has an important role in causing LN [6].
Fn14 ( TWEAK receptor) is interacted with TWEAK on renal mesangial, endothelial, tubular cells and podocytes [7]. This interactions produce multiple inflammatory mediators which lead to LN.
- Repair impairment and Tissue Scarring:
Impairment in regulation and repair may cause tissue scars like [8]:
- Necrosis and extracellular matrix production cause global glomerulosclerosis [9].
- Fibrinogen leakage in to Bowman’s space cause epithelial cell hyperproliferation. Accumulation of epithelial cells make glomerular crescent. In severe LN, epithelial cells produce extra amount of matrix in Bowman’s space, which result in glomerulosclerosis [10].
- Exaggerated healing response cause hyperproliferation of mesangial and endothelial cells, and podocyte loss cause damage in renal.
- Environmental factors:
- Geographical distribution :
LN is more severe in African, Hispanics and Asian patients with SLE. LN is associated with temperature and season [11].
- Infections
- May stimulate some antigen specific cells and increase apoptosis
- May induce anti-DNA antibodies
- May mimic lupus-like symptoms
- Associated with higher risk of SLE
- Associated with triggering the active courses and flare ups of disease in children
- Include:
- Parvovirus B19
- Epstein-Barr virus (EBV)
- Trypanosomiasis
- Mycobacterial infections
- SLE flares may follow bacterial infections
- Ultraviolet (UV) light:
- Can stimulate B-cells to produce more antibodies
- May activate macrophages, interfere with antigen processing, and therefore increase the degree of autoimmunity
- It is understood that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
- [Pathogen name] is usually transmitted via the [transmission route] route to the human host.
- Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
- [Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
- The progression to [disease name] usually involves the [molecular pathway].
- The pathophysiology of [disease/malignancy] depends on the histological subtype.
Genetics
interaction and mutation between below genes from multiple categories may cause severe LN[12] [13] [14] [15].
- STAT4 [16]- TNFSF4 (OX40L) [17]- IKZF1 [18]- IRF5 [19]- TLR9 [20]- TNFAIP3 (A20) [21]- TNIP3 (ABIN3) [22]- ACE [23]- KLK [24]- FCGR2A, 3A, 3B [25]- ITGAM [26] [27] [28]- HLA DR and BLK [29].
Epigenetic modification:
- DNA methylation:
Hypomethylated genes in B lymphocytes activate transcription, and cause production of many anti-DNA antibodies[30].
- Histone modifications:
Histone is a protein in chromatin that play role in gene regulation.
Acetylation of histones are concidered targets for autoantibodies in LN.
- MicroRNAs:
Non-coding RNA sequences that play role in gene regulation by degradation of mRNA and protein translation blockage.
Some miRNAs are increased in LN like miR-142-3p and miR-181 and some are decreased like miR-106a, miR-17, miR-20a, miR-92a and miR-203 [31].
These changes cause dysregulation of genes and LN.
- [Disease name] is transmitted in [mode of genetic transmission] pattern.
- Genes involved in the pathogenesis of [disease name] include [gene1], [gene2], and [gene3].
- The development of [disease name] is the result of multiple genetic mutations.
Associated Conditions
Gross Pathology
- On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
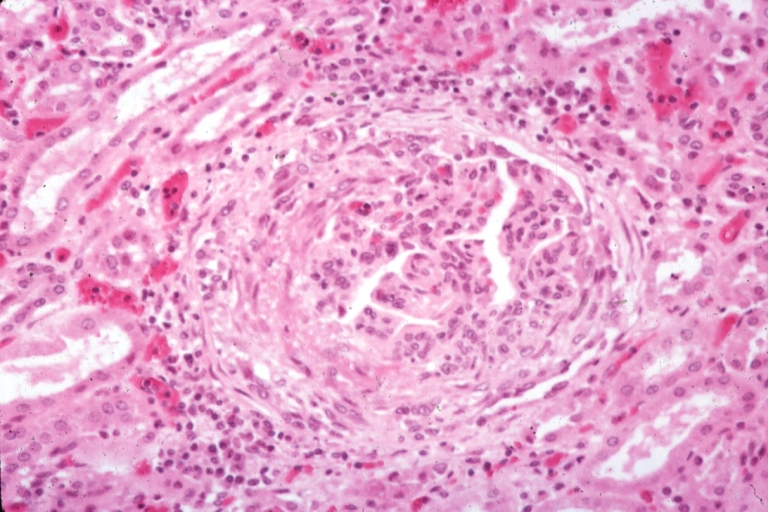
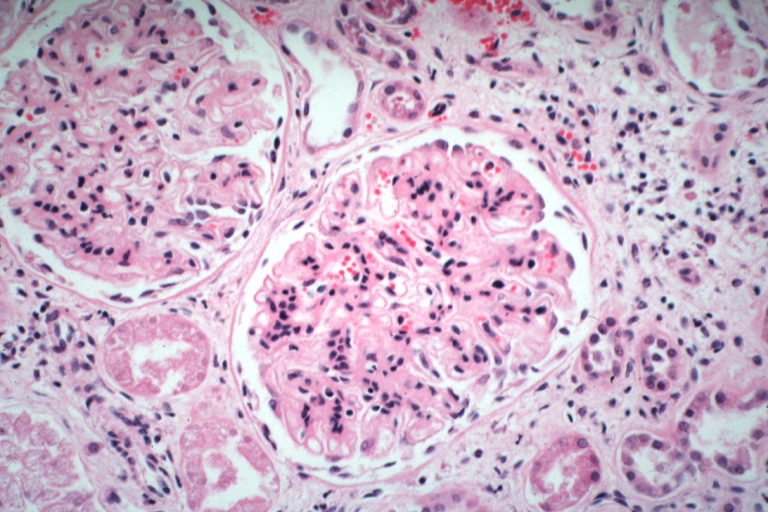
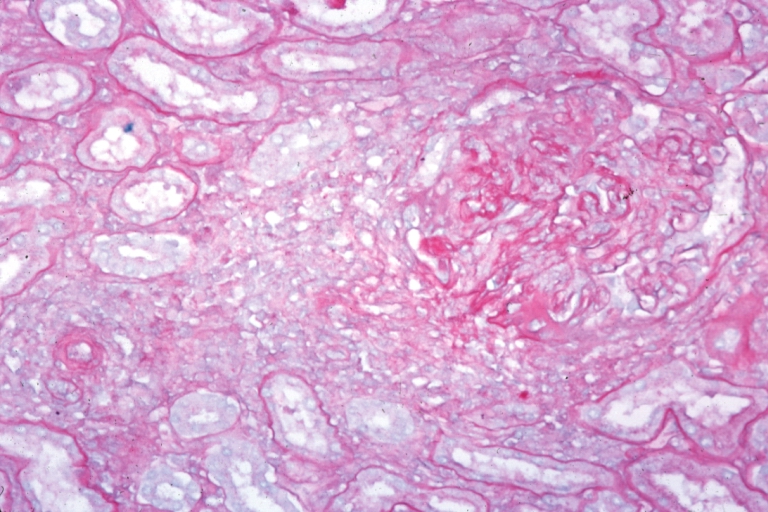
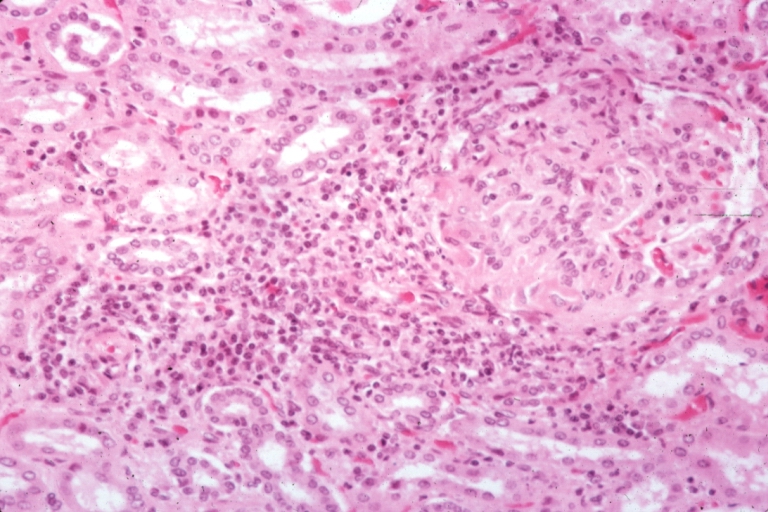
Microscopic Pathology
- On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
Videos
{{#ev:youtube|Tw07BFaDEo0}}
References
- ↑ Schwartz N, Goilav B, Putterman C (September 2014). "The pathogenesis, diagnosis and treatment of lupus nephritis". Curr Opin Rheumatol. 26 (5): 502–9. doi:10.1097/BOR.0000000000000089. PMC 4221732. PMID 25014039.
- ↑ He L, Hannon GJ (July 2004). "MicroRNAs: small RNAs with a big role in gene regulation". Nat. Rev. Genet. 5 (7): 522–31. doi:10.1038/nrg1379. PMID 15211354.
- ↑ Yu CC, Yen TS, Lowell CA, DeFranco AL (January 2001). "Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn". Curr. Biol. 11 (1): 34–8. PMID 11166177.
- ↑ Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, Li Z, Yang G (June 2013). "MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn". Arthritis Rheum. 65 (6): 1603–11. doi:10.1002/art.37912. PMID 23450709.
- ↑ Biesen R, Demir C, Barkhudarova F, Grün JR, Steinbrich-Zöllner M, Backhaus M, Häupl T, Rudwaleit M, Riemekasten G, Radbruch A, Hiepe F, Burmester GR, Grützkau A (April 2008). "Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus". Arthritis Rheum. 58 (4): 1136–45. doi:10.1002/art.23404. PMID 18383365.
- ↑ Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, Chow KM, Wang G, Li PK, Szeto CC (May 2011). "Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis". Nephrology (Carlton). 16 (4): 426–32. doi:10.1111/j.1440-1797.2011.01449.x. PMID 21303425.
- ↑ Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C (February 2006). "Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells". J. Immunol. 176 (3): 1889–98. PMID 16424220.
- ↑ Liu Y, Anders HJ (2014). "Lupus nephritis: from pathogenesis to targets for biologic treatment". Nephron Clin Pract. 128 (3–4): 224–31. doi:10.1159/000368581. PMID 25401461.
- ↑ Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ (July 2011). "Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis". J. Am. Soc. Nephrol. 22 (7): 1262–74. doi:10.1681/ASN.2010090970. PMC 3137574. PMID 21719782.
- ↑ Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ (December 2012). "Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury". J. Pathol. 228 (4): 482–94. doi:10.1002/path.4046. PMID 22553158.
- ↑ Li Y, Fang X, Li QZ (June 2013). "Biomarker profiling for lupus nephritis". Genomics Proteomics Bioinformatics. 11 (3): 158–65. doi:10.1016/j.gpb.2013.05.003. PMC 4357827. PMID 23732627.
- ↑ Mohan C, Putterman C (June 2015). "Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis". Nat Rev Nephrol. 11 (6): 329–41. doi:10.1038/nrneph.2015.33. PMID 25825084.
- ↑ Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK (June 2000). "Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains". Proc. Natl. Acad. Sci. U.S.A. 97 (12): 6670–5. PMC 18697. PMID 10841565.
- ↑ Xie S, Mohan C (February 2004). "Divide and conquer--the power of congenic strains". Clin. Immunol. 110 (2): 109–11. doi:10.1016/j.clim.2003.09.007. PMID 15003808.
- ↑ Henry T, Mohan C (2005). "Systemic lupus erythematosus--recent clues from congenic strains". Arch. Immunol. Ther. Exp. (Warsz.). 53 (3): 207–12. PMID 15995581.
- ↑ Amarilyo G, Lourenço EV, Shi FD, La Cava A (July 2014). "IL-17 promotes murine lupus". J. Immunol. 193 (2): 540–3. doi:10.4049/jimmunol.1400931. PMID 24920843.
- ↑ Crispín JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC (April 2012). "Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism". J. Immunol. 188 (8): 3567–71. doi:10.4049/jimmunol.1200143. PMC 3324672. PMID 22422882.
- ↑ Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, Satoh M, Koss M (August 2003). "Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice". J. Immunol. 171 (3): 1564–71. PMID 12874250.
- ↑ Zhou XJ, Cheng FJ, Qi YY, Zhao MH, Zhang H (2013). "A replication study from Chinese supports association between lupus-risk allele in TNFSF4 and renal disorder". Biomed Res Int. 2013: 597921. doi:10.1155/2013/597921. PMC 3713374. PMID 23936824.
- ↑ Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB (February 2010). "Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages". Proc. Natl. Acad. Sci. U.S.A. 107 (7): 3012–7. doi:10.1073/pnas.0914902107. PMC 2840310. PMID 20133703.
- ↑ Clynes R, Dumitru C, Ravetch JV (February 1998). "Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis". Science. 279 (5353): 1052–4. PMID 9461440.
- ↑ Dang J, Shan S, Li J, Zhao H, Xin Q, Liu Y, Bian X, Liu Q (June 2014). "Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus". Tissue Antigens. 83 (6): 401–8. doi:10.1111/tan.12349. PMID 24697319.
- ↑ Zhou TB, Liu YG, Lin N, Qin YH, Huang K, Shao MB, Peng DD (April 2012). "Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: a systematic review and metaanalysis". J. Rheumatol. 39 (4): 686–93. doi:10.3899/jrheum.110863. PMID 22337243.
- ↑ Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M, Ye Q, Liu S, Xie C, Zhou XJ, Chung SA, Pons-Estel B, Witte T, de Ramón E, Bae SC, Barizzone N, Sebastiani GD, Merrill JT, Gregersen PK, Gilkeson GG, Kimberly RP, Vyse TJ, Kim I, D'Alfonso S, Martin J, Harley JB, Criswell LA, Wakeland EK, Alarcón-Riquelme ME, Mohan C (April 2009). "Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans". J. Clin. Invest. 119 (4): 911–23. doi:10.1172/JCI36728. PMC 2662554. PMID 19307730.
- ↑ Brown EE, Edberg JC, Kimberly RP (December 2007). "Fc receptor genes and the systemic lupus erythematosus diathesis". Autoimmunity. 40 (8): 567–81. doi:10.1080/08916930701763710. PMID 18075791.
- ↑ Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC (September 2013). "Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling". J. Biol. Chem. 288 (37): 26775–84. doi:10.1074/jbc.M113.483743. PMC 3772223. PMID 23918926.
- ↑ Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R (November 2006). "FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis". J. Immunol. 177 (10): 7287–95. PMID 17082647.
- ↑ Zhou XJ, Lv JC, Cheng WR, Yu L, Zhao MH, Zhang H (2010). "Association of TLR9 gene polymorphisms with lupus nephritis in a Chinese Han population". Clin. Exp. Rheumatol. 28 (3): 397–400. PMID 20497632.
- ↑ Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B (October 2011). "Tolerogenic function of Blimp-1 in dendritic cells". J. Exp. Med. 208 (11): 2193–9. doi:10.1084/jem.20110658. PMC 3201204. PMID 21948081.
- ↑ Renaudineau Y, Youinou P (2011). "Epigenetics and autoimmunity, with special emphasis on methylation". Keio J Med. 60 (1): 10–6. PMID 21460598.
- ↑ Carlsen AL, Schetter AJ, Nielsen CT, Lood C, Knudsen S, Voss A, Harris CC, Hellmark T, Segelmark M, Jacobsen S, Bengtsson AA, Heegaard NH (May 2013). "Circulating microRNA expression profiles associated with systemic lupus erythematosus". Arthritis Rheum. 65 (5): 1324–34. doi:10.1002/art.37890. PMID 23401079.