Neuroendocrine tumors: Difference between revisions
Sara Mohsin (talk | contribs) |
Sara Mohsin (talk | contribs) |
||
| Line 112: | Line 112: | ||
* Parathyroid tumors | * Parathyroid tumors | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thymus and mediastinum | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Thymus and mediastinum | ||
| | | | ||
* Thymus and mediastinal carcinoid tumors | * Thymus and mediastinal carcinoid tumors | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Lungs | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Lungs<ref name="pmid18456740">{{cite journal| author=Oberg K, Jelic S, ESMO Guidelines Working Group| title=Neuroendocrine bronchial and thymic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. | journal=Ann Oncol | year= 2008 | volume= 19 Suppl 2 | issue= | pages= ii102-3 | pmid=18456740 | doi=10.1093/annonc/mdn116 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18456740 }} </ref> | ||
| | | | ||
* Pulmonary neuroendocrine tumors | * Pulmonary neuroendocrine tumors | ||
| Line 130: | Line 130: | ||
* Extrapulmonary small cell carcinomas (ESCC or EPSCC) | * Extrapulmonary small cell carcinomas (ESCC or EPSCC) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |GIT | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |GIT | ||
| | | | ||
* Gastroenteropancreatic neuroendocrine tumors (GEP-NET) | * Gastroenteropancreatic neuroendocrine tumors (GEP-NET) | ||
| Line 140: | Line 140: | ||
* Liver and gallbladder | * Liver and gallbladder | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Adrenal gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Adrenal gland | ||
| | | | ||
* Adrenal tumors, particularly adrenomedullary tumors | * Adrenal tumors, particularly adrenomedullary tumors | ||
| Line 151: | Line 151: | ||
* Neuroblastoma | * Neuroblastoma | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Mammary gland | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Mammary gland | ||
| | | | ||
* Breast tumors | * Breast tumors | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Genitourinary tract | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Genitourinary tract | ||
| | | | ||
* Urinary tract carcinoid tumor and neuroendocrine carcinoma | * Urinary tract carcinoid tumor and neuroendocrine carcinoma | ||
| Line 166: | Line 166: | ||
* Merkel cell carcinoma of skin (trabecular cancer) | * Merkel cell carcinoma of skin (trabecular cancer) | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Multiple organs involvement in inherited conditions | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Multiple organs involvement in inherited conditions | ||
| | | | ||
* Several inherited conditions: | * Several inherited conditions: | ||
| Line 179: | Line 179: | ||
===Classification of GEP-NETs by cell characteristics=== | ===Classification of GEP-NETs by cell characteristics=== | ||
The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology. In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. For example, the term ''atypical carcinoid'' is sometimes used to indicate an aggressive tumor without secretions, whether anaplastic or well-differentiated. | * The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology. | ||
* In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. For example, the term ''atypical carcinoid'' is sometimes used to indicate an aggressive tumor without secretions, whether anaplastic or well-differentiated. | |||
In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs, abandoning the term ''carcinoid'' in favor of ''neuroendocrine tumor'' (NET) and abandoning ''islet cell tumor'' or ''pancreatic endocrine tumor'' for ''neuroendocrine carcinoma'' (NEC). Judging from papers published into 2006, the medical community is accepting this new terminology with great sluggishness. (Perhaps one reason for the resistance is that the WHO chose to label the least aggressive subclass of neuroendocrine neoplasm with the term – ''neuroendocrine tumor'' – widely used previously either for the superclass or for the generally aggressive noncarcinoid subclass.) | * In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs, abandoning the term ''carcinoid'' in favor of ''neuroendocrine tumor'' (NET) and abandoning ''islet cell tumor'' or ''pancreatic endocrine tumor'' for ''neuroendocrine carcinoma'' (NEC). | ||
* Judging from papers published into 2006, the medical community is accepting this new terminology with great sluggishness. (Perhaps one reason for the resistance is that the WHO chose to label the least aggressive subclass of neuroendocrine neoplasm with the term – ''neuroendocrine tumor'' – widely used previously either for the superclass or for the generally aggressive noncarcinoid subclass.) | |||
Klöppel ''et alia'' have written an overview that clarifies the WHO classification and bridges the gap to the old terminology (Klöppel, Perren, and Heitz 2004) | * Klöppel ''et alia'' have written an overview that clarifies the WHO classification and bridges the gap to the old terminology (Klöppel, Perren, and Heitz 2004), this old terminology is given in the table below: | ||
====Summary of classification by cell characteristics (the WHO classification)==== | ====Summary of classification by cell characteristics (the WHO classification)==== | ||
| Line 220: | Line 220: | ||
| | | | ||
* '''WHO:''' Rare neuroendocrine-like lesions | * '''WHO:''' Rare neuroendocrine-like lesions | ||
|}<br /> | |||
{| class="wikitable" | |||
|+2010 World Health Organization International Classification and Distribution of Diseases for Oncology (3rd Ed. (ICD-O-3) Codes of Neuroendocrine Tumors (NETs) in the Gastrointestinal and Pancreatobiliary Tracts) | |||
! colspan="2" |NET (Neuroendocrine Tumor) Classification | |||
!Location | |||
!ICD-O-3 Code | |||
|- | |||
| colspan="2" |NET G1 (Grade 1) | |||
|All organs | |||
|8240/3 | |||
|- | |||
| colspan="2" |NET G2 (Grade 2) | |||
|All organs | |||
|8249/3 | |||
|- | |||
| rowspan="3" |Neuroendocrine carcinoma | |||
| | |||
|All organs | |||
|8246/3 | |||
|- | |||
|Large cell NEC | |||
|All organs | |||
|8013/3 | |||
|- | |||
|Small cell NEC | |||
|All organs | |||
|8041/3 | |||
|- | |||
| colspan="2" |Enterochromaffin cell serotonin-producing NET | |||
|All organs | |||
|8241/3 | |||
|- | |||
| colspan="2" |Gastrin-producing NET (gastrinoma) | |||
|Stomach, ampulla, small intestine, pancreas | |||
|8153/3 | |||
|- | |||
| colspan="2" |Glucagon-producing NET (glucagonoma) | |||
|Pancreas | |||
|8152/3 | |||
|- | |||
| colspan="2" |Gangliocytic paraganglioma | |||
|Ampulla, small intestine | |||
|8683/0 | |||
|- | |||
| colspan="2" |Somatostatin-producing NET (somatostatinoma) | |||
|Ampulla, small intestine, pancreas | |||
|8156/3 | |||
|- | |||
| colspan="2" |Insulin-producing NET (insulinoma) | |||
|Pancreas | |||
|8151/3 | |||
|- | |||
| colspan="2" |VIPoma | |||
|Pancreas | |||
|8155/3 | |||
|- | |||
| colspan="2" |L cell, Glucagon-like peptide and PP/PYY-producing NETs | |||
|Small intestine, appendix, colorectum | |||
|8152/1 | |||
|- | |||
| colspan="2" |Goblet cell carcinoid | |||
|Appendix, extrahepatic bile duct | |||
|8241/3 | |||
|- | |||
| colspan="2" |Tubular carcinoid | |||
|Appendix, extrahepatic bile duct | |||
|8245/1 | |||
|- | |||
| colspan="2" |Mixed adenoneuroendocrine carcinoma (MANEC) | |||
|All organs | |||
|8244/3 | |||
|- | |||
| colspan="2" |Neuroendocrine microadenoma | |||
|Pancreas | |||
|8150/0 | |||
|} | |} | ||
Revision as of 19:59, 26 August 2019
| Neuroendocrine tumors | |
| ICD-9 | 209 |
|---|---|
| ICD-O: | M8013/3, M8041/3, M8246/3, M8247/3, M8574/3 |
| MedlinePlus | 000393 |
| MeSH | D018358 |
|
Neuroendocrine tumors Microchapters |
For patient information, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [12] Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[13]
Overview
Neuroendocrine tumors, or more properly gastro-entero-pancreatic or gastroenteropancreatic neuroendocrine tumors (GEP-NETs), are cancers of the interface between the endocrine (hormonal) system and the nervous system.
Historical Perspective
Siegfried Oberndorfer, in 1907, was the first person to distinguish clearly what we now call GEP-NETs from other forms of cancer. He gave the term "carcinoid" to these tumors, because they were so slow-growing that he considered them to be "cancer-like" rather than truly cancerous. In 1929, he reported that some such tumors were not so indolent – these he distinguished as what we now call PETs from what most authorities call carcinoids. Despite the differences between the two categories, some doctors, including oncologists, persist in calling all GEP-NETs "carcinoid", even into the twenty-first century.
The earliest synthetic form of somatostatin used in the treatment of Neuroendocrine tumors was octreotide, first marketed, by Sandoz as Sandostatin, in 1988.
Classification
Human GEP-NETs by Site of Origin and by Symptom
| Human GEP-NETs by Site of Origin and by Symptom | Percentage | |
|---|---|---|
| Carcinoids (about two thirds of GEP-NETs) |
|
|
|
| |
| PETs (about one third of GEP-NETs) |
|
|
|
| |
| Rare GEP-NETs |
|
|
Simplified classification according to anatomic distribution
| Involved organ | Name/type of neuroendocrine tumor |
|---|---|
| Pituitary gland |
|
| Thyroid gland |
|
| Parathyroid glands |
|
| Thymus and mediastinum |
|
| Lungs[1] |
|
| Extrapulmonary |
|
| GIT |
|
| Adrenal gland |
|
| Peripheral nervous system | Peripheral nervous system tumors, such as:
|
| Mammary gland |
|
| Genitourinary tract |
|
| Skin |
|
| Multiple organs involvement in inherited conditions |
|
Classification of GEP-NETs by cell characteristics
- The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology.
- In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. For example, the term atypical carcinoid is sometimes used to indicate an aggressive tumor without secretions, whether anaplastic or well-differentiated.
- In 2000, the World Health Organization (WHO) revised the classification of GEP-NETs, abandoning the term carcinoid in favor of neuroendocrine tumor (NET) and abandoning islet cell tumor or pancreatic endocrine tumor for neuroendocrine carcinoma (NEC).
- Judging from papers published into 2006, the medical community is accepting this new terminology with great sluggishness. (Perhaps one reason for the resistance is that the WHO chose to label the least aggressive subclass of neuroendocrine neoplasm with the term – neuroendocrine tumor – widely used previously either for the superclass or for the generally aggressive noncarcinoid subclass.)
- Klöppel et alia have written an overview that clarifies the WHO classification and bridges the gap to the old terminology (Klöppel, Perren, and Heitz 2004), this old terminology is given in the table below:
Summary of classification by cell characteristics (the WHO classification)
- GEP-NETs are also sometimes called APUDomas, but that term is now considered to be misleading, since it is based on a discredited theory of the development of the tumors[2]
| Superclass:Öberg, WHO, Klöppel et alia: Gastro-entero-pancreatic neuroendocrine tumor (GEP-NET) | ||||
|---|---|---|---|---|
| Subclass 1 (less malignant) | Subclass 2 (more malignant) | Subclass 3 (most malignant) | Subclass 4 (mixed) | Subclass 5 (miscellaneous) |
|
|
|
|
|
| NET (Neuroendocrine Tumor) Classification | Location | ICD-O-3 Code | |
|---|---|---|---|
| NET G1 (Grade 1) | All organs | 8240/3 | |
| NET G2 (Grade 2) | All organs | 8249/3 | |
| Neuroendocrine carcinoma | All organs | 8246/3 | |
| Large cell NEC | All organs | 8013/3 | |
| Small cell NEC | All organs | 8041/3 | |
| Enterochromaffin cell serotonin-producing NET | All organs | 8241/3 | |
| Gastrin-producing NET (gastrinoma) | Stomach, ampulla, small intestine, pancreas | 8153/3 | |
| Glucagon-producing NET (glucagonoma) | Pancreas | 8152/3 | |
| Gangliocytic paraganglioma | Ampulla, small intestine | 8683/0 | |
| Somatostatin-producing NET (somatostatinoma) | Ampulla, small intestine, pancreas | 8156/3 | |
| Insulin-producing NET (insulinoma) | Pancreas | 8151/3 | |
| VIPoma | Pancreas | 8155/3 | |
| L cell, Glucagon-like peptide and PP/PYY-producing NETs | Small intestine, appendix, colorectum | 8152/1 | |
| Goblet cell carcinoid | Appendix, extrahepatic bile duct | 8241/3 | |
| Tubular carcinoid | Appendix, extrahepatic bile duct | 8245/1 | |
| Mixed adenoneuroendocrine carcinoma (MANEC) | All organs | 8244/3 | |
| Neuroendocrine microadenoma | Pancreas | 8150/0 | |
Pathophysiology
The neuroendocrine system
The endocrine system is a communication system in which hormones act as biochemical messengers to regulate physiological events in living organisms. The nervous system performs the same functions using electrical impulses as messengers. The neuroendocrine system is the combination of those two systems, or more specifically, the various interfaces between the two systems. A GEP-NET is a tumor of any such interface.
More specifically, the endocrine system is primarily a network of glands that produce and secrete hormones, usually into the bloodstream. It also includes cells that are not part of glands: the diffuse neuroendocrine system, scattered throughout other organs.
A hormone is a chemical that delivers a particular message to a particular organ, typically remote from the hormone's origin. For example, the hormone insulin, secreted by the pancreas, acts primarily to allow glucose to enter the body's cells for use as fuel. The hormone gastrin is secreted by the stomach to tell the stomach to produce acids to digest food.
Hormones can be divided into subtypes such as peptides, steroids, and neuroamines. For some researchers, there is no clear distinction between peptide hormones and peptides; the hormones are simply longer than other peptides. In the context of GEP-NETs, the terms hormone and peptide are often used interchangeably.
The vast majority of GEP-NETs fall into two nearly distinct categories: carcinoids, and pancreatic endocrine tumors (PETs). Despite great behavioral differences between the two, they are grouped together as GEP-NETs because of similarities in cell structure.
Pancreatic endocrine tumors (PETs) are also known as endocrine pancreatic tumors (EPTs) or islet cell tumors. PETs are assumed to originate generally in the islets of Langerhans within the pancreas – or, Arnold et alia suggest, from endocrine pancreatic precursor cells (Arnold et al. 2004, 199) – though they may originate outside of the pancreas. (The term pancreatic cancer almost always refers to adenopancreatic cancer, also known as exocrine pancreatic cancer. Adenopancreatic cancers are generally very aggressive, and are not a neuroendocrine cancers. About 95 percent of pancreatic tumors are adenopancreatic; about 1 or 2 percent are GEP-NETs.)
PETs may secrete hormones (as a result, perhaps, of impaired storage ability), and those hormones can wreak symptomatic havoc on the body. Those PETs that do not secrete hormones are called nonsecretory or nonfunctioning or nonfunctional tumors. Secretory tumors are classified by the hormone most strongly secreted – for example, insulinoma, which produces excessive insulin, and gastrinoma, which produces excessive gastrin (see more detail in the summary below).
Carcinoid tumors are further classified, depending on the point of origin, as foregut (lung, thymus, stomach, and duodenum) or midgut (distal ileum and proximal colon) or hindgut (distal colon and rectum). Less than one percent of carcinoid tumors originate in the pancreas. But for many tumors, the point of origin is unknown.
Carcinoid tumors, which secrete serotonin tend to grow much more slowly than PETs. Although this serotonin secretion is entirely different from a secretory PET's hormone secretion, carcinoid tumors with carcinoid syndrome are nevertheless sometimes called functioning, adding to the frequent confusion of carcinoids with PETs. Carcinoid syndrome is primarily associated with midgut carcinoids. A severe episode of carcinoid syndrome is called carcinoid crisis; it can be triggered by surgery or chemotherapy, among other factors. [4]
The mildest of the carcinoids are discovered only upon surgery for unrelated causes. These coincidental carcinoids are common; one study found that one person in ten has them. [5]
Neuroendocrine tumors other than coincidental carcinoids are rare. Incidence of PETs is estimated at one new case per 100,000 people per year; incidence of clinically significant carcinoids is twice that. Thus the total incidence of GEP-NETs in the United States would be about 9,000 new cases per year. But researchers differ widely in their estimates of incidence, especially at the level of the secretory subtypes (the various "-omas").
In addition to the two main categories, there are even rarer forms of GEP-NETs. At least one form – neuroendocrine lung tumors – arises from the respiratory rather than the gastro-entero-pancreatic system.
Non-human animals also suffer from GEP-NETs; for example, neuroendocrine cancer of the liver is a disease of dogs, and Devil facial tumor disease is a neuroendocrine tumor of Tasmanian Devils.
Rufini et alia summarize: "Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms originating from endocrine cells, which are characterized by the presence of secretory granules as well as the ability to produce biogenic amines and polypeptide hormones. These tumors originate from endocrine glands such as the adrenal medulla, the pituitary, and the parathyroids, as well as endocrine islets within the thyroid or the pancreas, and dispersed endocrine cells in the respiratory and gastrointestinal tract. The clinical behavior of NETs is extremely variable; they may be functioning or not functioning, ranging from very slow-growing tumors (well-differentiated NETs), which are the majority, to highly aggressive and very malignant tumors (poorly differentiated NETs).... Classically, NETs of the gastrointestinal tract are classified into 2 main groups: (1) carcinoids, ... and (2) endocrine pancreatic tumors (EPTs)" (Rufini, Calcagni, and Baum 2006). (Note that the definition of well-differentiated may be counterintuitive: a tumor is well-differentiated if its cells are similar to normal cells, which have a well-differentiated structure of nucleus, cytoplasm, membrane, etc.)
Ramage et alia provide a summary that differs somewhat from that of Rufini et alia: "NETs ... originate from pancreatic islet cells, gastroenteric tissue (from diffuse neuroendocrine cells distributed throughout the gut), neuroendocrine cells within the respiratory epithelium, and parafollicullar cells distributed within the thyroid (the tumours being referred to as medullary carcinomas of the thyroid). Pituitary, parathyroid, and adrenomedullary neoplasms have certain common characteristics with these tumours but are considered separately" (Ramage et al. 2005, [14]).
Metastases and malignancy
In the context of GEP-NETs, the terms metastatic and malignant are often used interchangeably.
GEP-NETs are often malignant, since the primary site often eludes detection for years, sometimes decades – during which time the tumor has the opportunity to metastasize. Researchers differ widely in their estimates of malignancy rates, especially at the level of the secretory subtypes (the various "-omas").
The most common metastatic sites are the liver, the lymph nodes, and the bones. Liver metastases are so frequent and so well-fed that for many patients, they dominate the course of the cancer. For a patient with a nonsecretory PET, for example, the primary threat to life may be the sheer bulk of the tumor load in the liver.
Causes
In the normal pancreas, cells called islet cells produce hormones that regulate a variety of bodily functions, such as blood sugar level and the production of stomach acid.
Islet cell tumors include:
- Gastrinomas (Zollinger-Ellison syndrome)
- Glucagonomas
- Insulinomas
Natural History, Complications and Prognosis
- Diabetes
- Hormone crises (if the tumor releases certain types of hormones)
- Severe low blood sugar (from insulinomas)
- Severe ulcers in the stomach and small intestine (from gastrinomas)
- Spread of the tumor to the liver
Prognosis
You may be cured if the tumors are surgically removed before they have spread to other organs. If tumors are cancerous, chemotherapy may be used, but it usually cannot cure patients.
Life-threatening problems (such as very low blood sugar) can occur due to excess hormone production, or if the cancer spreads throughout the body.
History and Symptoms
According to Arnold et alia, "many tumors are asymptomatic even in the presence of metastases" (Arnold et al. 2004, 197).
A carcinoid tumor may produce serotonin (5-HT), a biogenic amine that causes a specific set of symptoms including
- Flushing
- Diarrhea or increase in number of bowel movements
- Weight loss
- Weight gain
- Palpitations
- Congestive heart failure (CHF)
- Asthma
- Acromegaly
- Cushing's syndrome
This set of symptoms is called Carcinoid syndrome.
Laboratory Findings
- Cells that receive hormonal messages do so through receptors on the surface of the cells. For reasons that are not understood, many neuroendocrine tumor cells possess especially strong receptors; for example, PETs often have strong receptors for somatostatin, a very common hormone in the body. We say that such tumor cells overexpress the somatostatin receptors (SSTRs) and are thus avid for the hormone; their uptake of the hormone is strong. This avidity for somatostatin is a key for diagnosis – and it makes the tumors vulnerable to certain targeted therapies.
- Aside from their use in diagnosis, some markers can track the progress of therapy while the patient avoids the detrimental side-effects of CT-scan contrast.
| List of potential markers for GEP-NETs apart from hormones of secretory tumors | |||
|---|---|---|---|
| Most important markers | Other markers | Newer (as of 2005) markers | |
|
|
|
|
CT Scan
- CT-scan is a common diagnostic tool in the diagnosis of Neuroendocrine tumors.
- CT-scans using contrast medium can detect 95 percent of tumors over 3 cm in size, and no tumors under 1 cm (University of Michigan Medical School n. d., [15]).
PET Scan
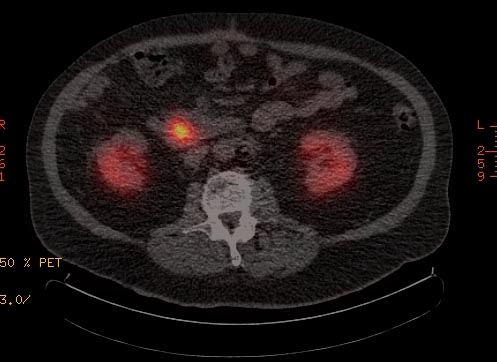
A gallium-68 receptor PET-CT, integrating a PET image with a CT image, is much more senstitive than an OctreoScan, and it generates objective (quantified) results in the form of a standardized uptake value (SUV).
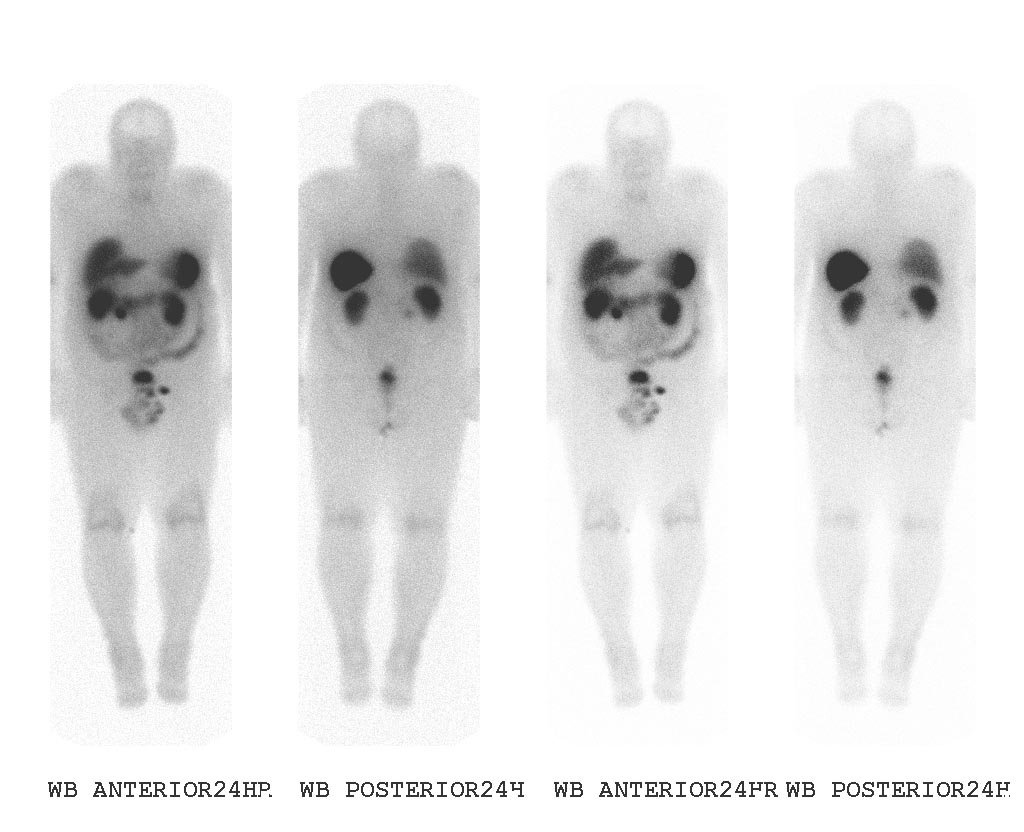
Octreoscan
The diagnostic procedure that utilizes a somatostatin analog is the OctreoScan, also called somatostatin receptor scintigraphy (SRS or SSRS): a patient is injected with octreotide chemically bound to a radioactive substance, often indium-111; for those patients whose tumor cells are avid for octreotide, a radiation-sensitive scan can then indicate the locations of the larger lesions.
An OctreoScan is a relatively crude test that generates subjective results.



Medical Therapy
Approach
According to Warner, the best care, at least for noncarcinoid GEP-NETs, is provided by "an active [as opposed to wait-and-see] approach using sequential multimodality treatment" delivered by a "multidisciplinary team, which also may include a surgeon, endocrinologist, oncologist, interventional radiologist, and other specialists". This recommendation is based on his view that, except for most insulinomas, "almost all" PETs "have long-term malignant potential" – and in sixty percent of cases, that potential is already manifest. "Indeed, the most common cause of death from PETs is hepatic [that is, liver] failure" (Warner 2005, 4).
Two tricky issues in evaluating therapies are durability (is the therapy long-lasting?) and stasis (are the tumors neither growing nor shrinking?). For example, one therapy might give good initial results – but within months the benefit evaporates. And another therapy might be disparaged by some for causing very little tumor shrinkage, but be championed by others for causing significant tumoristasis.
The half-life of somatostatin in circulation is under three minutes, making it useless for diagnosis and targeted therapies. For this reason, The synthetic forms are typically called somatostatin analogs (somatostatin analogues), but according to the US Food and Drug Administration (FDA), the proper term is somatostatin congeners. (In this article we conform to the old terminology, as the medical community has been slow to adopt the term congener.) The analogs have a much longer half-life than somatostatin, and other properties that make them more suitable for diagnosis and therapy.
Chemotherapy
The most common nonsurgical therapy for all GEP-NETs is chemotherapy, although chemotherapy is reported to be largely ineffective for carcinoids, not particularly durable (long-lasting) for PETs, and inappropriate for PETs of nonpancreatic origin. [6]
When chemotherapy fails, the most common therapy, in the United States, is more chemotherapy, with a different set of agents. Some studies have shown that the benefit from one agent is not highly predictive of the benefit from another agent, except that the long-term benefit of any agent is likely to be low.
Strong uptake of somatostatin analogs is a negative indication for chemo.
Symptomatic relief
There are two major somatostatin-analog-based targeted therapies. The first of the two therapies provides symptomatic relief for patients with secretory tumors. In effect, somatostatin given subcutaneously or intramuscularly "clogs up" the receptors, blocking the secretion of hormones from the tumor cells. Thus a patient who might otherwise die from severe diarrhea caused by a secretory tumor can gain additional years of life.
Specific counter-hormones or other hormone-blocking medications are sometimes also used to provide symptomatic relief.
Hormone-delivered radiotherapy – PRRT
The second of the two major somatostatin-analog-based targeted therapies is called peptide receptor radionuclide therapy (PRRT), though we might simply call it hormone-delivered radiotherapy. In this form of radioisotope therapy (RIT), radioactive substances (called radionuclides or radioligands) are chemically conjugated with hormones (peptides or neuroamines); the combination is given intravenously to a patient who has good uptake of the chosen hormone. The radioactive labelled hormones enter the tumor cells, and the attached radiation damages the tumor- and nearby cells. Not all cells are immediately killed this way. The process of tumor cells dying as result of this therapy can go on for several months, even up to two years. In patients with strongly overexpressing tumor cells, nearly all the radiation either gets into the tumors or is excreted in urine. As Rufini et alia say, GEP-NETs "are characterized by the presence of neuroamine uptake mechanisms and/or peptide receptors at the cell membrane, and these features constitute the basis of the clinical use of specific radiolabeled ligands, both for imaging and therapy" (Rufini, Calcagni, and Baum 2006, [16]).
The use of PRRT for GEP-NETs is similar to the use of iodine-131 as a standard therapy (in use since 1943) for nonmedullary thyroid tumors (which are not GEP-NETs). Thyroid cells (whether normal or neoplastic) tend to be avid for iodine, and nearby cells are killed when iodine-131 is infused into the bloodstream and is soon attracted to thyroid cells. Similarly, overexpressing GEP-NET cells (neoplastic cells only) are avid for somatostatin analogs, and nearby cells are killed when radionuclides attached to somatostatin analogs are infused into the bloodstream and are soon attracted to the tumor cells. In both therapies, hormonal targeting delivers a much higher dose of radiation than external beam radiation could safely deliver.
As of 2006, PRRT is available in at least dozen medical centers in Europe. In the USA it is FDA-approved, and available at the MD Anderson Cancer Center, but using a radionuclide, indium-111, that is much weaker than the lutetium-177 and the even stronger yttrium-90 used on the European continent. In the UK, only the radionuclide metaiodobenzylguanidine (I-MIBG) is licensed (but GEP-NETs are rarely avid for MIBG). Most patients (from all over the world) are treated (with lutetium-177) in The Netherlands, at the Erasmus Medical Center. PRRT with lutetium or yttrium is nowhere an "approved" therapy, but the German health insurance system, for example, covers the cost for German citizens.
PRRT using yttrium or lutetium was first applied to humans about 1999. Practitioners continue to refine their choices of radionuclides to maximize damage to tumors, of somatostatin analogs to maximize delivery, of chelators to bind the radionuclides with the hormones (and chelators can also increase uptake), and of protective mechanisms to minimize damage to healthy tissues (especially the kidneys).
Hepatic artery-delivered therapies
- One therapy for liver metastases of GEP-NETs is hepatic artery embolization (HAE). Larry Kvols, of the Moffitt Cancer Center and Research Institute in Tampa, Florida, says that "hepatic artery embolization has been quite successful. During that procedure a catheter is placed in the groin and then threaded up to the hepatic artery that supplies the tumors in the liver. We inject a material called embospheres [tiny spheres of glass or resin, also called microspheres] into the artery and it occludes the blood flow to the tumors, and in more than 80% of patients the tumors will show significant tumor shrinkage" (Kvols 2002, [17]). HAE is based on the observation that tumor cells get nearly all their nutrients from the hepatic artery, while the normal cells of the liver get about 75 percent of their nutrients (and about half of their oxygen) from the portal vein, and thus can survive with the hepatic artery effectively blocked.
- Another therapy is hepatic artery chemoinfusion, the injection of chemotherapy agents into the hepatic artery. Compared with systemic chemotherapy, a higher proportion of the chemotherapy agents are (in theory) delivered to the lesions in the liver.
- Hepatic artery chemoembolization (HACE), sometimes called transarterial chemoembolization (TACE), combines hepatic artery embolization with hepatic artery chemoinfusion: embospheres bound with chemotherapy agents, injected into the hepatic artery, lodge in downstream capillaries. The spheres not only block blood flow to the lesions, but by halting the chemotherapy agents in the neighborhood of the lesions, they provide a much better targeting leverage than chemoinfusion provides.
- Radioactive microsphere therapy (RMT) combines hepatic artery embolization with radiation therapy – microspheres bound with radionuclides, injected into the hepatic artery, lodge (as with HAE and HACE) in downstream capillaries. This therapy is also called selective internal radiation therapy, or SIRT. In contrast with PRRT, the lesions need not overexpress peptide receptors. (But PRRT can attack all lesions in the body, not just liver metastases.) Due to the mechanical targeting, the yttrium-labeled microspheres "are selectively taken up by the tumors, thus preserving normal liver" (Salem et al. 2002, [18]).
Other therapies
- Radiofrequency ablation (RFA) is used when a patient has relatively few metastases. In RFA, a needle is inserted into the center of the lesion and is vibrated at high frequency to generate heat; the tumor cells are killed by cooking.
- Cryoablation is similar to RFA; an endothermic substance is injected into the tumors to kill by freezing. Cryoablation has been considerably less successful for GEP-NETs than RFA.
- Interferon is sometimes used to treat GEP-NETs; its use was pioneered by Dr. Kjell Öberg at Uppsala. For GEP-NETs, Interferon is often used at low doses and in combination with other agents (especially somatostatin analogs such as octreotide). But some researchers claim that Interferon provides little value aside from symptom control.
- As described above, somatostatin analogs have been used for about two decades to alleviate symptoms by blocking the production of hormones from secretory tumors. They are also integral to PRRT. In addition, some doctors claim that, even without radiolabeling, even patients with nonsecretory tumors can benefit from somatostatin analogs, which purportedly can shrink or stabilize GEP-NETs. But some researchers claim that this "cold" octreotide provides little value aside from symptom control.
Surgery
Surgery is the only therapy that can cure GEP-NETs. However, the typical delay in diagnosis, giving the tumor the opportunity to metastasize, makes most GEP-NETs ineligible for surgery (non-resectable).
Case Studies
External links
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
- ↑ Oberg K, Jelic S, ESMO Guidelines Working Group (2008). "Neuroendocrine bronchial and thymic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up". Ann Oncol. 19 Suppl 2: ii102–3. doi:10.1093/annonc/mdn116. PMID 18456740.
- ↑
"The APUD concept led to the belief that these cells arise from the embryologic neural crest. This hypothesis eventually was found to be incorrect" (Warner 2005, 2).
"The APUD-concept is currently abandoned" (Öberg 1998, 2, [1]).
- ↑
"The main two groups of neuroendocrine GEP tumours are so-called carcinoid tumours and endocrine pancreatic tumours" (Öberg 2005a, 90, ).
"Less than 1% of carcinoids arise in the pancreas" (Warner 2005, 9).
Arnold et alia in effect define carcinoids as "extra-pancreatic endocrine gastronintestinal tumors" (Arnold et al. 2004, 196).
Some doctors believe that there is significant overlap between PETs and carcinoids. For example, endocrine surgeon Rodney Pommier says that "there are pancreatic carcinoids" (Pommier 2003, [2]). However, Pommier made his statement in a talk at a conference on carcinoids, not in a peer-reviewed journal; and in his talk he did not define the word carcinoid.
Another way to classify GEP-NETs is to separate those that begin in the glandular neuroendocrine system from those that begin in the diffuse neuroendocrine system. "Neuroendocrine tumors generally may be classified into two categories. The first category is an organ-specific group arising from neuroendocrine organs such as pituitary gland, thyroid, pancreas, and adrenal gland. The second group arises from the diffuse neuroendocrine cells/Kulchitsky cells that are widely distributed throughout the body and are highly concentrated in the pulmonary and gastrointestinal systems" (Liu et al. 2001, [3]).
- ↑ Larry Kvols, of the Moffitt Cancer Center and Research Institute in Tampa, Florida, lists flushing, diarrhea, CHF, and asthma as the four critical characteristics of carcinoid syndrome (Kvols 2002, [4]).
- ↑
"[In] 800 autopsy cases, ... incidence of tumor was 10% (6/60) in individuals having histological studies of all sections of the pancreas" (Kimura, Kuroda, and Morioka 1991, [5]).
Small tumors are not necessarily harmless: Rodney Pommier tells of a "chick pea-sized tumor causing [so much] hormonal effect" that the patient was wheelchair-bound, unable to walk (Pommier 2003, [6]).
- ↑
Ramage et alia say that "response to chemotherapy in patients with strongly positive carcinoid tumours was of the order of only 10% whereas patients with SSRS negative tumours had a response rate in excess of 70%. The highest response rates with chemotherapy are seen in the poorly differentiated and anaplastic NETs: response rates of 70% or more have been seen with cisplatin and etoposide based combinations. These responses may be relatively short lasting in the order of only 8–10 months. Response rates for pancreatic islet cell tumours vary between 40% and 70% and usually involve combinations of streptozotocin (or lomustine), dacarbazine, 5-fluorouracil, and adriamycin. However, the best results have been seen from the Mayo clinic where up to 70% response rates with remissions lasting several years have been seen by combining chemoembolisation of the hepatic artery with chemotherapy. The use of chemotherapy for midgut carcinoids has a much lower response rate, with 15–30% of patients deriving benefit, which may only last 6–8 months (Ramage et al. 2005, [7]).
For 125 patients with histologically proven unresectable islet-cell carcinomas, "median duration of regression was 18 months for the doxorubicin combination and 14 months for the 5-FU combination" (Arnold et al. 2004, 230).
- ↑
"The liver gets about 80% of its blood and half the oxygen from the portal vein, and only 20% of the blood and the other 50% of the oxygen from the artery.... The liver gets 80% of its blood from the portal vein and 20% from that little hepatic artery. But tumors get 100% of their blood off the hepatic artery, and this has been shown by multiple lines of evidence (Pommier 2003, [8]).
"The normal liver gets its blood supply from two sources; the portal vein (about 70%) and the hepatic artery (30%)" (Fong and Schoenfield n. d., [9]).
- ↑ "The theoretical advantage is that higher concentrations of the agents can be delivered to the tumors without subjecting the patients to the systemic toxicity of the agents.... In reality, however, much of the chemotherapeutic agents does end up in the rest of the body" (Fong and Schoenfield, [10]).
- ↑ The "microspheres preferentially cluster around the periphery of tumor nodules with a high tumor:normal tissue ratio of up to 200:1". The SIRT-spheres therapy is not FDA-approved for GEP-NETs; "it is FDA approved for liver metastases secondary to colorectal carcinoma and is under investigation for treatment of other liver malignancies, such as hepatocellular carcinoma and neuroendocrine malignancies" (Welsh, Kennedy, and Thomadsen 2006, [11]).