Lyme disease pathophysiology: Difference between revisions
| Line 29: | Line 29: | ||
*Only 20% of individuals [[Infection|infected]] with [[Lyme disease]] by the [[Ixodes scapularis|deer tick]] are aware of having had any [[tick]] bite, making early detection difficult in the absence of a [[rash]].<ref name="Wormser">{{cite journal | author=Wormser G, Masters E, Nowakowski J, ''et al'' | title=Prospective clinical evaluation of patients from missouri and New York with erythema migrans-like skin lesions. | journal=Clin Infect Dis | volume=41 | issue=7 | pages=958-65 | year=2005 | pmid= 16142659}}</ref> | *Only 20% of individuals [[Infection|infected]] with [[Lyme disease]] by the [[Ixodes scapularis|deer tick]] are aware of having had any [[tick]] bite, making early detection difficult in the absence of a [[rash]].<ref name="Wormser">{{cite journal | author=Wormser G, Masters E, Nowakowski J, ''et al'' | title=Prospective clinical evaluation of patients from missouri and New York with erythema migrans-like skin lesions. | journal=Clin Infect Dis | volume=41 | issue=7 | pages=958-65 | year=2005 | pmid= 16142659}}</ref> | ||
| style="width: 80px; background: #ADD8E6; text-align: center;" | | | style="width: 80px; background: #ADD8E6; text-align: center;" | | ||
[[Image:Ixodes scapularis.png|center|300px|thumb|'''''I. scapularis'', the primary vector of Lyme disease in Eastern North America''' Source | [[Image:Ixodes scapularis.png|center|300px|thumb|'''''I. scapularis'', the primary vector of Lyme disease in Eastern North America''' - Source: Gross L (2006) A New View on Lyme Disease: Rodents Hold the Key to Annual Risk. PLoS Biol 4(6): e182. https://doi.org/10.1371/journal.pbio.0040182 | ||
]] | ]] | ||
|- | |- | ||
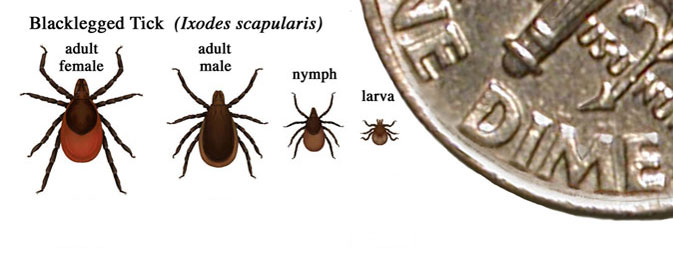
| colspan="2" |[[Image:Relative size ofblacklegged ticks at different life stages.jpg|center|400px|frame|Relative size of I. scapularis at different life stages [https://www.cdc.gov/lyme/transmission/index.html Source | | colspan="2" |[[Image:Relative size ofblacklegged ticks at different life stages.jpg|center|400px|frame|Relative size of I. scapularis at different life stages - [https://www.cdc.gov/lyme/transmission/index.html Source: CDC.gov]]] | ||
|} | |} | ||
Revision as of 14:25, 7 August 2017
| https://https://www.youtube.com/watch?v=rOQvpcpxbCs%7C350}} |
|
Lyme disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lyme disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Lyme disease pathophysiology |
|
Risk calculators and risk factors for Lyme disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Overview
Lyme disease is caused by Borrelia burgdorferi and is transmitted primarily by tick named Ixodes scapularis. Ticks can attach to any part of the human body but are often found in hard-to-see areas such as the groin, armpits, and scalp. In most cases, the tick must be attached for 36 to 48 hours or more before the spirochetes can be transmitted. Very few people affected with Lyme disease recall a tick bite. B. burgdorferi have two morphological forms, i.e. spiral form and spheroplast form. Survival strategies of B. burgdorferi include: antigenic variation, physical sequestration, intracellular invasion, and immune system supression.
Pathophysiology
Transmission
Primary Vector
|
 |
 | |
Other Potential Vectors
- The lone star tick (Amblyomma americanum), which is found throughout the southeastern U.S. as far west as Texas, and increasingly in northeastern states as well.[9]
- These tick bites usually go unnoticed due to the small size of the tick in its nymphal stage, as well as tick secretions that prevent the host from feeling any itch or pain from the bite.
- It was once thought to be a vector, although recent studies demonstrate that this tick species is not a competent vector of Borrelia burgdorferi sensu lato.[10]
- The lone star tick (Amblyomma americanum) is associated with southern tick-associated rash illness [STARI], Tularemia, and Ehrlichiosis. STARI is also known as Masters disease.[11]
Other Modes of Transmission
- While Lyme spirochetes have been found in insects other than ticks, reports of actual infectious transmission appear to be rare.[12][13]
- Sexual transmission have been reported; Lyme spirochetes have been found in semen and breast milk, however transmission of the spirochetes by these routes is not known to occur.[14][15][16]
- Congenital transmission of Lyme disease can occur from an infected mother to fetus through the placenta during pregnancy. However, prompt antibiotic treatment appears to prevent fetal harm.[17]
Reservoir host
- Primary reservoir host of B. burgdorferi are rodents. These rodents are infested by I. scapularis.
- The white-footed mice (Peromyscus leucopus) is the most common rodent infected by B. burgdorferi.[18]
- Other reservoirs may include voles, chipmunks, squirrels, raccoons, skunks, birds, and reptiles such as lizards in some cases.
- It is predicted that density of infected nymphal stage of ticks may be lower in areas where predators of primary reservoir host,particularly red fox(Vulpes vulpes), are more active. The reason may include:[19]
- Direct effect: Predation of reservoir hosts.
- Indirect effect: Decreased movement and increased refuge due to presence of active predator.
Coinfection
- Ixodes scapularis is also a vector for Anaplasma phagocytophilum (previously referred to as Ehrlichia phagocytophila) and Babesia microti.[20]
- Anaplasma phagocytophilum causes human granulocytic anaplasmosis(HGA), previously known as human granulocytic ehrlichiosis.
- Babesia microti causes babesiosis.
- Sometimes patients may be coinfected with ≥2 pathogens.
- Presence of flu-like symptoms(fever, chills ad headache) in patients of Lyme disease without erythema migrans may indicate concurrent infection with human granulocytic anaplasmosis(HGA) and/or babesiosis.[21]
- Coinfection shall be considered in case of prolonged flu-like symptoms that fail to respond to Lyme disease treatment.
- CBC should be considered as initial investigation for patient thought to have coinfection.
Pathogenesis
- B. burgdorferi enters bloodstream through saliva during tick bite.[22]
- After incubation period (around 3 - 30 days), B. burgdorferi migrates outwards in skin manifesting as erythema chronicum migrans. It is then disseminated to other organs including multiple skin sites manifesting as multiple erythema migrans.[23]
- B. burgdorferi is very slow growing organism. Its doubling time is 12-24 hours(in contrast bacterial pathogens such as Streptococcus and Staphylococcus, have a doubling time of 20-30 minutes).
- B. burgdorferi has an axial filament composed of flagella that run lengthwise between its cell wall and outer membrane like other spirochetes.This structure allows B. burgdorferi to move efficiently through viscous media(such as connective tissue) in a corkscrew fashion.
- This helps B. burgdorferi to disseminate throughout the body in days to weeks after infection.
- B. burgdorferi penetrates deep into the tissue where immune system and antibiotics are unable to reach.
- B. burgdorferi have two morphological forms, i.e. spiral form and spheroplast form (cysts, granules). The existence of B. burgdorferi spheroplasts, which lacks a cell wall, has been documented in the following models:
- The spiral form requires energy to convert to the altered form i.e. spheroplast form. This shows that the altered form have a survival function, and it is not merely an end stage degeneration products.[24]
- The spheroplasts are virulent and infectious and survives under adverse environmental conditions. Once the conditions are more favourable, they revert back to the spiral form in vitro.[31][32]
- B. burgdorferi spheroplasts plays a key factor in the relapsing, persistant nature of Lyme disease due to:
- Spheroplasts have dramatically reduced surface area for immune surveillance when compared to spiral form.
- Spheroplasts express different surface proteins; As current detects antibodies to surface proteins of the spiral form may result in seronegative disease (i.e. false-negative antibody tests).
- B. burgdorferi spheroplasts are generally not susceptible to the antibiotics traditionally used for Lyme disease. They have shown sensitivity in vitro to antiparasitic drugs to which the spiral form of B. burgdorferi is not sensitive. These drugs includes:
Mechanisms of persistence
- B. burgdorferi is susceptible to a number of antibiotics in vitro,but the efficacy of antibiotics in vivo have contradictory reports.B. burgdorferi have ability to persist in humans and animals for months to years even after a strong immune response and antibiotic treatment, especially when treatment is delayed and disease is disseminated.Numerous studies have demonstrated persistence of infection despite antibiotic therapy.[36][37][38]
- Survival strategies of B. burgdorferi include: [39]
- Antigenic variation
- B. burgdorferi has the ability to vary its surface proteins in response to attack by immune system.[40]
- This is due to complex genome of B. burgdorferi which helps evading the immune system and establish a presistant infection.[41]
- Physical sequestration
- B. burgdorferi is sequestered in sites such as central nervous system that are inaccessible to the immune system and antibiotics.[42]
- Evidence suggests that B. burgdorferi penetrates the blood-brain barrier by using fibrinolytic system of the host.[43]
- Intracellular invasion
- B. burgdorferi invades a variety of cells, including:
- B. burgdorferi hides inside these cells and evades the immune system .It is also protected against antibiotics to varying degrees, that allows the infection to persist.[50][51]
- Immune system suppression
- Mechanism of immune system suppression observed in B. burgdorferi includes:[39]
- Complement inhibition
- Induction of anti-inflammatory cytokines such as IL-10
- Formation of immune complexes
- Formation of immune complexes in B. burgdorferi might explain the seronegative disease (i.e. false-negative antibody tests of blood and cerebrospinal fluid). Studies have shown that a substantial numbers of seronegative Lyme patients have antibodies bound up in these complexes.[52]
- Mechanism of immune system suppression observed in B. burgdorferi includes:[39]
- Antigenic variation
Microscopic pathology
- Biopsy of erythema migrans shows:
- Dermal and epidermal involvement in center of lesion
- Dermal involvement at the periphery
References
- ↑ 1.0 1.1 1.2 1.3 Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH (2011). "Updates on Borrelia burgdorferi sensu lato complex with respect to public health". Ticks Tick Borne Dis. 2 (3): 123–8. doi:10.1016/j.ttbdis.2011.04.002. PMC 3167092. PMID 21890064.
- ↑ Schwartz, Ira; Fish, Durland; Daniels, Thomas J. (1997). "Prevalence of the Rickettsial Agent of Human Granulocytic Ehrlichiosis in Ticks from a Hyperendemic Focus of Lyme Disease". New England Journal of Medicine. 337 (1): 49–50. doi:10.1056/NEJM199707033370111. ISSN 0028-4793.
- ↑ Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D; et al. (1999). "Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans". Am J Epidemiol. 149 (8): 771–6. PMID 10206627.
- ↑ Piesman J, Maupin GO, Campos EG, Happ CM (1991). "Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method". J Infect Dis. 163 (4): 895–7. PMID 2010643.
- ↑ Ohnishi J, Piesman J, de Silva AM (2001). "Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks". Proc Natl Acad Sci U S A. 98 (2): 670–5. doi:10.1073/pnas.98.2.670. PMC 14646. PMID 11209063.
- ↑ 6.0 6.1 Girard YA, Travinsky B, Schotthoefer A, Fedorova N, Eisen RJ, Eisen L, Barbour AG, Lane RS (2009). "Population structure of the lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in Northern California". Appl. Environ. Microbiol. 75 (22): 7243–52. doi:10.1128/AEM.01704-09. PMC 2786521. PMID 19783741.
- ↑ Sui S, Yang Y, Sun Y, Wang X, Wang G, Shan G, Wang J, Yu J (2017). "On the core bacterial flora of Ixodes persulcatus (Taiga tick)". PLoS ONE. 12 (7): e0180150. doi:10.1371/journal.pone.0180150. PMC 5503197. PMID 28692666.
- ↑ Wormser G, Masters E, Nowakowski J; et al. (2005). "Prospective clinical evaluation of patients from missouri and New York with erythema migrans-like skin lesions". Clin Infect Dis. 41 (7): 958–65. PMID 16142659.
- ↑ Clark K (2004). "Borrelia species in host-seeking ticks and small mammals in northern Florida" (PDF). J Clin Microbiol. 42 (11): 5076–86. PMID 15528699.
- ↑ Ledin K, Zeidner N, Ribeiro J; et al. (2005). "Borreliacidal activity of saliva of the tick Amblyomma americanum". Med Vet Entomol. 19 (1): 90–95. PMID 15752182.
- ↑ Wormser GP, Masters E, Nowakowski J, McKenna D, Holmgren D, Ma K; et al. (2005). "Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions". Clin Infect Dis. 41 (7): 958–65. doi:10.1086/432935. PMID 16142659.
- ↑ Magnarelli L, Anderson J (1988). "Ticks and biting insects infected with the etiologic agent of Lyme disease, Borrelia burgdorferi" (PDF). J Clin Microbiol. 26 (8): 1482–6. PMID 3170711.
- ↑ Luger S (1990). "Lyme disease transmitted by a biting fly". N Engl J Med. 322 (24): 1752. PMID 2342543.
- ↑ Bach G (2001). "Recovery of Lyme spirochetes by PCR in semen samples of previously diagnosed Lyme disease patients.". 14th International Scientific Conference on Lyme Disease.
- ↑ Schmidt B, Aberer E, Stockenhuber C; et al. (1995). "Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis". Diagn Microbiol Infect Dis. 21 (3): 121–8. PMID 7648832.
- ↑ Steere AC (2003-02-01). "Lyme Disease: Questions and Answers" (PDF). Massachusetts General Hospital / Harvard Medical School. Retrieved 2007-03-22.
- ↑ Walsh CA, Mayer EW, Baxi LV (2007). "Lyme disease in pregnancy: case report and review of the literature". Obstetrical & gynecological survey. 62 (1): 41–50. doi:10.1097/01.ogx.0000251024.43400.9a. PMID 17176487.
- ↑ Anderson JF, Johnson RC, Magnarelli LA, Hyde FW (1986). "Culturing Borrelia burgdorferi from spleen and kidney tissues of wild-caught white-footed mice, Peromyscus leucopus". Zentralbl Bakteriol Mikrobiol Hyg A. 263 (1–2): 34–9. PMID 3577490.
- ↑ Hofmeester, Tim R.; Jansen, Patrick A.; Wijnen, Hendrikus J.; Coipan, Elena C.; Fonville, Manoj; Prins, Herbert H. T.; Sprong, Hein; van Wieren, Sipke E. (2017). "Cascading effects of predator activity on tick-borne disease risk". Proceedings of the Royal Society B: Biological Sciences. 284 (1859): 20170453. doi:10.1098/rspb.2017.0453. ISSN 0962-8452.
- ↑ Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS; et al. (2006). "The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America". Clin Infect Dis. 43 (9): 1089–134. doi:10.1086/508667. PMID 17029130.
- ↑ Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T; et al. (2002). "Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease". Clin Infect Dis. 34 (9): 1184–91. doi:10.1086/339813. PMID 11941544.
- ↑ Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW; et al. (1983). "The early clinical manifestations of Lyme disease". Ann Intern Med. 99 (1): 76–82. PMID 6859726.
- ↑ Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D; et al. (2005). "Brief communication: hematogenous dissemination in early Lyme disease". Ann Intern Med. 142 (9): 751–5. PMID 15867407.
- ↑ 24.0 24.1 Alban PS, Johnson PW, Nelson DR (2000). "Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi". Microbiology. 146 ( Pt 1): 119–27. PMID 10658658.
- ↑ 25.0 25.1 Mursic VP, Wanner G, Reinhardt S; et al. (1996). "Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants". Infection. 24 (3): 218–26. PMID 8811359.
- ↑ Kersten A, Poitschek C, Rauch S, Aberer E (1995). "Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi" (PDF). Antimicrob Agents Chemother. 39 (5): 1127–33. PMID 7625800.
- ↑ Schaller M, Neubert U (1994). "Ultrastructure of Borrelia burgdorferi after exposure to benzylpenicillin". Infection. 22 (6): 401–6. PMID 7698837.
- ↑ 28.0 28.1 Nanagara R, Duray PH, Schumacher HR Jr (1996). "Ultrastructural demonstration of spirochetal antigens in synovial fluid and synovial membrane in chronic Lyme disease: possible factors contributing to persistence of organisms". Hum Pathol. 27 (10): 1025–34. PMID 8892586.
- ↑ Phillips SE, Mattman LH, Hulinska D, Moayad H (1998). "A proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated". Infection. 26 (6): 364–7. PMID 9861561.
- ↑ Duray PH, Yin SR, Ito Y; et al. (2005). "Invasion of human tissue ex vivo by Borrelia burgdorferi". J Infect Dis. 191 (10): 1747–54. PMID 15838803.
- ↑ Gruntar I, Malovrh T, Murgia R, Cinco M (2001). "Conversion of Borrelia garinii cystic forms to motile spirochetes in vivo". APMIS. 109 (5): 383–8. PMID 11478686.
- ↑ Murgia R, Cinco M (2004). "Induction of cystic forms by different stress conditions in Borrelia burgdorferi". APMIS. 112 (1): 57–62. PMID 14961976.
- ↑ Brorson O, Brorson SH (1999). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole". APMIS. 107 (6): 566–76. PMID 10379684.
- ↑ Brorson O, Brorson SH (2004). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole" (PDF). Int Microbiol. 7 (2): 139–42. PMID 15248163.
- ↑ Brorson O, Brorson SH (2002). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to hydroxychloroquine". Int Microbiol. 5 (1): 25–31. PMID 12102233.
- ↑ Bayer ME, Zhang L, Bayer MH (1996). "Borrelia burgdorferi DNA in the urine of treated patients with chronic Lyme disease symptoms. A PCR study of 97 cases". Infection. 24 (5): 347–53. PMID 8923044.
- ↑ Preac-Mursic V, Weber K, Pfister HW; et al. (1989). "Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis". Infection. 17 (6): 355–9. PMID 2613324.
- ↑ Oksi J, Marjamaki M, Nikoskelainen J, Viljanen MK (1999). "Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis". Ann Med. 31 (3): 225–32. PMID 10442678.
- ↑ 39.0 39.1 Embers ME, Ramamoorthy R, Philipp MT (2004). "Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease". Microbes Infect. 6 (3): 312–8. PMID 15065567.
- ↑ Liang FT, Yan J, Mbow ML; et al. (2004). "Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses". Infect Immun. 72 (10): 5759–67. PMID 15385475.
- ↑ Gilmore RD, Howison RR, Schmit VL; et al. (2007). "Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice". Infect. Immun. 75 (6): 2753–64. doi:10.1128/IAI.00037-07. PMID 17371862.
- ↑ Miklossy J, Khalili K, Gern L; et al. (2004). "Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease". J Alzheimers Dis. 6 (6): 639–49, discussion 673-81. PMID 15665404.
- ↑ Grab DJ, Perides G, Dumler JS, Kim KJ, Park J, Kim YV, Nikolskaia O, Choi KS, Stins MF, Kim KS (2005). "Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier". Infect Immun. 73 (2): 1014–22. PMID 15664945.
- ↑ Ma Y, Sturrock A, Weis JJ (1991). "Intracellular localization of Borrelia burgdorferi within human endothelial cells" (PDF). Infect Immun. 59 (2): 671–8. PMID 1987083.</ref [[Fibroblasts]]<ref name="Klempner-b">Klempner MS, Noring R, Rogers RA (1993). "Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi". J Infect Dis. 167 (5): 1074–81. PMID 8486939.
- ↑ Dorward DW, Fischer ER, Brooks DM (1997). "Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease". Clin Infect Dis. 25 Suppl 1: S2–8. PMID 9233657.
- ↑ Montgomery RR, Nathanson MH, Malawista SE (1993). "The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery". J Immunol. 150 (3): 909–15. PMID 8423346.
- ↑ Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W (1996). "Heterogeneity of Borrelia burgdorferi in the skin". Am J Dermatopathol. 18 (6): 571–9. PMID 8989928.
- ↑ Girschick HJ, Huppertz HI, Russmann H, Krenn V, Karch H (1996). "Intracellular persistence of Borrelia burgdorferi in human synovial cells". Rheumatol Int. 16 (3): 125–32. PMID 8893378.
- ↑ Livengood JA, Gilmore RD (2006). "Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi". Microbes Infect. [Epub ahead of print]. PMID 17045505.
- ↑ Georgilis K, Peacocke M, Klempner MS (1992). "Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro". J Infect Dis. 166 (2): 440–4. PMID 1634816.
- ↑ Brouqui P, Badiaga S, Raoult D (1996). "Eucaryotic cells protect Borrelia burgdorferi from the action of penicillin and ceftriaxone but not from the action of doxycycline and erythromycin" (PDF). Antimicrob Agents Chemother. 40 (6): 1552–4. PMID 8726038.
- ↑ Schutzer SE, Coyle PK, Reid P, Holland B (1999). "Borrelia burgdorferi-specific immune complexes in acute Lyme disease". JAMA. 282 (20): 1942–6. PMID 10580460.