Phenacetin: Difference between revisions
Brian Blank (talk | contribs) No edit summary |
m (Protected "Phenacetin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
(No difference)
| |
Latest revision as of 18:17, 27 September 2011
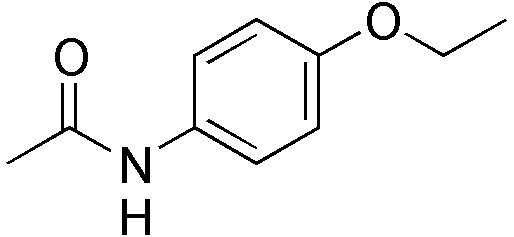 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol |
| Melting point | 134−136 °C (−78.8 °F) (decomposes) |
|
WikiDoc Resources for Phenacetin |
|
Articles |
|---|
|
Most recent articles on Phenacetin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Phenacetin at Clinical Trials.gov Clinical Trials on Phenacetin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phenacetin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phenacetin Discussion groups on Phenacetin Patient Handouts on Phenacetin Directions to Hospitals Treating Phenacetin Risk calculators and risk factors for Phenacetin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phenacetin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Phenacetin, introduced in 1887, was used principally as an analgesic, and was the first NSAID and fever reducer to go on the market. Typical doses of 300mg to 500mg a day result in an analgesic effect. Its analgesic effects are due to its actions on the sensory tracts of the spinal cord. In addition, phenacetin has a depressant action of the heart, where it acts as a negative inotrope. It is an antipyretic, acting on the brain to decrease the temperature set point. It is also used to treat rheumatoid arthritis (subacute type), intercostal neuralgia, and some forms of ataxia.
Phenacetin, and products containing phenacetin have been shown in an animal model to be carcinogenic. In humans, many case reports have implicated products containing phenacetin in urothelial neoplasms, especially transitional cell carcinoma of the renal pelvis. In one prospective series, phenacetin was associated with an increased risk of death due to urologic or renal diseases, death due to cancers, and death due to cardiovascular diseases.[1] In addition, people with glucose-6-phosphate dehydrogenase deficiency may experience acute hemolysis while taking this drug. Acute hemolysis is possible in the case of patients who develop an IgM response to Phenacetin leading to immune complexes that bind to erythrocytes in blood. The erythrocytes are then lysed when the complexes activate the complement cascade.
Synthesis (Northern Kentucky University)
ETHER SYNTHESIS: CONVERSION OF ACETAMINOPHEN INTO PHENACETIN Required Pre-lab readings: Ege, 5th Ed., sect 13.4, pp 498-501; Morhig, Chapter 19. Techniques you must be prepared to use: reflux; extraction; rotary evaporation; recrystallization. In the reaction today you will be converting 4-acetamidophenol (Acetaminophen) into ethyl 4- acetamidophenyl ether (Phenacetin). Both compounds are ingredients in many over-the-counter analgesics. This reaction is an example of the Williamson ether synthesis. For most ether syntheses strong bases such as amide ion are necessary to generate the nucleophile.
In a 50 mL round-bottomed flask place 12 mmol of liquid ethyl iodide, 15 mL of methyl ethyl ketone (2-butanone; MEK) as solvent, Acetaminophen (1.5 g; ?? mmol.), and powered anhydrous K2CO3 (2.5 g; ?? mmol). Mechanically stir this mixture and reflux for 1 hour. After the reflux is complete cool the flask in an ice/water bath and gravity filter the contents into a separatory funnel. Use small amounts of ether to insure that you have quantitatively transferred all the organic material from the flask to the funnel. Wash the organic phase with 5% aq. NaOH (what is the purpose of this step?), then dry it (Na2SO4) and decant into a flask and remove the solvents by rotary evaporation. The product is purified by recrystallization from water. Allow the purified product to air dry.
Phenacetin was widely used until the third quarter of the twentieth century, but was then largely replaced by non-carcinogenic drugs. Some branded phenacetin-based preparations continued to be sold, but with the phenacetin replaced by safer alternatives. A popular brand of phenacetin was Roche's Saridon, which was reformulated in 1983 to contain propyphenazone, paracetamol and caffeine. Paracetamol is a metabolite of phenacetin with similar analgesic and antipyretic effects, but the new formulation has not been found to have phenacetin's carcinogenicity.
Connection between chronic phenacetin use and renal papillary necrosis
Chronic use of phenacetin is known to lead to renal papillary necrosis.[2][3][4] This is a condition which results in destruction of some or all of the renal papillae in the kidneys.
Use as a filler for illegal cocaine
Phenacetin is now being widely used as a cutting agent to adulterate illegally supplied cocaine due to the similar physical features of the two drugs. [5]
Notes and references
- ↑ Dubach U, Rosner B, Stürmer T (1991). "An epidemiologic study of abuse of analgesic drugs. Effects of phenacetin and salicylate on mortality and cardiovascular morbidity (1968 to 1987)". N Engl J Med. 324 (3): 155–60. PMID 1984193.
- ↑ Cochran A, Lawson D, Linton A (1967). "Renal papillary necrosis following phenacetin excess". Scott Med J. 12 (7): 246–50. PMID 6036245.
- ↑ Tan G, Rabbino M, Hopper J (1964). "Is Phenacetin a Nephrotoxin? A Report on Twenty-three Users of the Drug". Calif Med. 101: 73–7. PMID 14180501.
- ↑ Brix A. "Renal papillary necrosis". Toxicol Pathol. 30 (6): 672–4. PMID 12512867.
- ↑ "Cancer chemical in street cocaine". BBC News. 23 November 2006.
- Pages with script errors
- Pages with non-numeric formatnum arguments
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Analgesics
- Antipyretics
- Acetanilides
- Drugs