Aortic aneurysm: Difference between revisions
No edit summary |
No edit summary |
||
| (115 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{| class="infobox" style="float:right;" | |||
|- | |||
| [[File:Siren.gif|30px|link=Aortic aneurysm resident survival guide]]|| <br> || <br> | |||
| [[Aortic aneurysm resident survival guide|'''Resident'''<br>'''Survival'''<br>'''Guide''']] | |||
|} | |||
{{Infobox_Disease | {{Infobox_Disease | ||
| Name = {{PAGENAME}} | | Name = {{PAGENAME}} | ||
| Image = Aortic aneurysm 22.jpg | | Image = Aortic aneurysm 22.jpg | ||
| Caption = Atherosclerotic Aneurysm: Gross, an excellent example, natural color, external view of typical thoracic aortic aneurysms <br> <small> [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] </small> | | Caption = Atherosclerotic Aneurysm: Gross, an excellent example, natural color, external view of typical thoracic aortic aneurysms <br> <small> [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] </small> | ||
}} | }} | ||
{{SI}} | {{SI}} | ||
'''For patient information on Thoracic aortic aneurysm, click [[Thoracic aortic aneurysm (patient information)|here]]''' | |||
'''For patient information on Abdominal aortic aneurysm, click [[Abdominal aortic aneurysm (patient information)|here]]''' | |||
{{CMG}}, {{AE}} [[User:Lina Ya'qoub|Lina Ya'qoub, MD]]; {{CZ}}; [[User:Gerald Chi|Gerald Chi, MD]] | |||
==Overview== | |||
An aortic aneurysm is a dilation of the [[aorta]] in which the aortic diameter is ≥ 3.0 cm if abdominal<ref name=":1">Kuivaniemi, Helena, et al. "Understanding the pathogenesis of abdominal aortic aneurysms." ''Expert review of cardiovascular therapy'' 13.9 (2015): 975-987.</ref> or >4 cm if thoracic<ref name=":6">Radiopaedia - Thoracic Aortic Aneurysms - <nowiki>https://radiopaedia.org/articles/thoracic-aortic-aneurysm?lang=us</nowiki> | |||
accessed at 06/08/2020</ref>, usually representing an underlying weakness in the wall of the aorta at that location. While the stretched vessel may occasionally cause discomfort, a greater concern is the risk of rupture which causes severe pain, massive internal [[hemorrhage]] which are often fatal. Aneurysms often are a source of blood clots ([[embolus|emboli]]) stemming from the most common etiology of [[atherosclerosis]]. | |||
==Classification== | |||
There are 2 types of aortic aneurysms: thoracic and abdominal. These can be further classified according to the respective part of the vessel that's been affected: | |||
*Thoracic | *[[Thoracic aortic aneurysm]], which occur in the thoracic aorta (runs through the chest); | ||
* | *[[Abdominal aortic aneurysm]], which occur in the abdominal aorta, are the most common. | ||
* | **Suprarenal - not as common, often more difficult to repair surgically due to the presence of many aortic branches; | ||
* | **Infrarenal - often more easily surgically repaired and more common; | ||
**Pararenal - aortic aneurysm is infrarenal but affects renal arteries; | |||
**Juxtarenal - infrarenal aortic aneurysm that affects the aorta just below the renal arteries. | |||
Aortic aneurysms may also be classified according to Crawford classification into 5 subtypes/groups: | |||
* | *Type 1: from the origin of left subclavian artery in descending thoracic aorta to the supra-renal abdominal aorta. | ||
** | *Type 2: from the left subclavian to the aorto-iliac bifurcation. | ||
** | *Type 3: from distal thoracic aorta to the aorto-iliac bifurcation | ||
*Type 4: limited to abdominal aorta below the diaphragm | |||
*Type 5: from distal thoracic aorta to celiac and superior mesenteric origins, but not the renal arteries.<ref name=":4">Frederick, John R., and Y. Joseph Woo. "Thoracoabdominal aortic aneurysm." ''Annals of cardiothoracic surgery'' 1.3 (2012): 277.</ref> | |||
==Historical Perspective== | |||
Aortic aneurysm was first recorded by Antyllus, a Greek surgeon, in the second century AD. In the Renaissaince era, in 1555, [[Vesalius]] first diagnosed an [[abdominal aortic aneurysm]]. The first publication on the pathology with case studies was published by Lancisi in 1728. Finally, in 1817, Astley Cooper was the first surgeon to ligate the abdominal aorta to treat a ruptured iliac aneurysm. In 1888, Rudoff Matas came up with the concept of endoaneurysmorrhaphy.<ref>Livesay, James J., Gregory N. Messner, and William K. Vaughn. "Milestones in treatment of aortic aneurysm: Denton A. Cooley, MD, and the Texas Heart Institute." ''Texas Heart Institute Journal'' 32.2 (2005): 130.</ref> | |||
</ | |||
==Pathophysiology== | |||
The aortic aneurysms are a multifactorial disease associated with genetic and environmental risk factors. [[Marfan's syndrome]] and [[Ehlers-Danlos syndrome]] are associated with the disease, but there are also rarer syndromes like the [[Loeys-Dietz syndrome]] that are associated as well. Even in patients that do not have genetic syndromes, it has been observed that genetics can also play a role on aortic aneurysms' development. There has been evidence of genetic heterogeneity as there has already been documented in [[intracranial aneurysms]].<ref name=":0" /> The genetic alterations associated with these genetic syndromes are the following: | |||
{| class="wikitable" | |||
|+Genetic diseases associated with aortic aneurysms <ref>Bhandari, R., Kanthi, Y. - The Genetics of Aortic Aneurysms - The American College of Cardiology - available at:https://www.acc.org/latest-in-cardiology/articles/2018/05/02/12/52/the-genetics-of-aortic-aneurysms accessed at 06/08/2020</ref> | |||
!Disease | |||
!Involved Cellular Pathway | |||
!Mutated Gene(s) | |||
!Affected Protein(s) | |||
|- | |||
|[[Ehlers-Danlos type IV syndrome]] | |||
|[[Extracellular Matrix Proteins]] | |||
|[[COL3A1]] | |||
|[[Collagen type III]] | |||
|- | |||
|[[Marfan's Syndrome]] | |||
|Extracellular Matrix Proteins | |||
|[[FBN1]] | |||
|Fibrillin-1 | |||
|- | |||
|[[Loeys-Dietz syndrome]] | |||
|[[TGF-β]] Pathway | |||
|[[TGFBR1]]/[[TGFBR2]] | |||
| | |||
|- | |||
|Aneurysm-Osteoarthritis Syndrome | |||
| | |||
|[[SMAD3]] | |||
|SMAD3 | |||
|- | |||
|[[Autosomal Dominant Polycystic Kidney Disease]] | |||
|[[Ciliopathy]] | |||
|''PKD1/PKD2'' | |||
|[[Polycystin 1]] | |||
[[Polycystin 2]] | |||
|- | |||
|[[Turner Syndrome]] | |||
|Meiotic Error with Monosomy, Mosaicism, or De Novo Germ Cell Mutation | |||
|45X | |||
45XO | |||
|Partial or Complete Absence of X Chromosome | |||
|- | |||
|[[Bicuspid Aortic Valve]] with TAA | |||
|[[Neural Crest]] Migration | |||
|''[[NOTCH1]]'' | |||
|Notch 1 | |||
|- | |||
|Familial TAA | |||
|Smooth Muscle Contraction Proteins | |||
|''[[ACTA2]]'' | |||
|α-Smooth Muscle Actin | |||
|- | |||
|Familial TAA with Patent Ductus Arteriosus | |||
|Smooth Muscle Contraction Proteins | |||
|''[[MYH11]]'' | |||
|Smooth Muscle Myosin | |||
|- | |||
|Familial TAA | |||
|Smooth Muscle Contraction Proteins | |||
|''[[MYLK]]'' | |||
|[[Myosin Light Chain Kinase]] | |||
|- | |||
|Familial TAA | |||
|Smooth Muscle Contraction Proteins | |||
|''[[PRKG1]]'' | |||
|Protein Kinase c-GMP Dependent, type I | |||
|- | |||
|Loeys-Dietz Syndrome variants | |||
|[[TGF-β]] Pathway | |||
|''[[TGF-βR1]]'' | |||
''TGF-βR2'' | |||
''[[SMAD3]]'' | |||
''TGF-β2'' | |||
''TGF-β3'' | |||
| | |||
|} | |||
These genetic diseases mostly affect either the synthesis of [[extracellular matrix protein]] or damage the smooth muscle cells both important component's of the aortic wall. Injury to any of these components lead to weakening of the aortic wall and dilation - resulting in aneurysm formation. | |||
< | The [[aorta]] is the largest vessel of the body, but it is not homogenous. Its upper segment is composed by a larger proportion of [[elastin]] in comparison to [[collagen]], therefore being more distensible. The lower segment has a larger proportion of [[collagen]], therefore it is less distensible. It is also where most of the atherosclerotic plaques of the [[aorta]] are located.<ref name=":1" /> Historically it was thought that abdominal and thoracic aortic aneurysms were caused by the same etiology: [[Atherosclerosis|atherosclerotic]] degeneration of the aortic wall, but recently it has been theorized that they are indeed different diseases.<ref name=":1" /> | ||
< | |||
The [[aortic arch]] mostly derives from the [[neural crest cell]] which differentiate into [[Smooth muscle cell|smooth muscle cells]]. These [[Smooth muscle cell|smooth muscle cells]] are probably more adapted to remodel the thoracic [[aorta]] and manage the higher [[pulse pressure]] and ejection volume due to increased production of elastic lamellae during development and growth.<ref name=":1" /> The [[abdominal aorta]] remains with cells of [[Mesoderm|mesodermal]] origin, which are more similar to that of the original primitive arterial. That difference results in the [[neural crest cell]] precursors of the thoracic aorta being able to respond differently to various [[cytokines]] and growth factors than the [[Mesoderm|mesodermal]] precursors of the abdominal aorta,<ref>Ruddy JM, Jones JA, Ikonomidis JS. Pathophysiology of thoracic aortic aneurysm (TAA): is it not one uniform aorta? Role of embryologic origin. Progress in cardiovascular diseases. 2013;56(1):68–73.</ref> such as [[homocysteine]]<ref>Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxidants & redox signaling. 2011;15(7):1927–1943. </ref> and [[Angiotensin|angiotensin II]].<ref>Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10.</ref> | |||
When [[neural crest]] vascular smooth muscle cells are treated with [[TGF-β]] they demonstrate increased [[collagen]] production, while mesodermal vascular [[smooth muscle cell]] did not.<ref>Gadson PF, Jr, Dalton ML, Patterson E, et al. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Experimental cell research. 1997;230(2):169–180.</ref> Not coincidently, mutations of the [[TGF-β]] receptor can cause thoracic aortic aneurysm but do not cause abdominal aortic ones. | |||
< | The thoracic and abdominal aorta are very structurally different. While they both have three layers: [[Tunica intima|intimal]], [[Tunica media|medial]] and [[Tunica externa (vessels)|adventitia]], the media of the thoracic aorta is comprised of approximately 60 units divided into vascular and [[avascular]] regions. The [[abdominal aorta]] consists of about 30 units and is entirely avascular, being dependent on trans-intimal diffusion of nutrients for its smooth muscle cells to survive.<ref>Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circulation research. 1969;25(6):677–686. </ref> It is believed that both differences explain why the [[abdominal aorta]] is more likely to form aneurysms. | ||
</ | |||
The development of aortic aneurysms is defined by: [[inflammation]]: infiltration of the vessel wall by [[lymphocytes]] and [[macrophage]]; extracellular matrix damage: destruction of [[elastin]] and [[collagen]] by [[proteases]] (also [[metalloproteinases]]) in the media and adventitia; cellular damage: loss of smooth muscle cells with thinning of the media; and insufficient repair: [[neovascularization]].<ref>Ailawadi G, Eliason JL, Upchurch GR Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg 2003;38:584-8.</ref> | |||
==Clinical Features== | |||
'''[[Thoracic aortic aneurysms]]:''' The aneurysms tend to grow slowly and most of them will never rupture. As they grow, however, their symptoms become more evident and present with mass effects over surrounding structures and pain. They may present with thoracic symptoms: interscapular or central pain, ripping chest pain and dyspnea. Atypical presentations include hoarseness, dizziness and dysphagia, due to esophageal compression.<ref>Hiller, H. G., and N. R. F. Lagattolla. "Thoracic aortic aneurysm presenting with dysphagia: a fatal delay in diagnosis." ''Thoracic surgical science'' 4 (2007).</ref> Aneurysm rupture lead to massive internal bleeding, hypovolemic shock and it is usually fatal. | |||
< | '''[[Abdominal aortic aneurysm|Abdominal aortic aneurysms]]:''' as the thoracic aneurysms, they begin [[asymptomatic]] but may cause symptoms as they grow and compress surrounding structures.<ref name=":2">Abdominal Aortic Aneurysm (AAA) Symptoms - Stanford Healthcare | ||
https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/abdominal-aortic-aneurysm/symptoms.html - accessed at 06/08/2020</ref>Even though they usually remain asymptomatic, when they rupture they present with an ensuing mortality of 85 to 90%., and symptomatic patients require urgent surgical repair.<ref>Kent, K. Craig. "Abdominal aortic aneurysms." ''New England journal of medicine'' 371.22 (2014): 2101-2108.</ref> | |||
< | |||
</ | |||
When symptomatic, abdominal aortic aneurysms present with: | |||
The | * Pain: in the chest, abdomen, lower back, or [[flanks]]. It may radiate to the [[groin]], [[buttocks]], or [[legs]]. The pain characteristics vary and may be deep, aching, gnawing, or throbbing It may also last for hours or days, not affected by movement. Occasionally, certain positions can be more comfortable and alleviate the symptoms; | ||
* Pulsating abdominal mass; | |||
*[[Ischemia]]: "cold foot" or a black or blue painful toe. This is usually the presentation when an aneurysm forms a blood cloth and it releases emboli to the lower extremities; | |||
* Fever or weight loss if caused by [[inflammatory]] states such as [[vasculitis]].<ref name=":2" /> | |||
If ruptured, the abdominal aortic aneurysm can present with sharp abdominal pain, often radiating to the back, discoloration of the skin and mucosa, [[tachycardia]] and low blood pressure due to [[hypovolemic shock]]. | |||
==Differentiating Aortic Aneurysm from other Diseases== | |||
'''Thoracic aortic aneurysms:''' differential diagnosis include other causes of chest pain: acute [[aortic dissection]], acute [[pericarditis]], [[aortic regurgitation]], [[heart failure]], [[Hypertensive Emergencies|hypertensive emergencies]], [[infective endocarditis]], [[myocardial Infarction]], [[pulmonary embolism]], [[superior vena cava syndrome]]. <ref>Thoracic Aneurysm Differential Diagnoses - Medscape available at: https://emedicine.medscape.com/article/761627-differential - accessed at 06/08/2020</ref> | |||
'''Abdominal aortic aneurysms:''' differential diagnosis include causes of pulsatile abdominal mass and/or abdominal pain such as [[ruptured viscus]], [[strangulated hernia]], ruptured visceral artery aneurysms, [[mesenteric ischemia]], acute [[cholecystitis]], ruptured hepatobiliary cancer, [[acute pancreatitis]], [[lymphomas]], and [[diverticular abscess]].<ref name=":3">Abdominal Aortic Aneurysm - Mayo Clinic<nowiki/>https://www.mayoclinic.org/diseases-conditions/abdominal-aortic-aneurysm/symptoms-causes/syc-20350688 - accessed at 06/08/2020</ref> | |||
These conditions can be easily differentiated using abdominal or thoracic imaging. | |||
==Epidemiology and Demographics== | |||
In the United States alone 15,000 people die yearly due to aortic aneurysms and it is the 13th leading cause of death. 1-2% of the population may have aortic aneurysms and [[prevalence]] rises up to 10% in older age groups. The disease varies according to where it takes place. In the thorax, the [[aortic arch]] is the less affected segment (10%) and the most common is the [[ascending aorta]] (50%). Regarding abdominal aneurysms, the infrarenal segment aortic aneurysms are three times more prevalent than the aortic aneurysms and [[Aortic dissection|dissections]].<ref name=":0">Kuivaniemi, Helena, Chris D. Platsoucas, and M. David Tilson III. "Aortic aneurysms: an immune disease with a strong genetic component." ''Circulation'' 117.2 (2008): 242-252.</ref> | |||
= | Regarding other factors as age, [[Abdominal aortic aneurysm|abdominal aortic aneurysms]] usually present 10 years later than [[thoracic aortic aneurysms]]. Both lesions are more present in men, but the proportion is much higher regarding abdominal aortic aneurysms (6:1 male:female ratio) in comparison to thoracic ones.<ref name=":0" /> | ||
[[Abdominal aortic aneurysm|Abdominal aortic aneurysms]] also affect patients differently regarding race, as they are more prevalent among whites than blacks, asians and hispanics. It also seems to be declining in prevalence as evidenced by a Swedish study that found out a 2% prevalence of abdominal aortic aneurysms in comparison to earlier studies which reported 4-8%, probably due to risk-factor modification. <ref name=":5">Ernst, Calvin B. "Abdominal aortic aneurysm." ''New England Journal of Medicine'' 328.16 (1993): 1167-1172.</ref> | |||
==Risk Factors== | |||
Many risk factors are common between both forms of aortic aneurysms, but some are specific for each presentation: | |||
</ | |||
*'''[[Abdominal aortic aneurysm]]:''' [[smoking]], male gender, age (>65 years), race (white), family history, other aneurysms.<ref name=":3" /> | |||
*'''[[Thoracic aortic aneurysm]]:''' [[smoking]], age (>65 years), [[hypertension]], [[atherosclerosis]], family history, [[Marfan's syndrome]], [[Bicuspid Aortic Valve|bicuspid aortic valve]]. <ref>Thoracic Aortic Aneurysm - Mayo Clinic available at: https://www.mayoclinic.org/diseases-conditions/thoracic-aortic-aneurysm/symptoms-causes/syc-20350188 - accessed at 06/08/2020</ref> | |||
< | == Natural History, Complications and Prognosis== | ||
< | Even though the majority of the aortic aneurysms remain asymptomatic for years, their natural history is [[Dissection of aorta|dissection]] or [[Rupture of the aorta|rupture]].<ref name=":4" /> According to Laplace's law, as the [[aneurysms]] grow larger they have a higher rate of expansion. Due to that, the frequency of monitoring changes with the diameter of the abdominal aortic aneurysm, being every 3 years for aneurysms with a 3-3.4cm diameter, yearly for diameters of 3.5-4.4cm, and every 6 months for larger than 4.5cm.<ref name=":5" /> For the thoracic one, up to 80% of the aneurysms will eventually rupture, and patients present with a 10-20% five-year survival rate if they remain untreated.<ref name=":4" /> Risk of rupture doubles every 1cm in growth over the 5cm diameter in descending thoracic aorta.<ref>Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg 1997;63:1533-45</ref> | ||
</ | |||
Besides rupturing and dissection of the aorta, aortic aneurysms can also present with systemic [[embolization]] and [[aortic regurgitation]] (if the thoracic aortic aneurysm is located in the [[ascending aorta]]). The altered blood flow in the aneurysm can also lead to the formation of [[blood cloths]] and [[embolization]]. <ref>Aortic Aneurysm: Symptoms and Complications - VeryWell Health available at: https://www.verywellhealth.com/aortic-aneurysm-symptoms-and-complications-4160769 - accessed at 06/08/2020</ref> | |||
== Diagnosis == | |||
===Diagnostic Criteria:=== | |||
'''[[Thoracic aortic aneurysm]]:''' considered an aneurysm when the diameter is >4 cm.<ref name=":6" /> | |||
</ | |||
'''[[Abdominal aortic aneurysm]]:''' considered an aneurysm when the diameter is >3 cm.<ref>Radiopaedia - Abdominal Aortic Aneurysms <nowiki>https://radiopaedia.org/articles/abdominal-aortic-aneurysm?lang=us</nowiki> | |||
Accessed at 06/08/2020</ref> | |||
</ | |||
== | === Symptoms: === | ||
'''[[Thoracic aortic aneurysm]]:''' as discussed above: most are asymptomatic. As they grow, they may cause: [[chest pain]], [[dyspnea]], [[hoarseness]], [[dizziness]], [[dysphagia]] and when they rupture: [[hypovolemic shock]] | |||
== | '''[[Abdominal aortic aneurysm]]:''' begin asymptomatic but may cause pain, pulsating abdominal mass, peripheral [[ischemia]], [[fever]] or [[weight loss]]. When they rupture, they cause [[acute abdominal pain]] and [[hypovolemic shock]]. | ||
=== Laboratory Findings === | |||
*There are no specific laboratory findings associated withaortic aneurysms. | |||
*[[Anemia]] can be seen in ruptured aortic aneurysms. | |||
== | ===Imaging Findings=== | ||
*An abdominal ultrasound can be diagnostic of abdominal aortic aneurysms and is the imaging tool used to screen for aortic aortic aneurysms. | |||
*[[CT]]A/[[MR]]A can accurately demonstrate aortic aneurysms extent. | |||
=== Other Diagnostic Studies === | |||
*Conventional [[angiogram]] can be used to diagnose aortic aneurysms. | |||
== 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines<ref name="pmid36334952">{{cite journal| author=Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW | display-authors=etal| title=2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. | journal=J Am Coll Cardiol | year= 2022 | volume= | issue= | pages= | pmid=36334952 | doi=10.1016/j.jacc.2022.08.004 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=36334952 }}</ref> == | |||
=== <u>Use of diagnostic imaging in Aortic Aneurysm</u> === | |||
==== Recommendations for AAA: Cause, Risk Factors, and Screening Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In men who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is recommended''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-R]])'' | |||
2. In men or women who are ≥65 years of age and who are first-degree relatives of patients with AAA, ultrasound screening for detection of AAA is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In women who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is reasonable''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |4. In men or women <65 years of age and who have multiple risk factors (Table 15) or a first-degree relative with AAA, ultrasound screening for AAA may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
=== | ==== Recommendations for Surveillance of Abdominal Aortic Dilation and Aneurysm Referenced studies that support the recommendations are summarized in the online date supplement ==== | ||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with an AAA of 3.0 cm to 3.9 cm, surveillance ultrasound is recommended every 3 years to assess for interval change''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
2. In men with an AAA of 4.0 cm to 4.9 cm and in women with an AAA of 4.0 cm to 4.4 cm, surveillance ultrasound is recommended annually to assess for interval change.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
3. In men with an AAA of ≥5.0 cm and women with an AAA of ≥4.5 cm, surveillance ultra-sound is recommended every 6 months to assess for interval change.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
4. In patients with an AAA that is inadequately defined with ultrasound, surveillance CT is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
5. In patients with an AAA that meets criteria for repair, CT is recommended for preoperative planning''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |4. | |||
-In such patients, when there is a contraindication to CT or to lower cumulative radiation risk, surveillance MRI is reasonable''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
In | === Recommendations for Surveillance of Thoracic Aortic Dilation and Aneurysm === | ||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with a dilated thoracic aorta, a TTE is recommended at the time of diagnosis to assess aortic valve anatomy, aortic valve function, and thoracic aortic diameters.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with a dilated thoracic aorta, a CT or MRI at the time of diagnosis is reasonable to assess thoracic aortic anatomy and diameters. | |||
3. In patients with a dilated thoracic aorta, follow-up imaging (with TTE, CT, or MRI, as appropriate based on individual anatomy) in 6 to 12 months is reasonable to determine the rate of aortic enlargement; if stable, surveillance imaging every 6 to 24 months (depending on aortic diameter) is reason-able | |||
|} | |||
=== | ==== Recommendations for HTAD: Genetic Testing and Screening of Family Members for TAD Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | ||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' 1. In patients with aortic root/ascending aortic aneurysms or aortic dissection, obtaining a multigenerational family history of TAD, unexplained sudden deaths, and peripheral and intracranial aneurysms is recommended. ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''2.'''In patients with aortic root/ascending aortic aneurysms or aortic dissection and risk factors for HTAD (Table 8, Figure 17), genetic testing to identify pathogenic/likely pathogenic variants (ie, mutations) is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''3.''' In patients with an established pathogenic or likely pathogenic variant in a gene predispos-ing to HTAD, it is recommended that genetic counseling be provided and the patient’s clini-cal management be informed by the specific gene and variant in the gene.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''4.''' In patients with TAD who have a pathogenic/likely pathogenic variant, genetic testing of at-risk biological relatives (ie, cascade testing) is recommended.6,10,11 In family members who are found by genetic screening to have inherited the pathogenic/likely pathogenic variant, aortic imaging with TTE (if aortic root and ascending aorta are adequately visualized, otherwise with CT or MRI) is recommended. ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''5.''' In a family with aortic root/ascending aor-tic aneurysms or aortic dissection, if the disease-causing variant is not identified with genetic testing, screening aortic imag-ing (as per recommendation 4) of at-risk biological relatives (ie, cascade testing) is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''6.''' In patients with aortic root/ascending aortic aneurysms or aortic dissection, in the absence of either a known family history of TAD or pathogenic/likely pathogenic variant, screening aortic imaging (as per recommendation 4) of first-degree relatives is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''7.''' In patients with acute type A aortic dissection, the diameter of the aortic root and ascending aorta should be recorded in the operative note and medical record to inform the management of affected relatives''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
==== Recommendations for Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' In patients with known or suspected aortic disease, aortic diameters should be measured at reproducible anatomic landmarks perpendicular to axis of blood flow, and these measurement methods should be reported in a clear and consistent manner. In cases of asymmetric or oval contour, the longest diameter and its perpendicular diameter should be reported. ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''2.''' In patients with known or suspected aortic disease, episodic and cumulative ionizing radiation doses should be kept as low as feasible while maintaining diagnostic image quality''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
'''3.'''In patients with known or suspected aortic disease, when performing CT or MR imaging, it is recommended that the root and ascending aortic diameters be measured from inner-edge to inner-edge, using an electrocardiographic-synchronized technique. If there are aortic wall abnormalities, such as atherosclerosis or discrete wall thickening (more common in the distal aorta), the outer-edge to outer-edge diameter should be reported''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
==== | '''4.''' In patients with known or suspected aortic disease, the aortic root diameter should be recorded as maximum sinus to sinus measurement. In the setting of known asymmetry, multiple measurements should be reported, and both short- and long-axis images of the root should be obtained to avoid underestimation of the diameter.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | ||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In patients with known or suspected aortic disease, it is reasonable that a dilated root or ascending aorta be indexed to patient height or BSA in the report, to aid in clinical risk assessment.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
'''2.''' In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow. Using inner-edge to inner-edge measurements may also be considered, particularly on short-axis imaging.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
: | ==== Recommendations for Diagnostic and Surveillance Aortic Imaging in Marfan Syndrome Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | ||
{| class="wikitable" | |||
|- | |||
: | | colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | ||
: | |- | ||
| bgcolor="LightGreen" |'''1.''' In patients with Marfan syndrome, a TTE is recommended at the time of initial diagnosis, to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter, to determine the rate of aortic growth; if the aortic diameters are stable, an annual surveillance TTE is recommended.1 If the aortic root, ascending aorta, or both are not adequately visualized on TTE, a CT or MRI of the thoracic aorta is recommended.22aC-EO2. In adults with Marfan syndrome, after the initial TTE, a CT or MRI of the thoracic aorta is reasonable to confirm the aortic diameters and assess the remainder of the thoracic aorta.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''2.'''In patients with Marfan syndrome who have undergone aortic root replacement, surveillance imaging of the thoracic aorta by MRI (or CT) is recommended to evaluate for distal TAD, initially annually and then, if normal in diameter and unchanged after 2 years, every other year''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" | [[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In adults with Marfan syndrome, after the initial TTE, a CT or MRI of the thoracic aorta is reasonable to confirm the aortic diameters and assess the remainder of the thoracic aorta.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
'''2.'''In patients with Marfan syndrome who have undergone aortic root replacement, surveil-lance imaging every 3 to 5 years for potential AAA is reasonable..''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
==== Recommendations for Imaging in Loeys-Dietz Syndrome ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' In patients with Loeys-Dietz syndrome, a baseline TTE is recommended to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter to determine the rate of aortic growth; if the aortic diameters are stable, annual surveillance TTE is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''2.''' In patients with Loeys-Dietz syndrome and a dilated or dissected aorta and/or arterial branches at baseline, annual surveillance imaging of the affected aorta and arteries with MRI or CT is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|- | |||
| bgcolor="LightGreen" |'''3.''' In patients with Loeys-Dietz syndrome, a baseline MRI or CT from head to pelvis is recommended to evaluate the entire aorta and its branches for aneurysm, dissection, and tortuosity.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.'''In patients with Loeys-Dietz syndrome with-out dilation of the aorta distal to the aortic root or ascending aorta and without dilated or dissected arterial branches, surveillance imaging from chest to pelvis with MRI (or CT) every 2 years is reasonable, but imaging may be more frequent depending on family history''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
'''2.'''In patients with Loeys-Dietz syndrome with-out dilation of the cerebral arteries on initial screening, periodic imaging surveillance for cerebral aneurysms with MRI or CT every 2 to 3 years is reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
==== Recommendations for Diagnostic Testing, Surveillance, and Surgical Intervention for Aortic Dilation in Turner Syndrome Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' In patients with Turner syndrome, TTE and cardiac MRI are recommended at the time of diagnosis to evaluate for BAV, aortic root and ascending aortic dilation, aortic coarctation, and other congenital heart defects.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''2.''' In patients with Turner syndrome who are ≥15 years old, the use of the ASI (ratio of aortic diameter [cm] to BSA [m2]) is recommended to define the degree of aortic dilation and assess the risk of aortic dissection''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''3.''' In patients with Turner syndrome without risk factors for aortic dissection (Table 12), sur-veillance imaging with TTE or MRI to evalu-ate the aorta is recommended every 5 years in children and every 10 years in adults, as well as before planning a pregnancy''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
'''4.''' In patients with Turner syndrome and an ASI >2.3 cm/m2, surveillance imaging of the aorta is recommended at least annually''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
'''5.''' In patients with Turner syndrome and risk factors for aortic dissection (Table 12), surveil-lance aortic imaging at an interval depending on the aortic diameter, ASI, and aortic growth rate is recommended ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In patients with Turner syndrome who are ≥15 years old and have an ASI of ≥2.5 cm/m2plus risk factors for aortic dissection (Table 12), surgical intervention to replace the aortic root, ascending aorta, or both is reasonable.9,102bC-EOIn those without risk factors for aortic dissection, surgical intervention to replace the aortic root, ascending aorta, or both may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In those without risk factors for aortic dissection, surgical intervention to replace the aortic root, ascending aorta, or both may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
=== | ==== Recommendations for HTAD: Genetic Testing and Screening of Family Members for TAD Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | ||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' In patients with aortic root/ascending aortic aneurysms or aortic dissection, obtaining a multigenerational family history of TAD, unexplained sudden deaths, and peripheral and intracranial aneurysms is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''2.''' In patients with aortic root/ascending aortic aneurysms or aortic dissection and risk factors for HTAD (Table 8, Figure 17), genetic testing to identify pathogenic/likely pathogenic variants (ie, mutations) is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''3.''' In patients with an established pathogenic or likely pathogenic variant in a gene predisposing to HTAD, it is recommended that genetic counseling be provided and the patient’s clinical management be informed by the specific gene and variant in the gene.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''4.''' In patients with TAD who have a pathogenic/likely pathogenic variant, genetic testing of at-risk biological relatives (ie, cascade testing) is recommended.6,10,11 In family members who are found by genetic screening to have inherited the pathogenic/likely pathogenic variant, aortic imaging with TTE (if aortic root and ascending aorta are adequately visualized, otherwise with CT or MRI) is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''5.''' In a family with aortic root/ascending aortic aneurysms or aortic dissection, if the disease-causing variant is not identified with genetic testing, screening aortic imaging (as per recommendation 4) of at-risk biological relatives (ie, cascade testing) is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR]])'' | |||
'''6.''' In patients with aortic root/ascending aortic aneurysms or aortic dissection, in the absence of either a known family history of TAD or pathogenic/likely pathogenic variant, screening aortic imaging (as per recommendation 4) of first-degree relatives is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD]])'' | |||
'''7.''' In patients with acute type A aortic dissection, the diameter of the aortic root and ascending aorta should be recorded in the operative note and medical record to inform the management of affected relatives.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO]])'' | |||
|} | |||
==== Recommendations for Inflammatory Aortitis: Diagnosis and Treatment of Takayasu Arteritis and GCA Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.'''In patients with large vessel vasculitis (LVV), prompt evaluation of the entire aorta and branch vessels with MRI or CT, with or without 18F-FDG positron emission tomography (FDG-PET), is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
==== Recommendations for Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.'''In patients with known or suspected aortic disease, aortic diameters should be measured at reproducible anatomic landmarks perpendicular to axis of blood flow, and these measurement methods should be reported in a clear and consistent manner. In cases of asymmetric or oval contour, the longest diam-eter and its perpendicular diameter should be reported''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
'''2.''' In patients with known or suspected aortic disease, episodic and cumulative ionizing radiation doses should be kept as low as feasible while maintaining diagnostic image quality.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
'''3.''' In patients with known or suspected aortic disease, when performing CT or MR imaging, it is recommended that the root and ascending aortic diameters be measured from inner-edge to inner-edge, using an electrocardiographic-synchronized technique. If there are aortic wall abnormalities, such as atherosclerosis or discrete wall thickening (more common in the distal aorta), the outer-edge to outer-edge diameter should be reported (Table 4)''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
'''4.''' In patients with known or suspected aortic disease, the aortic root diameter should be recorded as maximum sinus to sinus mea-surement. In the setting of known asymmetry, multiple measurements should be reported, and both short- and long-axis images of the root should be obtained to avoid underestima-tion of the diameter.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.'''In patients with known or suspected aortic disease, it is reasonable that a dilated root or ascending aorta be indexed to patient height or BSA in the report, to aid in clinical risk assessment''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
'''2.'''In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' Using inner-edge to inner-edge measure-ments may also be considered, particularly on short-axis imaging.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
== | ==== Recommendations for BAV Aortopathy Referenced studies that support the recommendations are summarized in the Online Data Supplement ==== | ||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |'''1.''' In patients with a BAV, TTE is indicated to evaluate valve morphology and function, to evaluate the diameter of the aortic root and ascending aorta, and to evaluate for aortic coarctation and other associated cardiovascular defects.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
'''2.''' In patients with a BAV, CT or MRI of the thoracic aorta is indicated when the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
'''3.''' In patients with a BAV and either HTAD or phenotypic features concerning for Loeys-Dietz syndrome, a medical genetics evaluation is recommended''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
[ | '''4.''' In patients with a BAV and a dilated aortic root or ascending aorta, screening of all first-degree relatives by TTE is recommended to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both; if the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE, a cardiac-gated CT or MRI of the thoracic aorta is indicated.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | ||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |'''1.''' In patients with a BAV, screening of all first-degree relatives by TTE is reasonable to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
== Treatment == | |||
=== <u>Medical Therapy in Aortic Aneurysm</u> === | |||
Focus is to reduce systemic blood pressure, inhibit [[MMP]] (zinc [[endopeptidases]] that degrade the [[Extracellular matrix protein|extracellular matrix]] in aortic aneurysms)<ref name=":7">Danyi, Peter, John A. Elefteriades, and Ion S. Jovin. "Medical therapy of thoracic aortic aneurysms: are we there yet?." ''Circulation'' 124.13 (2011): 1469-1476.</ref>, and contain the progression of [[atherosclerosis]]. | |||
< | *[[Beta-blockers]] may help in reducing the rate of expansion of the aortic aneurysm, reducing [[Shear (fluid)|shear]] stress - studies have been mostly on [[Marfan's syndrome|Marfan]] patients and they found a low compliance with [[propranolol]] due to a significant effect on quality of life<ref name=":7" />; | ||
*[[Tetracyclines]] inhibit the [[MMP]] [[endopeptidases]], and has been used in conditions in which MMP are overexpressed such as [[rheumatoid arthritis]]. There are studies in humans showing that [[doxycycline]] reduced the rate of expansion of aortic aneurysms. [[Roxithromycin]], a [[Macrolides|macrolide]] has been also show to reduce the expansion of the aortic aneurysms. | |||
*[[Statins]] may also be helpful due to their [[pleiotropic]] effecs, reducing the [[oxidative stress]] by blocking the [[reactive oxygen species]] on aneurysms, suppressing the [[NADH]]/[[NADPH]] oxidase system. | |||
*[[Angiotensin-converting enzyme inhibitors]] and [[Angiotensin receptor blocker|angiotensin receptor blockers]] promotes vascular hypertrophy, cell proliferation and production of extracellular matrix. It also activates the [[NADH]]/[[NADPH]] oxidase system, both stimulating and inhibiting [[MMP]]<nowiki/>s and degradation of [[Extracellular matrix protein|extracellular matrix]]. There is a controversy of which class is more effective, and ongoing trials are being run to further clarify these questions.<ref name=":7" /> | |||
</ | |||
</ | |||
There are no established guidelines for this matter, treatment is still controversial and should be individualized.<ref>Yoshimura, Koichi, et al. "Current status and perspectives on pharmacologic therapy for abdominal aortic aneurysm." ''Current drug targets'' 19.11 (2018): 1265-1275.</ref><ref name=":8">Clift, Paul F., and Elena Cervi. "A review of thoracic aortic aneurysm disease." ''Echo Research and Practice'' 7.1 (2020): R1-R10.</ref> | |||
< | == 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines<ref name="pmid363349522">{{cite journal| author=Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW | display-authors=etal| title=2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. | journal=J Am Coll Cardiol | year= 2022 | volume= | issue= | pages= | pmid=36334952 | doi=10.1016/j.jacc.2022.08.004 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=36334952 }}</ref> == | ||
</ | |||
=== Recommendations for BP Management in TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with TAA and an average systolic BP (SBP) of ≥130 mm Hg or an average diastolic BP (DBP) of ≥80 mm Hg, the use of antihypertensive medications is recommended to reduce risk of cardiovascular events''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with TAA, regardless of cause and in the absence of contraindications, use of beta blockers to achieve target BP goals is reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
3. In patients with TAA, regardless of etiology and in the absence of contraindications, ARB therapy is a reasonable adjunct to beta-blocker therapy to achieve target BP goals.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
=== Recommendations for Treatment of TAA With Statins === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |1. In patients with TAA and imaging or clinical evidence of atherosclerosis, statin therapy at moderate or high intensity is reasonable''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with TAA who have no evidence of atherosclerosis, the use of statin therapy may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendation for Antiplatelet Therapy in TAA === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |1. In patients with atherosclerotic TAA and concomitant aortic atheroma or PAU, the use of low-dose aspirin is reasonable, unless contraindicated''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
=== Recommendation for BP Management in AAA Referenced studies that support the recommendation are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with AAA and an average SBP of ≥130 mm Hg, or an average DBP of ≥80 mm Hg, the use of antihypertensive medication is recommended to reduce risk of cardiovascular events.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Treatment of AAA With Statins Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with AAA and evidence of aortic atherosclerosis, statin therapy at moderate or high intensity is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with AAA but no evidence of atherosclerosis, statin therapy may be considered ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendation for Antithrombotic Therapy in AAA === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |1. In patients with AAA with concomitant ather-oma and/or PAU, the use of low-dose aspirin may be considered, unless contraindicated ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== <u>Surgery in aortic aneurysm</u> === | |||
< | == 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines<ref name="pmid363349523">{{cite journal| author=Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW | display-authors=etal| title=2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. | journal=J Am Coll Cardiol | year= 2022 | volume= | issue= | pages= | pmid=36334952 | doi=10.1016/j.jacc.2022.08.004 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=36334952 }}</ref> == | ||
</ | |||
=== Recommendations for Surgery for Sporadic Aneurysms of the Aortic Root and Ascending AortaReferenced studies that support the recommendations are summarized in the Online Data supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with aneurysms of the aortic root and ascending aorta who have symptoms attributable to the aneurysm, surgery is indicated''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
2. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maxi-mum diameter of ≥5.5 cm, surgery is indicated.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
3. In patients with an aneurysm of the aortic root or ascending aorta of <5.5 cm, whose growth rate confirmed by tomographic imaging is ≥0.3 cm/y in 2 consecutive years, or ≥0.5 cm in 1 year, surgery is indicated''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |4. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maximum diameter of ≥5.0 cm, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
5. In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥4.5 cm, ascending aortic replacement is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
-In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥5.0 cm, ascending aortic replacement is reasonable''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
6. In patients with a height >1 standard deviation above or below the mean who have an asymptomatic aneurysm of the aortic root or ascending aorta and a maximal cross-sectional aortic area/height ratio of ≥10 cm2/m, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | -In patients undergoing cardiac surgery for indications other than aortic valve repair or replacement who have a concomitant aneurysm of ascending aorta with a maxi-mum diameter of ≥5.0 cm, ascending aortic replacement may be reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
7. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have either an ASI of ≥3.08 cm/m2 or AHI of ≥3.21 cm/m, surgery may be reasonable when per-formed by experienced surgeons in a Multidisciplinary Aortic Team.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendations for Surgical Approach for Patients With Sporadic Aneurysms of the Aortic Root and Ascending Aorta Meeting Criteria for SurgeryReferenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with an aneurysm isolated to the ascending aorta who meet criteria for surgery, aneurysm resection and replacement with an interposition graft should be performed.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
2. In patients undergoing aortic valve repair or replacement with a concomitant ascending aortic aneurysm, a separate aortic valve intervention and ascending aortic graft is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
3. In patients undergoing aortic root replacement with an aortic valve that is unsuitable for sparing or repair, a mechanical or biological valved conduit aortic root replacement is indicated.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |4. In patients undergoing aortic root replacement, valve-sparing aortic root replacement is reasonable if the aortic valve is suitable for sparing or repair and when performed by experienced surgeons in a Multidisciplinary Aortic Team | |||
''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Aortic Arch Aneurysms Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with an aortic arch aneurysm who have symptoms attributable to the aneurysm and are at low or intermediate operative risk, open surgical replacement is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with an isolated aortic arch aneurysm who are asymptomatic and have a low operative risk, open surgical replacement at an arch diameter of ≥5.5 cm is reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
3. In patients undergoing open surgical repair of an ascending aortic aneurysm, if the aneurys-mal disease extends into the proximal aortic arch, it is reasonable to extend the repair with a hemiarch replacement.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 4. In patients undergoing open surgical repair of an aortic arch aneurysm, if the aneurysmal disease extends into the proximal descending thoracic aorta, an elephant trunk procedure may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
5. In patients with an aortic arch aneurysm who are asymptomatic but meet criteria for inter-vention, but have a high risk from open surgi-cal repair, a hybrid or endovascular approach may be reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-EO)]]'' | |||
|} | |||
=== Recommendations for Size Thresholds for Repair of Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with intact descending TAA, repair is recommended when the diameter is ≥5.5 cm.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 2. In patients with intact descending TAA and risk factors for rupture (Table 17), repair may be considered at a diameter of <5.5 cm''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
3. In patients at increased risk for perioperative morbidity and mortality (Table 18), it may be reasonable to increase the size threshold for surgery accordingly''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Endovascular Versus Open Repair of Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients without Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome, who have a descending TAA that meets criteria for intervention and anatomy suitable for endovascular repair, TEVAR is recommended over open surgery''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
2. In patients with a descending TAA that meets criteria for repair with TEVAR, who have smaller or diseased access vessels, considerations for alternative vascular access are recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In patients with a descending TAA that meets criteria for intervention, who have anatomy unsuitable for endovascular repair, and who are without significant comorbidities and have a life expectancy of at least 10 years, open surgical repair is reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Left Subclavian Artery Management Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with descending TAA who undergo TEVAR with planned left subclavian artery coverage, revascularization of the left subclavian artery before TEVAR is recommended to prevent spinal cord injury (SCI)1,2 and poten-tially to reduce stroke risk2 and prevent other ischemic complications''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 2. In patients with descending TAA who have undergone TEVAR with left subclavian cover-age and develop SCI that is unresponsive to an increase in BP or a cerebrospinal fluid drain, left subclavian artery revascularization may be considered.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendation for Celiac Artery Management References that support the recommendation are included in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |1. In patients with descending TAA undergoing TEVAR in whom celiac artery coverage is being considered, it is reasonable to first con-firm adequate collateralization.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Ruptured Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with ruptured descending TAA who are anatomic candidates for endovascular repair, TEVAR is recommended over open repair because of decreased perioperative death and morbidity.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 2. In patients with ruptured descending TAA undergoing TEVAR, intentional coverage of the left subclavian artery, celiac artery, or both may be considered to increase the landing zone for endovascular repair.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Access Issues for TEVAR in Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with descending TAA undergoing TEVAR, review of preoperative CTA of the iliofemoral vessels should be performed to evaluate access.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
2. In patients with descending TAA undergoing TEVAR, if iliac access is marginal or inade-quate to prevent access-related complications, the use of alternative conduits is recommended''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In patients with descending TAA undergo-ing TEVAR who have suitable anatomy, total percutaneous femoral access is a reasonable alternative to open surgi-cal cutdown to avoid access-related complications''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Size Thresholds for Open Surgical Repair of TAAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with intact degenerative TAAA, repair is recommended when the diameter is ≥6.0 cm.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |2. In patients with intact degenerative TAAA, repair is reasonable when the diameter is ≥5.5 cm and the repair is performed by experienced surgeons in a Multidisciplinary Aortic Team.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
3. In patients with intact degenerative TAAA who have features associated with an increased risk of rupture (Table 19), repair is reasonable when the diameter is <5.5 cm''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for Open Versus Endovascular Repair of TAAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
''Ruptured TAAA'' | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with ruptured TAAA requiring inter-vention, open repair is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 2. In patients with ruptured TAAA requiring intervention, provided that the patient is hemodynamically stable, endovascular repair may be reasonable in centers with endovascular expertise and access to appropriate endovascular stent grafts''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
''Intact TAAA'' | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |3. In patients with Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syn-drome and intact TAAA requiring intervention, open repair is recommended over endovascular repair.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" | 4. In patients with intact degenerative TAAA and suitable anatomy, endovascular repair with fenestrated stent grafts, branched stent grafts, or both may be considered in centers with endovascular expertise and access to appropriate endovascular stent grafts.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for TAAA Spinal Cord Protection Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients undergoing open TAAA repair who are at high risk for SCI, cerebrospinal fluid drainage is recommended to reduce the incidence of temporary SCI, permanent SCI, or both.(''[[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: A)]]'' | |||
2. In patients who experience delayed spinal cord dysfunction after either open or endo-vascular TAAA repair, timely measures to optimize spinal cord perfusion and decrease intrathecal pressure are recommended (Table 20).''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for TAAA Renal and Visceral Organ Protection Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients undergoing open repair of TAAA involving the renal arteries, cold blood or crystalloid renal perfusion is recommended to pro-vide effective protection against renal injury.(''[[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: A)]]'' | |||
2. In patients undergoing open or endovascular TAAA repair who have end-organ ischemia or significant stenoses from atherosclerotic visceral or renal artery disease, additional revascularization procedures are recommended''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendation for Access During Endovascular Repair of AAA Referenced studies that support the recommendation are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients undergoing endovascular repair of AAA who have suitable common femoral artery anatomy, ultrasound-guided percutaneous access and closure is recommended over open cutdown to reduce operative time, blood loss, length of stay, time to wound healing, and pain.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-R)]]'' | |||
|} | |||
=== Recommendations for Repair of Ruptured AAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients presenting with ruptured AAA who are hemodynamically stable, CT imaging is recommended to evaluate whether the AAA is amenable to endovascular repair.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-R)]]'' | |||
2. In patients presenting with ruptured AAA who have suitable anatomy, endovascular repair is recommended over open repair to reduce the risk of morbidity and mortality.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-R)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In patients undergoing endovascular repair for ruptured AAA, local anesthesia is preferred to general anesthesia to reduce risk of perioperative mortality.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
4. In patients with ruptured AAA, permissive hypotension can be beneficial to decrease the rate of bleeding''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendations for the Threshold for AAA Repair Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with unruptured AAA, repair is recommended in those with a maximal aneurysm diameter of ≥5.5 cm in men or ≥5.0 cm in women''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: A)]]'' | |||
2. In patients with unruptured AAA who have symptoms that are attributable to the aneurysm, repair is recommended to reduce the risk of rupture.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In patients with unruptured saccular AAA, intervention to reduce the risk of rupture may be reasonable.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
4. In patients with unruptured AAA and aneurysm growth of ≥0.5 cm in 6 months, repair to reduce the risk of rupture may be reason-able''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
=== Recommendations for Open Versus Endovascular Repair of AAA Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with non ruptured AAA with low to moderate operative risk and who have anatomy suitable for either open or EVAR, a shared decision-making process weighing the risks and benefits of each approach is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: A)]]'' | |||
2. In patients undergoing elective endovascular repair for non ruptured AAA, adherence to manufacturer’s instructions for use is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | |||
|- | |||
| bgcolor="LemonChiffon" |3. In patients with non ruptured AAA and a high perioperative risk, EVAR is reasonable to reduce the risk of 30-day morbidity, mortality, or both''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
4. For patients with non ruptured AAA, a moderate to high perioperative risk, and anatomy suitable for an FDA-approved fenestrated endovascular device, endovascular repair is reasonable over open repair to reduce the risk of perioperative complications.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendations for the Treatment of Concomitant Common Iliac Aneurysms Referenced studies that support the recommendations are summarized in the Online Data Supplement === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. For patients with asymptomatic small AAA and concomitant common iliac artery aneurysm(s) ≥3.5 cm, elective repair of both abdominal and iliac aneurysms is recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
2. When treating common iliac artery aneurysms or ectasia as part of AAA repair, preservation of at least 1 hypogastric artery is recommended, if anatomically feasible, to decrease the risk of pelvic ischemia.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
Decision to perform elective surgery to prevent aneurysm rupture is complicated as there must be an appropriate patient selection and timing for repair of the aneurysm which demands selecting patients at the greatest risk of aneurysm rupture. Once rupture occurs, mortality is extremely high. Fatality rates of emergency surgical repair is 50% if the patient manages to reach the hospital, in comparison to 1-5% fatality rate in elective surgical repair.<ref name=":9">Aggarwal, Sourabh, et al. "Abdominal aortic aneurysm: A comprehensive review." ''Experimental & Clinical Cardiology'' 16.1 (2011): 11.</ref> | |||
According to the 2005 AHA/ACC guidelines - it is recommended surgical repair of abdominal aortic aneurysms: | |||
* 5.5 cm in diameter or greater in asymptomatic patients; | |||
* Increase by 0.5 cm or greater in diameter in 6 months; | |||
* Symptomatic aneurysms. | |||
Endovascular repair may be performed with better short-term morbidity and mortality rates but with failed long-term benefits over surgical repair. Endovascular is preferred in high-risk patients while surgical repair is generally indicated for low/average-risk patients.<ref name=":9" /> | |||
In thoracic aortic aneurysms, surgery is indicated in [[Marfan's syndrome]] when the aortic diameter reaches 5.0cm, or the rate of increase of the aortic root diameter approaches 1.0 cm per year, or progressive and severe aortic regurgitation. If family history is positive for aortic aneurysms, aggressive therapy may be indicated in individuals with Marfan and [[Loeys-Dietz syndrome|Loeys Dietz syndrome]]. Surgery consists in replacing the affected portion of the aorta. <ref name=":8" /> | |||
=== Prevention === | |||
[[Smoking]] cessation is an important measure to prevent aortic aneurysm progression and rupture, as is control of the other cardiovascular risks, such as [[Hypertension, systemic|hypertension]], sedentarism and [[dyslipidemia]].<ref name=":3" /> | |||
=== Recommendation for Smoking Cessation in AAA === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with AAA who smoke cigarettes, smoking cessation efforts are recommended''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |||
=== Recommendation for Smoking Cessation in TAA === | |||
{| class="wikitable" | |||
|- | |||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |||
| bgcolor="LightGreen" |1. In patients with TAA who smoke cigarettes, smoking cessation efforts are recommended.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
|} | |||
==Related Chapters== | |||
* [[Aortic dissection|Aortic Dissection]] | |||
* [[Thoracic aortic aneurysms|Thoracic Aortic Aneurysm]] | |||
* [[Abdominal aortic aneurysm|Abdominal Aortic Aneurysm]] | |||
* [[Aneurysm]] | |||
==References== | ==References== | ||
{{ | {{reflist|2}} | ||
[[es:Aneurisma de aorta]] | |||
<br /> | |||
{{ | {{WikiDoc Help Menu}} | ||
{{ | {{WikiDoc Sources}} | ||
[[CME Category::Cardiology]] | |||
[[pl:Tętniak aorty]] | |||
[[Category:Vascular surgery]] | [[Category:Vascular surgery]] | ||
[[Category:Cardiology]] | [[Category:Cardiology]] | ||
[[Category:Emergency medicine]] | [[Category:Emergency medicine]] | ||
[[Category:Disease]] | |||
[[ | |||
Latest revision as of 20:21, 6 December 2022
| Resident Survival Guide |
| Aortic aneurysm | |
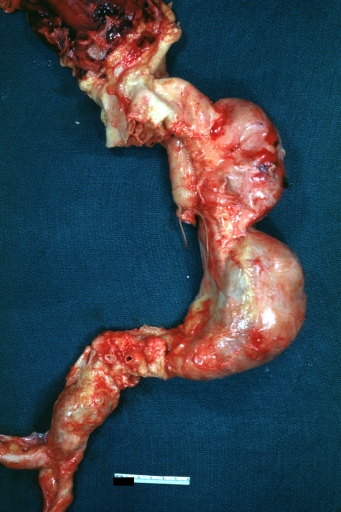 | |
|---|---|
| Atherosclerotic Aneurysm: Gross, an excellent example, natural color, external view of typical thoracic aortic aneurysms Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
|
WikiDoc Resources for Aortic aneurysm |
|
Articles |
|---|
|
Most recent articles on Aortic aneurysm Most cited articles on Aortic aneurysm |
|
Media |
|
Powerpoint slides on Aortic aneurysm |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Aortic aneurysm at Clinical Trials.gov Trial results on Aortic aneurysm Clinical Trials on Aortic aneurysm at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Aortic aneurysm NICE Guidance on Aortic aneurysm
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Aortic aneurysm Discussion groups on Aortic aneurysm Patient Handouts on Aortic aneurysm Directions to Hospitals Treating Aortic aneurysm Risk calculators and risk factors for Aortic aneurysm
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Aortic aneurysm |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
For patient information on Thoracic aortic aneurysm, click here
For patient information on Abdominal aortic aneurysm, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1], Associate Editor(s)-in-Chief: Lina Ya'qoub, MD; Cafer Zorkun, M.D., Ph.D. [2]; Gerald Chi, MD
Overview
An aortic aneurysm is a dilation of the aorta in which the aortic diameter is ≥ 3.0 cm if abdominal[1] or >4 cm if thoracic[2], usually representing an underlying weakness in the wall of the aorta at that location. While the stretched vessel may occasionally cause discomfort, a greater concern is the risk of rupture which causes severe pain, massive internal hemorrhage which are often fatal. Aneurysms often are a source of blood clots (emboli) stemming from the most common etiology of atherosclerosis.
Classification
There are 2 types of aortic aneurysms: thoracic and abdominal. These can be further classified according to the respective part of the vessel that's been affected:
- Thoracic aortic aneurysm, which occur in the thoracic aorta (runs through the chest);
- Abdominal aortic aneurysm, which occur in the abdominal aorta, are the most common.
- Suprarenal - not as common, often more difficult to repair surgically due to the presence of many aortic branches;
- Infrarenal - often more easily surgically repaired and more common;
- Pararenal - aortic aneurysm is infrarenal but affects renal arteries;
- Juxtarenal - infrarenal aortic aneurysm that affects the aorta just below the renal arteries.
Aortic aneurysms may also be classified according to Crawford classification into 5 subtypes/groups:
- Type 1: from the origin of left subclavian artery in descending thoracic aorta to the supra-renal abdominal aorta.
- Type 2: from the left subclavian to the aorto-iliac bifurcation.
- Type 3: from distal thoracic aorta to the aorto-iliac bifurcation
- Type 4: limited to abdominal aorta below the diaphragm
- Type 5: from distal thoracic aorta to celiac and superior mesenteric origins, but not the renal arteries.[3]
Historical Perspective
Aortic aneurysm was first recorded by Antyllus, a Greek surgeon, in the second century AD. In the Renaissaince era, in 1555, Vesalius first diagnosed an abdominal aortic aneurysm. The first publication on the pathology with case studies was published by Lancisi in 1728. Finally, in 1817, Astley Cooper was the first surgeon to ligate the abdominal aorta to treat a ruptured iliac aneurysm. In 1888, Rudoff Matas came up with the concept of endoaneurysmorrhaphy.[4]
Pathophysiology
The aortic aneurysms are a multifactorial disease associated with genetic and environmental risk factors. Marfan's syndrome and Ehlers-Danlos syndrome are associated with the disease, but there are also rarer syndromes like the Loeys-Dietz syndrome that are associated as well. Even in patients that do not have genetic syndromes, it has been observed that genetics can also play a role on aortic aneurysms' development. There has been evidence of genetic heterogeneity as there has already been documented in intracranial aneurysms.[5] The genetic alterations associated with these genetic syndromes are the following:
| Disease | Involved Cellular Pathway | Mutated Gene(s) | Affected Protein(s) |
|---|---|---|---|
| Ehlers-Danlos type IV syndrome | Extracellular Matrix Proteins | COL3A1 | Collagen type III |
| Marfan's Syndrome | Extracellular Matrix Proteins | FBN1 | Fibrillin-1 |
| Loeys-Dietz syndrome | TGF-β Pathway | TGFBR1/TGFBR2 | |
| Aneurysm-Osteoarthritis Syndrome | SMAD3 | SMAD3 | |
| Autosomal Dominant Polycystic Kidney Disease | Ciliopathy | PKD1/PKD2 | Polycystin 1 |
| Turner Syndrome | Meiotic Error with Monosomy, Mosaicism, or De Novo Germ Cell Mutation | 45X
45XO |
Partial or Complete Absence of X Chromosome |
| Bicuspid Aortic Valve with TAA | Neural Crest Migration | NOTCH1 | Notch 1 |
| Familial TAA | Smooth Muscle Contraction Proteins | ACTA2 | α-Smooth Muscle Actin |
| Familial TAA with Patent Ductus Arteriosus | Smooth Muscle Contraction Proteins | MYH11 | Smooth Muscle Myosin |
| Familial TAA | Smooth Muscle Contraction Proteins | MYLK | Myosin Light Chain Kinase |
| Familial TAA | Smooth Muscle Contraction Proteins | PRKG1 | Protein Kinase c-GMP Dependent, type I |
| Loeys-Dietz Syndrome variants | TGF-β Pathway | TGF-βR1
TGF-βR2 TGF-β2 TGF-β3 |
These genetic diseases mostly affect either the synthesis of extracellular matrix protein or damage the smooth muscle cells both important component's of the aortic wall. Injury to any of these components lead to weakening of the aortic wall and dilation - resulting in aneurysm formation.
The aorta is the largest vessel of the body, but it is not homogenous. Its upper segment is composed by a larger proportion of elastin in comparison to collagen, therefore being more distensible. The lower segment has a larger proportion of collagen, therefore it is less distensible. It is also where most of the atherosclerotic plaques of the aorta are located.[1] Historically it was thought that abdominal and thoracic aortic aneurysms were caused by the same etiology: atherosclerotic degeneration of the aortic wall, but recently it has been theorized that they are indeed different diseases.[1]
The aortic arch mostly derives from the neural crest cell which differentiate into smooth muscle cells. These smooth muscle cells are probably more adapted to remodel the thoracic aorta and manage the higher pulse pressure and ejection volume due to increased production of elastic lamellae during development and growth.[1] The abdominal aorta remains with cells of mesodermal origin, which are more similar to that of the original primitive arterial. That difference results in the neural crest cell precursors of the thoracic aorta being able to respond differently to various cytokines and growth factors than the mesodermal precursors of the abdominal aorta,[7] such as homocysteine[8] and angiotensin II.[9]
When neural crest vascular smooth muscle cells are treated with TGF-β they demonstrate increased collagen production, while mesodermal vascular smooth muscle cell did not.[10] Not coincidently, mutations of the TGF-β receptor can cause thoracic aortic aneurysm but do not cause abdominal aortic ones.
The thoracic and abdominal aorta are very structurally different. While they both have three layers: intimal, medial and adventitia, the media of the thoracic aorta is comprised of approximately 60 units divided into vascular and avascular regions. The abdominal aorta consists of about 30 units and is entirely avascular, being dependent on trans-intimal diffusion of nutrients for its smooth muscle cells to survive.[11] It is believed that both differences explain why the abdominal aorta is more likely to form aneurysms.
The development of aortic aneurysms is defined by: inflammation: infiltration of the vessel wall by lymphocytes and macrophage; extracellular matrix damage: destruction of elastin and collagen by proteases (also metalloproteinases) in the media and adventitia; cellular damage: loss of smooth muscle cells with thinning of the media; and insufficient repair: neovascularization.[12]
Clinical Features
Thoracic aortic aneurysms: The aneurysms tend to grow slowly and most of them will never rupture. As they grow, however, their symptoms become more evident and present with mass effects over surrounding structures and pain. They may present with thoracic symptoms: interscapular or central pain, ripping chest pain and dyspnea. Atypical presentations include hoarseness, dizziness and dysphagia, due to esophageal compression.[13] Aneurysm rupture lead to massive internal bleeding, hypovolemic shock and it is usually fatal.
Abdominal aortic aneurysms: as the thoracic aneurysms, they begin asymptomatic but may cause symptoms as they grow and compress surrounding structures.[14]Even though they usually remain asymptomatic, when they rupture they present with an ensuing mortality of 85 to 90%., and symptomatic patients require urgent surgical repair.[15]
When symptomatic, abdominal aortic aneurysms present with:
- Pain: in the chest, abdomen, lower back, or flanks. It may radiate to the groin, buttocks, or legs. The pain characteristics vary and may be deep, aching, gnawing, or throbbing It may also last for hours or days, not affected by movement. Occasionally, certain positions can be more comfortable and alleviate the symptoms;
- Pulsating abdominal mass;
- Ischemia: "cold foot" or a black or blue painful toe. This is usually the presentation when an aneurysm forms a blood cloth and it releases emboli to the lower extremities;
- Fever or weight loss if caused by inflammatory states such as vasculitis.[14]
If ruptured, the abdominal aortic aneurysm can present with sharp abdominal pain, often radiating to the back, discoloration of the skin and mucosa, tachycardia and low blood pressure due to hypovolemic shock.
Differentiating Aortic Aneurysm from other Diseases
Thoracic aortic aneurysms: differential diagnosis include other causes of chest pain: acute aortic dissection, acute pericarditis, aortic regurgitation, heart failure, hypertensive emergencies, infective endocarditis, myocardial Infarction, pulmonary embolism, superior vena cava syndrome. [16]
Abdominal aortic aneurysms: differential diagnosis include causes of pulsatile abdominal mass and/or abdominal pain such as ruptured viscus, strangulated hernia, ruptured visceral artery aneurysms, mesenteric ischemia, acute cholecystitis, ruptured hepatobiliary cancer, acute pancreatitis, lymphomas, and diverticular abscess.[17]
These conditions can be easily differentiated using abdominal or thoracic imaging.
Epidemiology and Demographics
In the United States alone 15,000 people die yearly due to aortic aneurysms and it is the 13th leading cause of death. 1-2% of the population may have aortic aneurysms and prevalence rises up to 10% in older age groups. The disease varies according to where it takes place. In the thorax, the aortic arch is the less affected segment (10%) and the most common is the ascending aorta (50%). Regarding abdominal aneurysms, the infrarenal segment aortic aneurysms are three times more prevalent than the aortic aneurysms and dissections.[5]
Regarding other factors as age, abdominal aortic aneurysms usually present 10 years later than thoracic aortic aneurysms. Both lesions are more present in men, but the proportion is much higher regarding abdominal aortic aneurysms (6:1 male:female ratio) in comparison to thoracic ones.[5]
Abdominal aortic aneurysms also affect patients differently regarding race, as they are more prevalent among whites than blacks, asians and hispanics. It also seems to be declining in prevalence as evidenced by a Swedish study that found out a 2% prevalence of abdominal aortic aneurysms in comparison to earlier studies which reported 4-8%, probably due to risk-factor modification. [18]
Risk Factors
Many risk factors are common between both forms of aortic aneurysms, but some are specific for each presentation:
- Abdominal aortic aneurysm: smoking, male gender, age (>65 years), race (white), family history, other aneurysms.[17]
- Thoracic aortic aneurysm: smoking, age (>65 years), hypertension, atherosclerosis, family history, Marfan's syndrome, bicuspid aortic valve. [19]
Natural History, Complications and Prognosis
Even though the majority of the aortic aneurysms remain asymptomatic for years, their natural history is dissection or rupture.[3] According to Laplace's law, as the aneurysms grow larger they have a higher rate of expansion. Due to that, the frequency of monitoring changes with the diameter of the abdominal aortic aneurysm, being every 3 years for aneurysms with a 3-3.4cm diameter, yearly for diameters of 3.5-4.4cm, and every 6 months for larger than 4.5cm.[18] For the thoracic one, up to 80% of the aneurysms will eventually rupture, and patients present with a 10-20% five-year survival rate if they remain untreated.[3] Risk of rupture doubles every 1cm in growth over the 5cm diameter in descending thoracic aorta.[20]
Besides rupturing and dissection of the aorta, aortic aneurysms can also present with systemic embolization and aortic regurgitation (if the thoracic aortic aneurysm is located in the ascending aorta). The altered blood flow in the aneurysm can also lead to the formation of blood cloths and embolization. [21]
Diagnosis
Diagnostic Criteria:
Thoracic aortic aneurysm: considered an aneurysm when the diameter is >4 cm.[2]
Abdominal aortic aneurysm: considered an aneurysm when the diameter is >3 cm.[22]
Symptoms:
Thoracic aortic aneurysm: as discussed above: most are asymptomatic. As they grow, they may cause: chest pain, dyspnea, hoarseness, dizziness, dysphagia and when they rupture: hypovolemic shock
Abdominal aortic aneurysm: begin asymptomatic but may cause pain, pulsating abdominal mass, peripheral ischemia, fever or weight loss. When they rupture, they cause acute abdominal pain and hypovolemic shock.
Laboratory Findings
- There are no specific laboratory findings associated withaortic aneurysms.
- Anemia can be seen in ruptured aortic aneurysms.
Imaging Findings
- An abdominal ultrasound can be diagnostic of abdominal aortic aneurysms and is the imaging tool used to screen for aortic aortic aneurysms.
- CTA/MRA can accurately demonstrate aortic aneurysms extent.
Other Diagnostic Studies
- Conventional angiogram can be used to diagnose aortic aneurysms.
2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines[23]
Use of diagnostic imaging in Aortic Aneurysm
Recommendations for AAA: Cause, Risk Factors, and Screening Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In men who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is recommended(Level of Evidence: B-R)
2. In men or women who are ≥65 years of age and who are first-degree relatives of patients with AAA, ultrasound screening for detection of AAA is recommended.(Level of Evidence: C-LD) |
| Class IIa |
| 3. In women who are ≥65 years of age who have ever smoked, ultrasound screening for detection of AAA is reasonable(Level of Evidence: C-EO) |
| Class IIb |
| 4. In men or women <65 years of age and who have multiple risk factors (Table 15) or a first-degree relative with AAA, ultrasound screening for AAA may be considered.(Level of Evidence: C-LD) |
Recommendations for Surveillance of Abdominal Aortic Dilation and Aneurysm Referenced studies that support the recommendations are summarized in the online date supplement
| Class I |
| 1. In patients with an AAA of 3.0 cm to 3.9 cm, surveillance ultrasound is recommended every 3 years to assess for interval change(Level of Evidence: B-NR)
2. In men with an AAA of 4.0 cm to 4.9 cm and in women with an AAA of 4.0 cm to 4.4 cm, surveillance ultrasound is recommended annually to assess for interval change.(Level of Evidence: B-NR) 3. In men with an AAA of ≥5.0 cm and women with an AAA of ≥4.5 cm, surveillance ultra-sound is recommended every 6 months to assess for interval change.(Level of Evidence: B-NR) 4. In patients with an AAA that is inadequately defined with ultrasound, surveillance CT is recommended.(Level of Evidence: C-EO) 5. In patients with an AAA that meets criteria for repair, CT is recommended for preoperative planning(Level of Evidence: C-EO) |
| Class IIa |
| 4.
-In such patients, when there is a contraindication to CT or to lower cumulative radiation risk, surveillance MRI is reasonable(Level of Evidence: C-LD) |
Recommendations for Surveillance of Thoracic Aortic Dilation and Aneurysm
| Class I |
| 1. In patients with a dilated thoracic aorta, a TTE is recommended at the time of diagnosis to assess aortic valve anatomy, aortic valve function, and thoracic aortic diameters.(Level of Evidence: C-LD) |
| Class IIa |
| 2. In patients with a dilated thoracic aorta, a CT or MRI at the time of diagnosis is reasonable to assess thoracic aortic anatomy and diameters.
3. In patients with a dilated thoracic aorta, follow-up imaging (with TTE, CT, or MRI, as appropriate based on individual anatomy) in 6 to 12 months is reasonable to determine the rate of aortic enlargement; if stable, surveillance imaging every 6 to 24 months (depending on aortic diameter) is reason-able |
Recommendations for HTAD: Genetic Testing and Screening of Family Members for TAD Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. 1. In patients with aortic root/ascending aortic aneurysms or aortic dissection, obtaining a multigenerational family history of TAD, unexplained sudden deaths, and peripheral and intracranial aneurysms is recommended. (Level of Evidence: B-NR) |
| 2.In patients with aortic root/ascending aortic aneurysms or aortic dissection and risk factors for HTAD (Table 8, Figure 17), genetic testing to identify pathogenic/likely pathogenic variants (ie, mutations) is recommended.(Level of Evidence: B-NR) |
| 3. In patients with an established pathogenic or likely pathogenic variant in a gene predispos-ing to HTAD, it is recommended that genetic counseling be provided and the patient’s clini-cal management be informed by the specific gene and variant in the gene.(Level of Evidence: B-NR) |
| 4. In patients with TAD who have a pathogenic/likely pathogenic variant, genetic testing of at-risk biological relatives (ie, cascade testing) is recommended.6,10,11 In family members who are found by genetic screening to have inherited the pathogenic/likely pathogenic variant, aortic imaging with TTE (if aortic root and ascending aorta are adequately visualized, otherwise with CT or MRI) is recommended. (Level of Evidence: B-NR) |
| 5. In a family with aortic root/ascending aor-tic aneurysms or aortic dissection, if the disease-causing variant is not identified with genetic testing, screening aortic imag-ing (as per recommendation 4) of at-risk biological relatives (ie, cascade testing) is recommended.(Level of Evidence: B-NR) |
| 6. In patients with aortic root/ascending aortic aneurysms or aortic dissection, in the absence of either a known family history of TAD or pathogenic/likely pathogenic variant, screening aortic imaging (as per recommendation 4) of first-degree relatives is recommended.(Level of Evidence: C-LD) |
| 7. In patients with acute type A aortic dissection, the diameter of the aortic root and ascending aorta should be recorded in the operative note and medical record to inform the management of affected relatives(Level of Evidence: C-EO) |
Recommendations for Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with known or suspected aortic disease, aortic diameters should be measured at reproducible anatomic landmarks perpendicular to axis of blood flow, and these measurement methods should be reported in a clear and consistent manner. In cases of asymmetric or oval contour, the longest diameter and its perpendicular diameter should be reported. (Level of Evidence: B-NR)
2. In patients with known or suspected aortic disease, episodic and cumulative ionizing radiation doses should be kept as low as feasible while maintaining diagnostic image quality(Level of Evidence: C-LD) 3.In patients with known or suspected aortic disease, when performing CT or MR imaging, it is recommended that the root and ascending aortic diameters be measured from inner-edge to inner-edge, using an electrocardiographic-synchronized technique. If there are aortic wall abnormalities, such as atherosclerosis or discrete wall thickening (more common in the distal aorta), the outer-edge to outer-edge diameter should be reported(Level of Evidence: C-EO) 4. In patients with known or suspected aortic disease, the aortic root diameter should be recorded as maximum sinus to sinus measurement. In the setting of known asymmetry, multiple measurements should be reported, and both short- and long-axis images of the root should be obtained to avoid underestimation of the diameter.(Level of Evidence: C-EO) |
| Class IIa |
| 1. In patients with known or suspected aortic disease, it is reasonable that a dilated root or ascending aorta be indexed to patient height or BSA in the report, to aid in clinical risk assessment.(Level of Evidence: C-LD)
2. In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow.(Level of Evidence: C-EO) |
| Class IIb |
| 1. In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow. Using inner-edge to inner-edge measurements may also be considered, particularly on short-axis imaging.(Level of Evidence: C-EO) |
Recommendations for Diagnostic and Surveillance Aortic Imaging in Marfan Syndrome Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with Marfan syndrome, a TTE is recommended at the time of initial diagnosis, to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter, to determine the rate of aortic growth; if the aortic diameters are stable, an annual surveillance TTE is recommended.1 If the aortic root, ascending aorta, or both are not adequately visualized on TTE, a CT or MRI of the thoracic aorta is recommended.22aC-EO2. In adults with Marfan syndrome, after the initial TTE, a CT or MRI of the thoracic aorta is reasonable to confirm the aortic diameters and assess the remainder of the thoracic aorta.(Level of Evidence: C-EO) |
| 2.In patients with Marfan syndrome who have undergone aortic root replacement, surveillance imaging of the thoracic aorta by MRI (or CT) is recommended to evaluate for distal TAD, initially annually and then, if normal in diameter and unchanged after 2 years, every other year(Level of Evidence: C-LD) |
| Class IIa |
| 1. In adults with Marfan syndrome, after the initial TTE, a CT or MRI of the thoracic aorta is reasonable to confirm the aortic diameters and assess the remainder of the thoracic aorta.(Level of Evidence: C-EO)
2.In patients with Marfan syndrome who have undergone aortic root replacement, surveil-lance imaging every 3 to 5 years for potential AAA is reasonable..(Level of Evidence: C-LD) |
Recommendations for Imaging in Loeys-Dietz Syndrome
| Class I |
| 1. In patients with Loeys-Dietz syndrome, a baseline TTE is recommended to determine the diameters of the aortic root and ascending aorta, and 6 months thereafter to determine the rate of aortic growth; if the aortic diameters are stable, annual surveillance TTE is recommended.(Level of Evidence: C-EO) |
| 2. In patients with Loeys-Dietz syndrome and a dilated or dissected aorta and/or arterial branches at baseline, annual surveillance imaging of the affected aorta and arteries with MRI or CT is recommended.(Level of Evidence: C-EO) |
| 3. In patients with Loeys-Dietz syndrome, a baseline MRI or CT from head to pelvis is recommended to evaluate the entire aorta and its branches for aneurysm, dissection, and tortuosity.(Level of Evidence: C-EO) |
| Class IIa |
| 1.In patients with Loeys-Dietz syndrome with-out dilation of the aorta distal to the aortic root or ascending aorta and without dilated or dissected arterial branches, surveillance imaging from chest to pelvis with MRI (or CT) every 2 years is reasonable, but imaging may be more frequent depending on family history(Level of Evidence: C-EO)
2.In patients with Loeys-Dietz syndrome with-out dilation of the cerebral arteries on initial screening, periodic imaging surveillance for cerebral aneurysms with MRI or CT every 2 to 3 years is reasonable.(Level of Evidence: C-EO) |
Recommendations for Diagnostic Testing, Surveillance, and Surgical Intervention for Aortic Dilation in Turner Syndrome Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with Turner syndrome, TTE and cardiac MRI are recommended at the time of diagnosis to evaluate for BAV, aortic root and ascending aortic dilation, aortic coarctation, and other congenital heart defects.(Level of Evidence: B-NR)
2. In patients with Turner syndrome who are ≥15 years old, the use of the ASI (ratio of aortic diameter [cm] to BSA [m2]) is recommended to define the degree of aortic dilation and assess the risk of aortic dissection(Level of Evidence: B-NR) 3. In patients with Turner syndrome without risk factors for aortic dissection (Table 12), sur-veillance imaging with TTE or MRI to evalu-ate the aorta is recommended every 5 years in children and every 10 years in adults, as well as before planning a pregnancy(Level of Evidence: C-LD) 4. In patients with Turner syndrome and an ASI >2.3 cm/m2, surveillance imaging of the aorta is recommended at least annually(Level of Evidence: C-EO) 5. In patients with Turner syndrome and risk factors for aortic dissection (Table 12), surveil-lance aortic imaging at an interval depending on the aortic diameter, ASI, and aortic growth rate is recommended (Level of Evidence: C-EO) |
| Class IIa |
| 1. In patients with Turner syndrome who are ≥15 years old and have an ASI of ≥2.5 cm/m2plus risk factors for aortic dissection (Table 12), surgical intervention to replace the aortic root, ascending aorta, or both is reasonable.9,102bC-EOIn those without risk factors for aortic dissection, surgical intervention to replace the aortic root, ascending aorta, or both may be considered.(Level of Evidence: C-LD) |
| Class IIb |
| 1. In those without risk factors for aortic dissection, surgical intervention to replace the aortic root, ascending aorta, or both may be considered.(Level of Evidence: C-EO) |
Recommendations for HTAD: Genetic Testing and Screening of Family Members for TAD Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with aortic root/ascending aortic aneurysms or aortic dissection, obtaining a multigenerational family history of TAD, unexplained sudden deaths, and peripheral and intracranial aneurysms is recommended.(Level of Evidence: B-NR)
2. In patients with aortic root/ascending aortic aneurysms or aortic dissection and risk factors for HTAD (Table 8, Figure 17), genetic testing to identify pathogenic/likely pathogenic variants (ie, mutations) is recommended.(Level of Evidence: B-NR) 3. In patients with an established pathogenic or likely pathogenic variant in a gene predisposing to HTAD, it is recommended that genetic counseling be provided and the patient’s clinical management be informed by the specific gene and variant in the gene.(Level of Evidence: B-NR) 4. In patients with TAD who have a pathogenic/likely pathogenic variant, genetic testing of at-risk biological relatives (ie, cascade testing) is recommended.6,10,11 In family members who are found by genetic screening to have inherited the pathogenic/likely pathogenic variant, aortic imaging with TTE (if aortic root and ascending aorta are adequately visualized, otherwise with CT or MRI) is recommended.(Level of Evidence: B-NR) 5. In a family with aortic root/ascending aortic aneurysms or aortic dissection, if the disease-causing variant is not identified with genetic testing, screening aortic imaging (as per recommendation 4) of at-risk biological relatives (ie, cascade testing) is recommended.(Level of Evidence: B-NR) 6. In patients with aortic root/ascending aortic aneurysms or aortic dissection, in the absence of either a known family history of TAD or pathogenic/likely pathogenic variant, screening aortic imaging (as per recommendation 4) of first-degree relatives is recommended.(Level of Evidence: C-LD) 7. In patients with acute type A aortic dissection, the diameter of the aortic root and ascending aorta should be recorded in the operative note and medical record to inform the management of affected relatives.(Level of Evidence: C-EO) |
Recommendations for Inflammatory Aortitis: Diagnosis and Treatment of Takayasu Arteritis and GCA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1.In patients with large vessel vasculitis (LVV), prompt evaluation of the entire aorta and branch vessels with MRI or CT, with or without 18F-FDG positron emission tomography (FDG-PET), is recommended.(Level of Evidence: C-LD) |
Recommendations for Aortic Imaging Techniques to Determine Presence and Progression of Aortic Disease Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1.In patients with known or suspected aortic disease, aortic diameters should be measured at reproducible anatomic landmarks perpendicular to axis of blood flow, and these measurement methods should be reported in a clear and consistent manner. In cases of asymmetric or oval contour, the longest diam-eter and its perpendicular diameter should be reported(Level of Evidence: B-NR)
2. In patients with known or suspected aortic disease, episodic and cumulative ionizing radiation doses should be kept as low as feasible while maintaining diagnostic image quality.(Level of Evidence: C-LD) 3. In patients with known or suspected aortic disease, when performing CT or MR imaging, it is recommended that the root and ascending aortic diameters be measured from inner-edge to inner-edge, using an electrocardiographic-synchronized technique. If there are aortic wall abnormalities, such as atherosclerosis or discrete wall thickening (more common in the distal aorta), the outer-edge to outer-edge diameter should be reported (Table 4)(Level of Evidence: C-EO) 4. In patients with known or suspected aortic disease, the aortic root diameter should be recorded as maximum sinus to sinus mea-surement. In the setting of known asymmetry, multiple measurements should be reported, and both short- and long-axis images of the root should be obtained to avoid underestima-tion of the diameter.(Level of Evidence: C-EO) |
| Class IIa |
| 1.In patients with known or suspected aortic disease, it is reasonable that a dilated root or ascending aorta be indexed to patient height or BSA in the report, to aid in clinical risk assessment(Level of Evidence: C-LD)
2.In patients with known or suspected aortic disease, when performing echocardiography, it is reasonable to measure the aorta from leading-edge to leading-edge, perpendicular to the axis of blood flow.(Level of Evidence: C-EO) |
| Class IIb |
| 1. Using inner-edge to inner-edge measure-ments may also be considered, particularly on short-axis imaging.(Level of Evidence: C-EO) |
Recommendations for BAV Aortopathy Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with a BAV, TTE is indicated to evaluate valve morphology and function, to evaluate the diameter of the aortic root and ascending aorta, and to evaluate for aortic coarctation and other associated cardiovascular defects.(Level of Evidence: B-NR)
2. In patients with a BAV, CT or MRI of the thoracic aorta is indicated when the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE.(Level of Evidence: C-LD) 3. In patients with a BAV and either HTAD or phenotypic features concerning for Loeys-Dietz syndrome, a medical genetics evaluation is recommended(Level of Evidence: C-LD) 4. In patients with a BAV and a dilated aortic root or ascending aorta, screening of all first-degree relatives by TTE is recommended to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both; if the diameter and morphology of the aortic root, ascending aorta, or both cannot be assessed accurately or completely by TTE, a cardiac-gated CT or MRI of the thoracic aorta is indicated.(Level of Evidence: C-LD) |
| Class IIa |
| 1. In patients with a BAV, screening of all first-degree relatives by TTE is reasonable to evaluate for the presence of a BAV, dilation of the aortic root and ascending aorta, or both (Level of Evidence: B-NR) |
Treatment
Medical Therapy in Aortic Aneurysm
Focus is to reduce systemic blood pressure, inhibit MMP (zinc endopeptidases that degrade the extracellular matrix in aortic aneurysms)[24], and contain the progression of atherosclerosis.
- Beta-blockers may help in reducing the rate of expansion of the aortic aneurysm, reducing shear stress - studies have been mostly on Marfan patients and they found a low compliance with propranolol due to a significant effect on quality of life[24];
- Tetracyclines inhibit the MMP endopeptidases, and has been used in conditions in which MMP are overexpressed such as rheumatoid arthritis. There are studies in humans showing that doxycycline reduced the rate of expansion of aortic aneurysms. Roxithromycin, a macrolide has been also show to reduce the expansion of the aortic aneurysms.
- Statins may also be helpful due to their pleiotropic effecs, reducing the oxidative stress by blocking the reactive oxygen species on aneurysms, suppressing the NADH/NADPH oxidase system.
- Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers promotes vascular hypertrophy, cell proliferation and production of extracellular matrix. It also activates the NADH/NADPH oxidase system, both stimulating and inhibiting MMPs and degradation of extracellular matrix. There is a controversy of which class is more effective, and ongoing trials are being run to further clarify these questions.[24]
There are no established guidelines for this matter, treatment is still controversial and should be individualized.[25][26]
2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines[27]
Recommendations for BP Management in TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with TAA and an average systolic BP (SBP) of ≥130 mm Hg or an average diastolic BP (DBP) of ≥80 mm Hg, the use of antihypertensive medications is recommended to reduce risk of cardiovascular events(Level of Evidence: B-NR) |
| Class IIa |
| 2. In patients with TAA, regardless of cause and in the absence of contraindications, use of beta blockers to achieve target BP goals is reasonable.(Level of Evidence: C-LD)
3. In patients with TAA, regardless of etiology and in the absence of contraindications, ARB therapy is a reasonable adjunct to beta-blocker therapy to achieve target BP goals.(Level of Evidence: C-EO) |
Recommendations for Treatment of TAA With Statins
| Class IIa |
| 1. In patients with TAA and imaging or clinical evidence of atherosclerosis, statin therapy at moderate or high intensity is reasonable(Level of Evidence: C-LD) |
| Class IIb |
| 2. In patients with TAA who have no evidence of atherosclerosis, the use of statin therapy may be considered.(Level of Evidence: C-LD) |
Recommendation for Antiplatelet Therapy in TAA
| Class IIa |
| 1. In patients with atherosclerotic TAA and concomitant aortic atheroma or PAU, the use of low-dose aspirin is reasonable, unless contraindicated(Level of Evidence: C-EO) |
Recommendation for BP Management in AAA Referenced studies that support the recommendation are summarized in the Online Data Supplement
| Class I |
| 1. In patients with AAA and an average SBP of ≥130 mm Hg, or an average DBP of ≥80 mm Hg, the use of antihypertensive medication is recommended to reduce risk of cardiovascular events.(Level of Evidence: B-NR) |
Recommendations for Treatment of AAA With Statins Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with AAA and evidence of aortic atherosclerosis, statin therapy at moderate or high intensity is recommended.(Level of Evidence: B-NR) |
| Class IIb |
| 2. In patients with AAA but no evidence of atherosclerosis, statin therapy may be considered (Level of Evidence: C-LD) |
Recommendation for Antithrombotic Therapy in AAA
| Class IIb |
| 1. In patients with AAA with concomitant ather-oma and/or PAU, the use of low-dose aspirin may be considered, unless contraindicated (Level of Evidence: C-LD) |
Surgery in aortic aneurysm
2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines[28]
Recommendations for Surgery for Sporadic Aneurysms of the Aortic Root and Ascending AortaReferenced studies that support the recommendations are summarized in the Online Data supplement
| Class I |
| 1. In patients with aneurysms of the aortic root and ascending aorta who have symptoms attributable to the aneurysm, surgery is indicated(Level of Evidence: C-LD)
2. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maxi-mum diameter of ≥5.5 cm, surgery is indicated.(Level of Evidence: B-NR) 3. In patients with an aneurysm of the aortic root or ascending aorta of <5.5 cm, whose growth rate confirmed by tomographic imaging is ≥0.3 cm/y in 2 consecutive years, or ≥0.5 cm in 1 year, surgery is indicated(Level of Evidence: C-LD) |
| Class IIa |
| 4. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have a maximum diameter of ≥5.0 cm, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.(Level of Evidence: B-NR)
5. In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥4.5 cm, ascending aortic replacement is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.(Level of Evidence: B-NR) -In patients undergoing repair or replacement of a tricuspid aortic valve who have a concomitant aneurysm of the ascending aorta with a maximum diameter of ≥5.0 cm, ascending aortic replacement is reasonable(Level of Evidence: B-NR) 6. In patients with a height >1 standard deviation above or below the mean who have an asymptomatic aneurysm of the aortic root or ascending aorta and a maximal cross-sectional aortic area/height ratio of ≥10 cm2/m, surgery is reasonable when performed by experienced surgeons in a Multidisciplinary Aortic Team.(Level of Evidence: C-LD) |
| Class IIb |
| -In patients undergoing cardiac surgery for indications other than aortic valve repair or replacement who have a concomitant aneurysm of ascending aorta with a maxi-mum diameter of ≥5.0 cm, ascending aortic replacement may be reasonable.(Level of Evidence: C-LD)
7. In asymptomatic patients with aneurysms of the aortic root or ascending aorta who have either an ASI of ≥3.08 cm/m2 or AHI of ≥3.21 cm/m, surgery may be reasonable when per-formed by experienced surgeons in a Multidisciplinary Aortic Team.(Level of Evidence: C-LD) |
Recommendations for Surgical Approach for Patients With Sporadic Aneurysms of the Aortic Root and Ascending Aorta Meeting Criteria for SurgeryReferenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with an aneurysm isolated to the ascending aorta who meet criteria for surgery, aneurysm resection and replacement with an interposition graft should be performed.(Level of Evidence: B-NR)
2. In patients undergoing aortic valve repair or replacement with a concomitant ascending aortic aneurysm, a separate aortic valve intervention and ascending aortic graft is recommended.(Level of Evidence: B-NR) 3. In patients undergoing aortic root replacement with an aortic valve that is unsuitable for sparing or repair, a mechanical or biological valved conduit aortic root replacement is indicated.(Level of Evidence: B-NR) |
| Class IIa |
| 4. In patients undergoing aortic root replacement, valve-sparing aortic root replacement is reasonable if the aortic valve is suitable for sparing or repair and when performed by experienced surgeons in a Multidisciplinary Aortic Team |
Recommendations for Aortic Arch Aneurysms Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with an aortic arch aneurysm who have symptoms attributable to the aneurysm and are at low or intermediate operative risk, open surgical replacement is recommended.(Level of Evidence: C-EO) |
| Class IIa |
| 2. In patients with an isolated aortic arch aneurysm who are asymptomatic and have a low operative risk, open surgical replacement at an arch diameter of ≥5.5 cm is reasonable.(Level of Evidence: B-NR)
3. In patients undergoing open surgical repair of an ascending aortic aneurysm, if the aneurys-mal disease extends into the proximal aortic arch, it is reasonable to extend the repair with a hemiarch replacement.(Level of Evidence: C-LD) |
| Class IIb |
| 4. In patients undergoing open surgical repair of an aortic arch aneurysm, if the aneurysmal disease extends into the proximal descending thoracic aorta, an elephant trunk procedure may be considered.(Level of Evidence: C-LD)
5. In patients with an aortic arch aneurysm who are asymptomatic but meet criteria for inter-vention, but have a high risk from open surgi-cal repair, a hybrid or endovascular approach may be reasonable.(Level of Evidence: C-EO) |
Recommendations for Size Thresholds for Repair of Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with intact descending TAA, repair is recommended when the diameter is ≥5.5 cm.(Level of Evidence: B-NR) |
| Class IIb |
| 2. In patients with intact descending TAA and risk factors for rupture (Table 17), repair may be considered at a diameter of <5.5 cm(Level of Evidence: B-NR)
3. In patients at increased risk for perioperative morbidity and mortality (Table 18), it may be reasonable to increase the size threshold for surgery accordingly(Level of Evidence: B-NR) |
Recommendations for Endovascular Versus Open Repair of Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients without Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syndrome, who have a descending TAA that meets criteria for intervention and anatomy suitable for endovascular repair, TEVAR is recommended over open surgery(Level of Evidence: B-NR)
2. In patients with a descending TAA that meets criteria for repair with TEVAR, who have smaller or diseased access vessels, considerations for alternative vascular access are recommended.(Level of Evidence: B-NR) |
| Class IIa |
| 3. In patients with a descending TAA that meets criteria for intervention, who have anatomy unsuitable for endovascular repair, and who are without significant comorbidities and have a life expectancy of at least 10 years, open surgical repair is reasonable.(Level of Evidence: B-NR) |
Recommendations for Left Subclavian Artery Management Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with descending TAA who undergo TEVAR with planned left subclavian artery coverage, revascularization of the left subclavian artery before TEVAR is recommended to prevent spinal cord injury (SCI)1,2 and poten-tially to reduce stroke risk2 and prevent other ischemic complications(Level of Evidence: B-NR) |
| Class IIb |
| 2. In patients with descending TAA who have undergone TEVAR with left subclavian cover-age and develop SCI that is unresponsive to an increase in BP or a cerebrospinal fluid drain, left subclavian artery revascularization may be considered.(Level of Evidence: C-LD) |
Recommendation for Celiac Artery Management References that support the recommendation are included in the Online Data Supplement
| Class IIa |
| 1. In patients with descending TAA undergoing TEVAR in whom celiac artery coverage is being considered, it is reasonable to first con-firm adequate collateralization.(Level of Evidence: B-NR) |
Recommendations for Ruptured Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with ruptured descending TAA who are anatomic candidates for endovascular repair, TEVAR is recommended over open repair because of decreased perioperative death and morbidity.(Level of Evidence: B-NR) |
| Class IIb |
| 2. In patients with ruptured descending TAA undergoing TEVAR, intentional coverage of the left subclavian artery, celiac artery, or both may be considered to increase the landing zone for endovascular repair.(Level of Evidence: B-NR) |
Recommendations for Access Issues for TEVAR in Descending TAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with descending TAA undergoing TEVAR, review of preoperative CTA of the iliofemoral vessels should be performed to evaluate access.(Level of Evidence: B-NR)
2. In patients with descending TAA undergoing TEVAR, if iliac access is marginal or inade-quate to prevent access-related complications, the use of alternative conduits is recommended(Level of Evidence: B-NR) |
| Class IIa |
| 3. In patients with descending TAA undergo-ing TEVAR who have suitable anatomy, total percutaneous femoral access is a reasonable alternative to open surgi-cal cutdown to avoid access-related complications(Level of Evidence: B-NR) |
Recommendations for Size Thresholds for Open Surgical Repair of TAAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with intact degenerative TAAA, repair is recommended when the diameter is ≥6.0 cm.(Level of Evidence: B-NR) |
| Class IIa |
| 2. In patients with intact degenerative TAAA, repair is reasonable when the diameter is ≥5.5 cm and the repair is performed by experienced surgeons in a Multidisciplinary Aortic Team.(Level of Evidence: B-NR)
3. In patients with intact degenerative TAAA who have features associated with an increased risk of rupture (Table 19), repair is reasonable when the diameter is <5.5 cm(Level of Evidence: B-NR) |
Recommendations for Open Versus Endovascular Repair of TAAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
Ruptured TAAA
| Class I |
| 1. In patients with ruptured TAAA requiring inter-vention, open repair is recommended.(Level of Evidence: B-NR) |
| Class IIb |
| 2. In patients with ruptured TAAA requiring intervention, provided that the patient is hemodynamically stable, endovascular repair may be reasonable in centers with endovascular expertise and access to appropriate endovascular stent grafts(Level of Evidence: C-LD) |
Intact TAAA
| Class I |
| 3. In patients with Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehlers-Danlos syn-drome and intact TAAA requiring intervention, open repair is recommended over endovascular repair.(Level of Evidence: C-LD) |
| Class IIb |
| 4. In patients with intact degenerative TAAA and suitable anatomy, endovascular repair with fenestrated stent grafts, branched stent grafts, or both may be considered in centers with endovascular expertise and access to appropriate endovascular stent grafts.(Level of Evidence: B-NR) |
Recommendations for TAAA Spinal Cord Protection Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients undergoing open TAAA repair who are at high risk for SCI, cerebrospinal fluid drainage is recommended to reduce the incidence of temporary SCI, permanent SCI, or both.(Level of Evidence: A)
2. In patients who experience delayed spinal cord dysfunction after either open or endo-vascular TAAA repair, timely measures to optimize spinal cord perfusion and decrease intrathecal pressure are recommended (Table 20).(Level of Evidence: B-NR) |
Recommendations for TAAA Renal and Visceral Organ Protection Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients undergoing open repair of TAAA involving the renal arteries, cold blood or crystalloid renal perfusion is recommended to pro-vide effective protection against renal injury.(Level of Evidence: A)
2. In patients undergoing open or endovascular TAAA repair who have end-organ ischemia or significant stenoses from atherosclerotic visceral or renal artery disease, additional revascularization procedures are recommended(Level of Evidence: B-NR) |
Recommendation for Access During Endovascular Repair of AAA Referenced studies that support the recommendation are summarized in the Online Data Supplement
| Class I |
| 1. In patients undergoing endovascular repair of AAA who have suitable common femoral artery anatomy, ultrasound-guided percutaneous access and closure is recommended over open cutdown to reduce operative time, blood loss, length of stay, time to wound healing, and pain.(Level of Evidence: B-R) |
Recommendations for Repair of Ruptured AAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients presenting with ruptured AAA who are hemodynamically stable, CT imaging is recommended to evaluate whether the AAA is amenable to endovascular repair.(Level of Evidence: B-R)
2. In patients presenting with ruptured AAA who have suitable anatomy, endovascular repair is recommended over open repair to reduce the risk of morbidity and mortality.(Level of Evidence: B-R) |
| Class IIa |
| 3. In patients undergoing endovascular repair for ruptured AAA, local anesthesia is preferred to general anesthesia to reduce risk of perioperative mortality.(Level of Evidence: B-NR)
4. In patients with ruptured AAA, permissive hypotension can be beneficial to decrease the rate of bleeding(Level of Evidence: C-LD) |
Recommendations for the Threshold for AAA Repair Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with unruptured AAA, repair is recommended in those with a maximal aneurysm diameter of ≥5.5 cm in men or ≥5.0 cm in women(Level of Evidence: A)
2. In patients with unruptured AAA who have symptoms that are attributable to the aneurysm, repair is recommended to reduce the risk of rupture.(Level of Evidence: B-NR) |
| Class IIb |
| 3. In patients with unruptured saccular AAA, intervention to reduce the risk of rupture may be reasonable.(Level of Evidence: C-LD)
4. In patients with unruptured AAA and aneurysm growth of ≥0.5 cm in 6 months, repair to reduce the risk of rupture may be reason-able(Level of Evidence: C-LD) |
Recommendations for Open Versus Endovascular Repair of AAA Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. In patients with non ruptured AAA with low to moderate operative risk and who have anatomy suitable for either open or EVAR, a shared decision-making process weighing the risks and benefits of each approach is recommended.(Level of Evidence: A)
2. In patients undergoing elective endovascular repair for non ruptured AAA, adherence to manufacturer’s instructions for use is recommended.(Level of Evidence: B-NR) |
| Class IIa |
| 3. In patients with non ruptured AAA and a high perioperative risk, EVAR is reasonable to reduce the risk of 30-day morbidity, mortality, or both(Level of Evidence: B-NR)
4. For patients with non ruptured AAA, a moderate to high perioperative risk, and anatomy suitable for an FDA-approved fenestrated endovascular device, endovascular repair is reasonable over open repair to reduce the risk of perioperative complications.(Level of Evidence: B-NR) |
Recommendations for the Treatment of Concomitant Common Iliac Aneurysms Referenced studies that support the recommendations are summarized in the Online Data Supplement
| Class I |
| 1. For patients with asymptomatic small AAA and concomitant common iliac artery aneurysm(s) ≥3.5 cm, elective repair of both abdominal and iliac aneurysms is recommended.(Level of Evidence: C-LD)
2. When treating common iliac artery aneurysms or ectasia as part of AAA repair, preservation of at least 1 hypogastric artery is recommended, if anatomically feasible, to decrease the risk of pelvic ischemia.(Level of Evidence: B-NR) |
Decision to perform elective surgery to prevent aneurysm rupture is complicated as there must be an appropriate patient selection and timing for repair of the aneurysm which demands selecting patients at the greatest risk of aneurysm rupture. Once rupture occurs, mortality is extremely high. Fatality rates of emergency surgical repair is 50% if the patient manages to reach the hospital, in comparison to 1-5% fatality rate in elective surgical repair.[29]
According to the 2005 AHA/ACC guidelines - it is recommended surgical repair of abdominal aortic aneurysms:
- 5.5 cm in diameter or greater in asymptomatic patients;
- Increase by 0.5 cm or greater in diameter in 6 months;
- Symptomatic aneurysms.
Endovascular repair may be performed with better short-term morbidity and mortality rates but with failed long-term benefits over surgical repair. Endovascular is preferred in high-risk patients while surgical repair is generally indicated for low/average-risk patients.[29]
In thoracic aortic aneurysms, surgery is indicated in Marfan's syndrome when the aortic diameter reaches 5.0cm, or the rate of increase of the aortic root diameter approaches 1.0 cm per year, or progressive and severe aortic regurgitation. If family history is positive for aortic aneurysms, aggressive therapy may be indicated in individuals with Marfan and Loeys Dietz syndrome. Surgery consists in replacing the affected portion of the aorta. [26]
Prevention
Smoking cessation is an important measure to prevent aortic aneurysm progression and rupture, as is control of the other cardiovascular risks, such as hypertension, sedentarism and dyslipidemia.[17]
Recommendation for Smoking Cessation in AAA
| Class I |
| 1. In patients with AAA who smoke cigarettes, smoking cessation efforts are recommended(Level of Evidence: B-NR) |
Recommendation for Smoking Cessation in TAA
| Class I |
| 1. In patients with TAA who smoke cigarettes, smoking cessation efforts are recommended.(Level of Evidence: C-LD) |
Related Chapters
References
- ↑ 1.0 1.1 1.2 1.3 Kuivaniemi, Helena, et al. "Understanding the pathogenesis of abdominal aortic aneurysms." Expert review of cardiovascular therapy 13.9 (2015): 975-987.
- ↑ 2.0 2.1 Radiopaedia - Thoracic Aortic Aneurysms - https://radiopaedia.org/articles/thoracic-aortic-aneurysm?lang=us accessed at 06/08/2020
- ↑ 3.0 3.1 3.2 Frederick, John R., and Y. Joseph Woo. "Thoracoabdominal aortic aneurysm." Annals of cardiothoracic surgery 1.3 (2012): 277.
- ↑ Livesay, James J., Gregory N. Messner, and William K. Vaughn. "Milestones in treatment of aortic aneurysm: Denton A. Cooley, MD, and the Texas Heart Institute." Texas Heart Institute Journal 32.2 (2005): 130.
- ↑ 5.0 5.1 5.2 Kuivaniemi, Helena, Chris D. Platsoucas, and M. David Tilson III. "Aortic aneurysms: an immune disease with a strong genetic component." Circulation 117.2 (2008): 242-252.
- ↑ Bhandari, R., Kanthi, Y. - The Genetics of Aortic Aneurysms - The American College of Cardiology - available at:https://www.acc.org/latest-in-cardiology/articles/2018/05/02/12/52/the-genetics-of-aortic-aneurysms accessed at 06/08/2020
- ↑ Ruddy JM, Jones JA, Ikonomidis JS. Pathophysiology of thoracic aortic aneurysm (TAA): is it not one uniform aorta? Role of embryologic origin. Progress in cardiovascular diseases. 2013;56(1):68–73.
- ↑ Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxidants & redox signaling. 2011;15(7):1927–1943.
- ↑ Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10.
- ↑ Gadson PF, Jr, Dalton ML, Patterson E, et al. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-beta1: regulation of c-myb and alpha1 (I) procollagen genes. Experimental cell research. 1997;230(2):169–180.
- ↑ Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circulation research. 1969;25(6):677–686.
- ↑ Ailawadi G, Eliason JL, Upchurch GR Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg 2003;38:584-8.
- ↑ Hiller, H. G., and N. R. F. Lagattolla. "Thoracic aortic aneurysm presenting with dysphagia: a fatal delay in diagnosis." Thoracic surgical science 4 (2007).
- ↑ 14.0 14.1 Abdominal Aortic Aneurysm (AAA) Symptoms - Stanford Healthcare https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/abdominal-aortic-aneurysm/symptoms.html - accessed at 06/08/2020
- ↑ Kent, K. Craig. "Abdominal aortic aneurysms." New England journal of medicine 371.22 (2014): 2101-2108.
- ↑ Thoracic Aneurysm Differential Diagnoses - Medscape available at: https://emedicine.medscape.com/article/761627-differential - accessed at 06/08/2020
- ↑ 17.0 17.1 17.2 Abdominal Aortic Aneurysm - Mayo Clinichttps://www.mayoclinic.org/diseases-conditions/abdominal-aortic-aneurysm/symptoms-causes/syc-20350688 - accessed at 06/08/2020
- ↑ 18.0 18.1 Ernst, Calvin B. "Abdominal aortic aneurysm." New England Journal of Medicine 328.16 (1993): 1167-1172.
- ↑ Thoracic Aortic Aneurysm - Mayo Clinic available at: https://www.mayoclinic.org/diseases-conditions/thoracic-aortic-aneurysm/symptoms-causes/syc-20350188 - accessed at 06/08/2020
- ↑ Juvonen T, Ergin MA, Galla JD, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg 1997;63:1533-45
- ↑ Aortic Aneurysm: Symptoms and Complications - VeryWell Health available at: https://www.verywellhealth.com/aortic-aneurysm-symptoms-and-complications-4160769 - accessed at 06/08/2020
- ↑ Radiopaedia - Abdominal Aortic Aneurysms https://radiopaedia.org/articles/abdominal-aortic-aneurysm?lang=us Accessed at 06/08/2020
- ↑ Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW; et al. (2022). "2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines". J Am Coll Cardiol. doi:10.1016/j.jacc.2022.08.004. PMID 36334952 Check
|pmid=value (help). - ↑ 24.0 24.1 24.2 Danyi, Peter, John A. Elefteriades, and Ion S. Jovin. "Medical therapy of thoracic aortic aneurysms: are we there yet?." Circulation 124.13 (2011): 1469-1476.
- ↑ Yoshimura, Koichi, et al. "Current status and perspectives on pharmacologic therapy for abdominal aortic aneurysm." Current drug targets 19.11 (2018): 1265-1275.
- ↑ 26.0 26.1 Clift, Paul F., and Elena Cervi. "A review of thoracic aortic aneurysm disease." Echo Research and Practice 7.1 (2020): R1-R10.
- ↑ Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW; et al. (2022). "2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines". J Am Coll Cardiol. doi:10.1016/j.jacc.2022.08.004. PMID 36334952 Check
|pmid=value (help). - ↑ Writing Committee Members. Isselbacher EM, Preventza O, Hamilton Black J, Augoustides JG, Beck AW; et al. (2022). "2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines". J Am Coll Cardiol. doi:10.1016/j.jacc.2022.08.004. PMID 36334952 Check
|pmid=value (help). - ↑ 29.0 29.1 Aggarwal, Sourabh, et al. "Abdominal aortic aneurysm: A comprehensive review." Experimental & Clinical Cardiology 16.1 (2011): 11.