Zollinger-Ellison syndrome pathophysiology: Difference between revisions
Jump to navigation
Jump to search
| (34 intermediate revisions by 5 users not shown) | |||
| Line 4: | Line 4: | ||
{{CMG}} {{AE}} {{ARK}} {{MJK}} | {{CMG}} {{AE}} {{ARK}} {{MJK}} | ||
==Overview== | ==Overview== | ||
Zollinger-Ellison syndrome results from increased levels of [[gastrin]] due to an existing [[gastrinoma]] in the [[duodenum]] or [[pancreas]]. | |||
== | ==Pathophysiology== | ||
* | |||
* | === Physiology === | ||
* | |||
* | * Chemotransmitters, which are delivered to the [[gastric mucosa]], have a main role in the stimulation and inhibition of [[gastric acid]] and [[pepsin]] production.<ref name="pmid18474247">{{cite journal| author=Schubert ML, Peura DA| title=Control of gastric acid secretion in health and disease. | journal=Gastroenterology | year= 2008 | volume= 134 | issue= 7 | pages= 1842-60 | pmid=18474247 | doi=10.1053/j.gastro.2008.05.021 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18474247 }} </ref> | ||
* | *[[Gastric acid]] is responsible for [[protein]] [[digestion]], absorption of [[calcium]], [[iron]], [[vitamin B12]], [[Thyroid hormone|thyroid hormones]] and some drugs ([[itraconazole]] and [[ketoconazole]]).<ref name="pmid25040647">{{cite journal| author=Irving SA, Vadiveloo T, Leese GP| title=Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS). | journal=Clin Endocrinol (Oxf) | year= 2015 | volume= 82 | issue= 1 | pages= 136-41 | pmid=25040647 | doi=10.1111/cen.12559 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25040647 }} </ref> | ||
*[[Gastric acid]] is responsible for lowering [[Stomach|gastric]] [[pH]]. | |||
*[[Acid|Acidic]] [[pH]] kills [[Microorganism|microorganisms]], reduces [[bacterial growth]], and prevents [[Intestine|intestinal]] [[infection]] and [[bacterial peritonitis]].<ref name="pmid25151556">{{cite journal| author=Hegarty JP, Sangster W, Harris LR, Stewart DB| title=Proton pump inhibitors induce changes in colonocyte gene expression that may affect Clostridium difficile infection. | journal=Surgery | year= 2014 | volume= 156 | issue= 4 | pages= 972-8 | pmid=25151556 | doi=10.1016/j.surg.2014.06.074 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25151556 }} </ref><ref name="pmid24674763">{{cite journal| author=Buendgens L, Bruensing J, Matthes M, Dückers H, Luedde T, Trautwein C et al.| title=Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. | journal=J Crit Care | year= 2014 | volume= 29 | issue= 4 | pages= 696.e11-5 | pmid=24674763 | doi=10.1016/j.jcrc.2014.03.002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24674763 }} </ref> | |||
*[[Gastric acid|Acid]] [[secretion]] has 3 phases:<ref name="pmid15703599">{{cite journal| author=Schubert ML| title=Gastric secretion. | journal=Curr Opin Gastroenterol | year= 2003 | volume= 19 | issue= 6 | pages= 519-25 | pmid=15703599 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15703599 }} </ref> | |||
:1. Cephalic | |||
:*Mediated by [[Vagus nerve|vagal]] stimulation during thinking about, smelling, and seeing food. | |||
:2. Gastric | |||
:*The major mediator for acid secretion due to [[stomach]] distension and [[chemical]] effects related to the food. | |||
:3. Intestinal | |||
:*Small mediator for acid secretion due to chemical effects of food | |||
* Acid secretion mediated by some pathways:<ref name="pmid16149129">{{cite journal| author=Geibel JP| title=Role of potassium in acid secretion. | journal=World J Gastroenterol | year= 2005 | volume= 11 | issue= 34 | pages= 5259-65 | pmid=16149129 | doi= | pmc=4622792 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16149129 }} </ref><ref name="pmid17928547">{{cite journal| author=Heitzmann D, Warth R| title=No potassium, no acid: K+ channels and gastric acid secretion. | journal=Physiology (Bethesda) | year= 2007 | volume= 22 | issue= | pages= 335-41 | pmid=17928547 | doi=10.1152/physiol.00016.2007 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17928547 }} </ref> | |||
**[[Parietal cell|Parietal cells]] | |||
*** Contains the hydrogen-potassium-ATPase acid-secreting pump which controls acid secretion | |||
**[[Gastrin]]<ref name="pmid24279703">{{cite journal| author=Waldum HL, Hauso Ø, Fossmark R| title=The regulation of gastric acid secretion - clinical perspectives. | journal=Acta Physiol (Oxf) | year= 2014 | volume= 210 | issue= 2 | pages= 239-56 | pmid=24279703 | doi=10.1111/apha.12208 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24279703 }} </ref> | |||
*** Main [[hormone]] involved in acid secretion | |||
***[[G cell|Gastrin-expressing cells (G cells)]] are located in the [[antrum]] and are responsible for [[gastrin]] [[secretion]]. | |||
***[[Gastrin]] stimulates [[gastrin]] [[secretion]] from [[Parietal cell|parietal cells]] by [[histamine]] release from [[Enterochromaffin cells|enterochromaffin-like (ECL) cells]]. | |||
***[[Gastrin]] activates [[Cholecystokinin receptor|cholecystokinin (CCK) 2 receptor]] and somatostatin-secreting D cells.<ref name="pmid2859810">{{cite journal| author=Soll AH, Amirian DA, Park J, Elashoff JD, Yamada T| title=Cholecystokinin potently releases somatostatin from canine fundic mucosal cells in short-term culture. | journal=Am J Physiol | year= 1985 | volume= 248 | issue= 5 Pt 1 | pages= G569-73 | pmid=2859810 | doi=10.1152/ajpgi.1985.248.5.G569 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2859810 }} </ref><ref name="pmid1373504">{{cite journal| author=Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY et al.| title=Expression cloning and characterization of the canine parietal cell gastrin receptor. | journal=Proc Natl Acad Sci U S A | year= 1992 | volume= 89 | issue= 8 | pages= 3605-9 | pmid=1373504 | doi= | pmc=48917 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1373504 }} </ref> | |||
* Acid secretion is stimulated by [[histamine]] release, [[gastrin]] release, and [[acetylcholine]] release.<ref name="pmid7502535">{{cite journal| author=Sachs G, Prinz C, Loo D, Bamberg K, Besancon M, Shin JM| title=Gastric acid secretion: activation and inhibition. | journal=Yale J Biol Med | year= 1994 | volume= 67 | issue= 3-4 | pages= 81-95 | pmid=7502535 | doi= | pmc=2588922 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7502535 }} </ref>. | |||
* Acid secretion is inhibited by somatostatin secretion from oxyntic glands and antral D cells. | |||
===Pathogenesis=== | |||
*[[Embryology|Embryologic]] [[endoderm]] produces [[enteroendocrine cells]] and these [[Cell (biology)|cells]] are considered as the origin of [[Gastrinoma|gastrinomas]].<ref name="pmid7904550">{{cite journal| author=Norton JA| title=Neuroendocrine tumors of the pancreas and duodenum. | journal=Curr Probl Surg | year= 1994 | volume= 31 | issue= 2 | pages= 77-156 | pmid=7904550 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7904550 }}</ref> | |||
* Symptoms of Zollinger-Ellison syndrome are related to hypergastrinemia.<ref name="pmid17108778">{{cite journal| author=Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT| title=Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. | journal=Medicine (Baltimore) | year= 2006 | volume= 85 | issue= 6 | pages= 295-330 | pmid=17108778 | doi=10.1097/01.md.0000236956.74128.76 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17108778 }}</ref> | |||
*[[Hypertrophy (medical)|Hypertrophy]] of [[gastrin]] [[Mucous membrane|mucosa]] results in hypergastrinemia. | |||
*[[Gastric acid]] [[secretion]] increases four to six-fold. | |||
* Hypergastrinemia results from increase activity of parietal cells and histamine-secreting enterochromaffin-like cells. | |||
*Gastric acid secretion overrides the mucosal defense of the [[gastric]] and [[duodenal]] wall which may cause [[ulceration]] and inactivation of [[pancreatic]] enzymes. | |||
*The majority of patients have large and multiple peptic ulcers located in distal duodenum and proximal jejunum.<ref name="pmid7439637">{{cite journal| author=McGuigan JE, Wolfe MM| title=Secretin injection test in the diagnosis of gastrinoma. | journal=Gastroenterology | year= 1980 | volume= 79 | issue= 6 | pages= 1324-31 | pmid=7439637 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7439637 }}</ref> | |||
*Inactivation of pancreatic enzymes leads to fat [[malabsorption]] and [[diarrhea]].<ref name="urlGastrinoma - StatPearls - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK441842/ |title=Gastrinoma - StatPearls - NCBI Bookshelf |format= |work= |accessdate=}}</ref> | |||
*High gastric acid secretion does not reabsorb in small intestine and colon; therefore, it results in chronic diarrhea.<ref name="pmid17108778" /> | |||
*Sodium and water do not reabsorb in presence of high volume of gastric acids which results in secretory diarrhea. | |||
*The major factors related to fat malabsorption are as following:<ref name="pmid6824402">{{cite journal| author=King CE, Toskes PP| title=Nutrient malabsorption in the Zollinger-Ellison syndrome. Normalization during long-term cimetidine therapy. | journal=Arch Intern Med | year= 1983 | volume= 143 | issue= 2 | pages= 349-51 | pmid=6824402 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6824402 }} </ref> | |||
** Gastric mucosal damage | |||
** Inactivation of Pancreatic enzymes | |||
** Bile salts precipitation | |||
==Genetics== | ==Genetics== | ||
*Approximately | *Approximately 75% of Zollinger-Ellison syndrome (ZES) patients develop sporadically. <ref name="pmid22723327">{{cite journal| author=Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR et al.| title=Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). | journal=J Clin Endocrinol Metab | year= 2012 | volume= 97 | issue= 9 | pages= 2990-3011 | pmid=22723327 | doi=10.1210/jc.2012-1230 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22723327 }} </ref> | ||
*Approximately 25% of patients are associated with [[MEN, type 1|Multiple Endocrine Neoplasia-type 1 syndrome]].<ref name="pmid171087783">{{cite journal| author=Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT| title=Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. | journal=Medicine (Baltimore) | year= 2006 | volume= 85 | issue= 6 | pages= 295-330 | pmid=17108778 | doi=10.1097/01.md.0000236956.74128.76 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17108778 }}</ref> | |||
*[[MEN, type 1|MEN-1]]<nowiki/> is considered as an [[Autosomal dominant inheritance|autosomal dominant]] disorder defining by tumors of the [[pituitary]], the [[parathyroid]], and the [[pancreas]].<ref name="pmid171087782">{{cite journal| author=Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT| title=Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. | journal=Medicine (Baltimore) | year= 2006 | volume= 85 | issue= 6 | pages= 295-330 | pmid=17108778 | doi=10.1097/01.md.0000236956.74128.76 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17108778 }}</ref> | |||
*[[MEN1]] results from [[mutations]] in an 10-exon [[gene]] at [[chromosome 11]]q13. <ref name="pmid23363383">{{cite journal |vauthors=Ito T, Igarashi H, Uehara H, Jensen RT |title=Pharmacotherapy of Zollinger-Ellison syndrome |journal=Expert Opin Pharmacother |volume=14 |issue=3 |pages=307–21 |year=2013 |pmid=23363383 |pmc=3580316 |doi=10.1517/14656566.2013.767332 |url=}}</ref> | |||
==Associated Conditions== | ==Associated Conditions== | ||
*Multiple endocrine neoplasia type 1 ([[MEN 1]]) | *[[Multiple endocrine neoplasia type 1]] ([[MEN 1]])<ref name="pmid9354421">{{cite journal| author=Zhuang Z, Vortmeyer AO, Pack S, Huang S, Pham TA, Wang C et al.| title=Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas. | journal=Cancer Res | year= 1997 | volume= 57 | issue= 21 | pages= 4682-6 | pmid=9354421 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9354421 }}</ref> | ||
*[[Gastrinoma]] | *[[Gastrinoma]] (duodenal gastrinoma and pancreatic gastrinomas)<ref name="pmid79045502">{{cite journal| author=Norton JA| title=Neuroendocrine tumors of the pancreas and duodenum. | journal=Curr Probl Surg | year= 1994 | volume= 31 | issue= 2 | pages= 77-156 | pmid=7904550 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7904550 }}</ref> | ||
*Usually these gastriomas are small (less < 1 cm), multiple ones,less often to metastasize to liver rather than pancreatic gastrinomas. | |||
*[[Peptic ulcer disease]] | *[[Peptic ulcer disease]] | ||
==Gross Pathology== | ==Gross Pathology== | ||
*Gross pathology presents as enlarged fundic mucosal folds with cerebriform pattern. | *Gross pathology presents as enlarged [[Fundus (stomach)|fundic mucosal folds]] with cerebriform pattern. | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
* | *A well-differentiated [[neuroendocrine tumor]] (NET) histologically typically shows an organ like arrangement of cells with nesting, trabecular, or gyriform patterns. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
*The tumor cells are round with regular bland nuclei | *The tumor cells are usually round with regular bland nuclei which produce large number of secretory granules with diffuse immunoexpression of [[neuroendocrine]] markers where as, the poorly differentiated [[neuroendocrine tumor]] (NET) shows a atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules, and limited biomarker immunoexpression. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
* | *Immunostaining for [[chromogranin A]] and [[synaptophysin]] is an important step in the diagnosis of [[neuroendocrine]] tumors. In order to differentiate from other [[neuroendocrine tumors]] [[gastrin]] [[immunostaining]] may be used. [[somatostatin]] [[scintigraphy]] is considered an effective localizing tool as [[Gastrinoma|gastrinomas]] tend to express a high density of [[somatostatin]] receptors. <ref name="pmid28722872">{{cite journal |vauthors=Cingam S, Karanchi H |title= |journal= |volume= |issue= |pages= |year= |pmid=28722872 |doi= |url=}}</ref> | ||
[[image:ZES_NET_Duodenum.jpg|thumb|500px|center|By Ed Uthman from Houston, TX, USA [CC BY 2.0 (http://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons]] | |||
==References== | ==References== | ||
Latest revision as of 01:55, 12 September 2019
|
Zollinger-Ellison syndrome Microchapters |
|
Differentiating Zollinger-Ellison syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Zollinger-Ellison syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Zollinger-Ellison syndrome pathophysiology |
|
Risk calculators and risk factors for Zollinger-Ellison syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Aravind Reddy Kothagadi M.B.B.S[2] Mohamad Alkateb, MBBCh [3]
Overview
Zollinger-Ellison syndrome results from increased levels of gastrin due to an existing gastrinoma in the duodenum or pancreas.
Pathophysiology
Physiology
- Chemotransmitters, which are delivered to the gastric mucosa, have a main role in the stimulation and inhibition of gastric acid and pepsin production.[1]
- Gastric acid is responsible for protein digestion, absorption of calcium, iron, vitamin B12, thyroid hormones and some drugs (itraconazole and ketoconazole).[2]
- Gastric acid is responsible for lowering gastric pH.
- Acidic pH kills microorganisms, reduces bacterial growth, and prevents intestinal infection and bacterial peritonitis.[3][4]
- Acid secretion has 3 phases:[5]
- 1. Cephalic
- Mediated by vagal stimulation during thinking about, smelling, and seeing food.
- 2. Gastric
- 3. Intestinal
- Small mediator for acid secretion due to chemical effects of food
- Acid secretion mediated by some pathways:[6][7]
- Parietal cells
- Contains the hydrogen-potassium-ATPase acid-secreting pump which controls acid secretion
- Gastrin[8]
- Main hormone involved in acid secretion
- Gastrin-expressing cells (G cells) are located in the antrum and are responsible for gastrin secretion.
- Gastrin stimulates gastrin secretion from parietal cells by histamine release from enterochromaffin-like (ECL) cells.
- Gastrin activates cholecystokinin (CCK) 2 receptor and somatostatin-secreting D cells.[9][10]
- Parietal cells
- Acid secretion is stimulated by histamine release, gastrin release, and acetylcholine release.[11].
- Acid secretion is inhibited by somatostatin secretion from oxyntic glands and antral D cells.
Pathogenesis
- Embryologic endoderm produces enteroendocrine cells and these cells are considered as the origin of gastrinomas.[12]
- Symptoms of Zollinger-Ellison syndrome are related to hypergastrinemia.[13]
- Hypertrophy of gastrin mucosa results in hypergastrinemia.
- Gastric acid secretion increases four to six-fold.
- Hypergastrinemia results from increase activity of parietal cells and histamine-secreting enterochromaffin-like cells.
- Gastric acid secretion overrides the mucosal defense of the gastric and duodenal wall which may cause ulceration and inactivation of pancreatic enzymes.
- The majority of patients have large and multiple peptic ulcers located in distal duodenum and proximal jejunum.[14]
- Inactivation of pancreatic enzymes leads to fat malabsorption and diarrhea.[15]
- High gastric acid secretion does not reabsorb in small intestine and colon; therefore, it results in chronic diarrhea.[13]
- Sodium and water do not reabsorb in presence of high volume of gastric acids which results in secretory diarrhea.
- The major factors related to fat malabsorption are as following:[16]
- Gastric mucosal damage
- Inactivation of Pancreatic enzymes
- Bile salts precipitation
Genetics
- Approximately 75% of Zollinger-Ellison syndrome (ZES) patients develop sporadically. [17]
- Approximately 25% of patients are associated with Multiple Endocrine Neoplasia-type 1 syndrome.[18]
- MEN-1 is considered as an autosomal dominant disorder defining by tumors of the pituitary, the parathyroid, and the pancreas.[19]
- MEN1 results from mutations in an 10-exon gene at chromosome 11q13. [20]
Associated Conditions
- Multiple endocrine neoplasia type 1 (MEN 1)[21]
- Gastrinoma (duodenal gastrinoma and pancreatic gastrinomas)[22]
- Usually these gastriomas are small (less < 1 cm), multiple ones,less often to metastasize to liver rather than pancreatic gastrinomas.
- Peptic ulcer disease
Gross Pathology
- Gross pathology presents as enlarged fundic mucosal folds with cerebriform pattern.
Microscopic Pathology
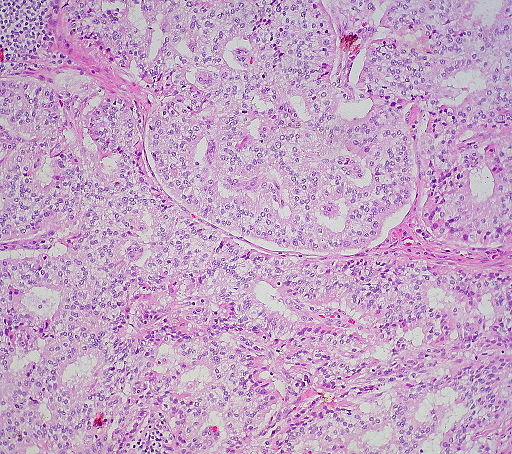
- A well-differentiated neuroendocrine tumor (NET) histologically typically shows an organ like arrangement of cells with nesting, trabecular, or gyriform patterns. [23]
- The tumor cells are usually round with regular bland nuclei which produce large number of secretory granules with diffuse immunoexpression of neuroendocrine markers where as, the poorly differentiated neuroendocrine tumor (NET) shows a atypical, sheet-like, diffuse and irregular nuclei, less cytoplasmic secretory granules, and limited biomarker immunoexpression. [23]
- Immunostaining for chromogranin A and synaptophysin is an important step in the diagnosis of neuroendocrine tumors. In order to differentiate from other neuroendocrine tumors gastrin immunostaining may be used. somatostatin scintigraphy is considered an effective localizing tool as gastrinomas tend to express a high density of somatostatin receptors. [23]
References
- ↑ Schubert ML, Peura DA (2008). "Control of gastric acid secretion in health and disease". Gastroenterology. 134 (7): 1842–60. doi:10.1053/j.gastro.2008.05.021. PMID 18474247.
- ↑ Irving SA, Vadiveloo T, Leese GP (2015). "Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS)". Clin Endocrinol (Oxf). 82 (1): 136–41. doi:10.1111/cen.12559. PMID 25040647.
- ↑ Hegarty JP, Sangster W, Harris LR, Stewart DB (2014). "Proton pump inhibitors induce changes in colonocyte gene expression that may affect Clostridium difficile infection". Surgery. 156 (4): 972–8. doi:10.1016/j.surg.2014.06.074. PMID 25151556.
- ↑ Buendgens L, Bruensing J, Matthes M, Dückers H, Luedde T, Trautwein C; et al. (2014). "Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea". J Crit Care. 29 (4): 696.e11–5. doi:10.1016/j.jcrc.2014.03.002. PMID 24674763.
- ↑ Schubert ML (2003). "Gastric secretion". Curr Opin Gastroenterol. 19 (6): 519–25. PMID 15703599.
- ↑ Geibel JP (2005). "Role of potassium in acid secretion". World J Gastroenterol. 11 (34): 5259–65. PMC 4622792. PMID 16149129.
- ↑ Heitzmann D, Warth R (2007). "No potassium, no acid: K+ channels and gastric acid secretion". Physiology (Bethesda). 22: 335–41. doi:10.1152/physiol.00016.2007. PMID 17928547.
- ↑ Waldum HL, Hauso Ø, Fossmark R (2014). "The regulation of gastric acid secretion - clinical perspectives". Acta Physiol (Oxf). 210 (2): 239–56. doi:10.1111/apha.12208. PMID 24279703.
- ↑ Soll AH, Amirian DA, Park J, Elashoff JD, Yamada T (1985). "Cholecystokinin potently releases somatostatin from canine fundic mucosal cells in short-term culture". Am J Physiol. 248 (5 Pt 1): G569–73. doi:10.1152/ajpgi.1985.248.5.G569. PMID 2859810.
- ↑ Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY; et al. (1992). "Expression cloning and characterization of the canine parietal cell gastrin receptor". Proc Natl Acad Sci U S A. 89 (8): 3605–9. PMC 48917. PMID 1373504.
- ↑ Sachs G, Prinz C, Loo D, Bamberg K, Besancon M, Shin JM (1994). "Gastric acid secretion: activation and inhibition". Yale J Biol Med. 67 (3–4): 81–95. PMC 2588922. PMID 7502535.
- ↑ Norton JA (1994). "Neuroendocrine tumors of the pancreas and duodenum". Curr Probl Surg. 31 (2): 77–156. PMID 7904550.
- ↑ 13.0 13.1 Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT (2006). "Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature". Medicine (Baltimore). 85 (6): 295–330. doi:10.1097/01.md.0000236956.74128.76. PMID 17108778.
- ↑ McGuigan JE, Wolfe MM (1980). "Secretin injection test in the diagnosis of gastrinoma". Gastroenterology. 79 (6): 1324–31. PMID 7439637.
- ↑ "Gastrinoma - StatPearls - NCBI Bookshelf".
- ↑ King CE, Toskes PP (1983). "Nutrient malabsorption in the Zollinger-Ellison syndrome. Normalization during long-term cimetidine therapy". Arch Intern Med. 143 (2): 349–51. PMID 6824402.
- ↑ Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR; et al. (2012). "Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1)". J Clin Endocrinol Metab. 97 (9): 2990–3011. doi:10.1210/jc.2012-1230. PMID 22723327.
- ↑ Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT (2006). "Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature". Medicine (Baltimore). 85 (6): 295–330. doi:10.1097/01.md.0000236956.74128.76. PMID 17108778.
- ↑ Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT (2006). "Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature". Medicine (Baltimore). 85 (6): 295–330. doi:10.1097/01.md.0000236956.74128.76. PMID 17108778.
- ↑ Ito T, Igarashi H, Uehara H, Jensen RT (2013). "Pharmacotherapy of Zollinger-Ellison syndrome". Expert Opin Pharmacother. 14 (3): 307–21. doi:10.1517/14656566.2013.767332. PMC 3580316. PMID 23363383.
- ↑ Zhuang Z, Vortmeyer AO, Pack S, Huang S, Pham TA, Wang C; et al. (1997). "Somatic mutations of the MEN1 tumor suppressor gene in sporadic gastrinomas and insulinomas". Cancer Res. 57 (21): 4682–6. PMID 9354421.
- ↑ Norton JA (1994). "Neuroendocrine tumors of the pancreas and duodenum". Curr Probl Surg. 31 (2): 77–156. PMID 7904550.
- ↑ 23.0 23.1 23.2 Cingam S, Karanchi H. PMID 28722872. Missing or empty
|title=(help)