Mesothelioma pathophysiology: Difference between revisions
Feham Tariq (talk | contribs) |
|||
| (44 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Mesothelioma}} | {{Mesothelioma}} | ||
{{CMG}}{{AE}}{{ | {{CMG}}; {{AE}}{{FT}},{{SR}} | ||
==Overview== | ==Overview== | ||
[[Asbestos]] causes DNA damage directly by mechanically interfering with the segregation of [[chromosomes]] during [[mitosis]] and indirectly by inducing [[mesothelial cells]] and [[macrophages]], to release mutagenic [[reactive oxygen]] and nitrogen species. Asbestos fibres have been shown to alter the function and secretory properties of [[macrophages]], ultimately creating conditions which favor the development of mesothelioma. Following asbestos [[phagocytosis]], macrophages generate increased amounts of hydroxyl radicals, which are normal by-products of cellular anaerobic metabolism. However, these free radicals are also known clastogenic and membrane-active agents thought to promote asbestos carcinogenicity. These oxidants can participate in the oncogenic process by directly and indirectly interacting with DNA, modifying membrane-associated cellular events, including oncogene activation and perturbation of cellular antioxidant defences. Genes involved in the pathogenesis of mesothelioma include ''[[BAP1]]'', ''[[CDKN2A]]'', ''[[WT1]]'', ''[[NF2 gene|NF2]]'', and ''[[TP53]]''. On gross pathology, pleural mesothelioma is characterized by discrete plaques and nodules that coalesce to produce a sheet-like tumor, with the pleural surface seeding of malignant mesothelioma cells. Based on the histology, mesothelioma may be classified into 3 subtypes: epithelial, sarcomatoid, and biphagic. Mesothelioma is demonstrated by positivity to [[tumor marker]]s, such as [[calretinin]], epithelial membrane antigen, [[cytokeratin]], and [[mesothelin]]. | |||
==Pathogenesis== | ==Pathogenesis== | ||
The pathogenesis of mesothelioma is influenced by the following factors:<ref name="pmid7448712">{{cite journal| author=Selikoff IJ, Hammond EC, Seidman H| title=Latency of asbestos disease among insulation workers in the United States and Canada. | journal=Cancer | year= 1980 | volume= 46 | issue= 12 | pages= 2736-40 | pmid=7448712 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7448712 }} </ref><ref name="pmid20068227">{{cite journal| author=Heintz NH, Janssen-Heininger YM, Mossman BT| title=Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. | journal=Am J Respir Cell Mol Biol | year= 2010 | volume= 42 | issue= 2 | pages= 133-9 | pmid=20068227 | doi=10.1165/rcmb.2009-0206TR | pmc=2822975 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20068227 }} </ref><ref name="ThomasChen2015">{{cite journal|last1=Thomas|first1=Anish|last2=Chen|first2=Yuanbin|last3=Yu|first3=Tinghui|last4=Gill|first4=Ammara|last5=Prasad|first5=Vinay|title=Distinctive clinical characteristics of malignant mesothelioma in young patients|journal=Oncotarget|volume=6|issue=18|year=2015|pages=16766–16773|issn=1949-2553|doi=10.18632/oncotarget.4414}}</ref><ref name="pmid12706492">{{cite journal| author=Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT| title=Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. | journal=Free Radic Biol Med | year= 2003 | volume= 34 | issue= 9 | pages= 1117-29 | pmid=12706492 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12706492 }} </ref> | |||
*Asbestos fibres | |||
*[[Radiation therapy|Radiation]] therapy | |||
*[[Smoking]] | |||
===Types of Asbestos fibres=== | |||
There are two types of asbestos fibers: | |||
* [[Amphibole]] (sharp, rod-like) | |||
* Serpentine | |||
=== Role of Asbestos fibers === | |||
[[Asbestos]] fibers play the following role in the development of mesothelioma: | |||
* The most common type of fibres found in US are the serpentine fibers, which are considered less [[Carcinogen|carcinogenic]] than the amphibole ones. | |||
* Following inhalation from the environment, these fibres are trapped in the lower third zone of the [[lung]]. | |||
* They are [[Phagocytosis|phagocytosed]] by the [[Mesothelium|mesothelial]] cells and initiate an [[oncogenic]] cascade of events which include: | |||
** Activation of c-Myc and c-Jun [[oncogene]] | |||
** Promotion of antiapoptotic genes such as [[Bcl-xL|Bcl]]-xL | |||
** Binding with epidermal growth factor receptor ([[EGFR]]) | |||
* [[Asbestos]] causes DNA damage directly by mechanically interfering with the segregation of [[chromosomes]] during [[mitosis]] and indirectly by inducing [[mesothelial cells]] and [[macrophages]], to release mutagenic [[reactive oxygen]] and nitrogen species. | |||
*Asbestos fibres have been shown to alter the function and secretory properties of [[macrophages]], ultimately creating conditions which favor the development of mesothelioma. | |||
*Following asbestos [[phagocytosis]], macrophages generate increased amounts of hydroxyl radicals, which are normal by-products of cellular anaerobic metabolism. | |||
*However, these free radicals are also known clastogenic and membrane-active agents thought to promote asbestos carcinogenicity. These oxidants can participate in the oncogenic process by directly and indirectly interacting with DNA, modifying membrane-associated cellular events, including oncogene activation and perturbation of cellular antioxidant defences. | |||
*The [[mesothelium]] consists of a single layer of flattened to cuboidal cells forming the [[epithelium|epithelial]] lining of the serous cavities of the body including the [[peritoneum|peritoneal]], [[pericardium|pericardial]] and [[pleura]]l cavities. | |||
*Deposition of asbestos fibres in the parenchyma of the lung may result in the penetration of the visceral pleura from where the fibre can then be carried to the pleural surface, thus leading to the development of malignant mesothelial plaques. | |||
*The processes leading to the development of peritoneal mesothelioma remain unresolved, although it has been proposed that asbestos fibres from the lung are transported to the abdomen and associated organs via the [[lymphatic system]]. | |||
*Additionally, asbestos fibres may be deposited in the gut after ingestion of sputum contaminated with asbestos fibres. | |||
*Asbestos also may possess [[immunosuppressant|immunosuppressive]] properties. For example, chrysotile fibres have been shown to depress the in-vitro proliferation of phytohemagglutinin-stimulated peripheral blood lymphocytes, suppress natural killer cell lysis, and significantly reduce [[lymphokine-activated killer cell]] viability and recovery. | |||
*Furthermore, genetic alterations in asbestos-activated macrophages may result in the release of potent mesothelial cell mitogens such as [[platelet-derived growth factor]] ([[PDGF]]) and [[transforming growth factor]]-β ([[TGF-β]]) which in turn, may induce the chronic stimulation and proliferation of mesothelial cells after injury by asbestos fibres. | |||
===Radiation therapy=== | |||
Survivors of radiation therapy have been found to develop mesothelioma according to several studies. Following cancers have been studied post-radiation therapy which are seen to develop mesothelioma:<ref name="pmid16174857">{{cite journal| author=Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H et al.| title=Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. | journal=J Natl Cancer Inst | year= 2005 | volume= 97 | issue= 18 | pages= 1354-65 | pmid=16174857 | doi=10.1093/jnci/dji278 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16174857 }} </ref><ref name="pmid14663775">{{cite journal| author=Matesich SM, Shapiro CL| title=Second cancers after breast cancer treatment. | journal=Semin Oncol | year= 2003 | volume= 30 | issue= 6 | pages= 740-8 | pmid=14663775 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14663775 }} </ref><ref name="pmid16708354">{{cite journal| author=Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK| title=The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. | journal=Cancer | year= 2006 | volume= 107 | issue= 1 | pages= 108-15 | pmid=16708354 | doi=10.1002/cncr.21971 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16708354 }} </ref> | |||
* Non-Hodgkins lymphoma<ref name="pmid8230284">{{cite journal| author=Travis LB, Curtis RE, Glimelius B, Holowaty E, Van Leeuwen FE, Lynch CF et al.| title=Second cancers among long-term survivors of non-Hodgkin's lymphoma. | journal=J Natl Cancer Inst | year= 1993 | volume= 85 | issue= 23 | pages= 1932-7 | pmid=8230284 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8230284 }} </ref> | |||
* Testicular cancer | |||
* Breast cancer | |||
===Smoking=== | |||
* Smoking has a synergistic effect on asbestos fibre inhalation in the pathogenesis of mesothelioma. | |||
==Genetics== | ==Genetics== | ||
*Development of mesothelioma is the result of multiple [[mutation|genetic mutations]]. | *Development of mesothelioma is the result of multiple [[mutation|genetic mutations]]. | ||
*Genes involved in the pathogenesis of mesothelioma include:<ref name=" | *Genes involved in the pathogenesis of mesothelioma include:<ref name="pmid15950811">{{cite journal| author=Ladanyi M| title=Implications of P16/CDKN2A deletion in pleural mesotheliomas. | journal=Lung Cancer | year= 2005 | volume= 49 Suppl 1 | issue= | pages= S95-8 | pmid=15950811 | doi=10.1016/j.lungcan.2005.03.017 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15950811 }} </ref><ref name="pmid9072005">{{cite journal| author=Kumar-Singh S, Segers K, Rodeck U, Backhovens H, Bogers J, Weyler J et al.| title=WT1 mutation in malignant mesothelioma and WT1 immunoreactivity in relation to p53 and growth factor receptor expression, cell-type transition, and prognosis. | journal=J Pathol | year= 1997 | volume= 181 | issue= 1 | pages= 67-74 | pmid=9072005 | doi=10.1002/(SICI)1096-9896(199701)181:1<67::AID-PATH723>3.0.CO;2-Z | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9072005 }} </ref><ref name="pmid22825583">{{cite journal| author=Ladanyi M, Zauderer MG, Krug LM, Ito T, McMillan R, Bott M et al.| title=New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment. | journal=Clin Cancer Res | year= 2012 | volume= 18 | issue= 17 | pages= 4485-90 | pmid=22825583 | doi=10.1158/1078-0432.CCR-11-2375 | pmc=PMC3432735 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22825583 }} </ref><ref name="pmid23435014">{{cite journal| author=Andujar P, Pairon JC, Renier A, Descatha A, Hysi I, Abd-Alsamad I et al.| title=Differential mutation profiles and similar intronic TP53 polymorphisms in asbestos-related lung cancer and pleural mesothelioma. | journal=Mutagenesis | year= 2013 | volume= 28 | issue= 3 | pages= 323-31 | pmid=23435014 | doi=10.1093/mutage/get008 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23435014 }} </ref><ref name="pmid18248818">{{cite journal| author=Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst-Hensch N, Stahel R et al.| title=PTEN expression is a strong predictor of survival in mesothelioma patients. | journal=Eur J Cardiothorac Surg | year= 2008 | volume= 33 | issue= 3 | pages= 502-6 | pmid=18248818 | doi=10.1016/j.ejcts.2007.09.045 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18248818 }} </ref><ref name="pmid21245096">{{cite journal| author=Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T et al.| title=LATS2 is a tumor suppressor gene of malignant mesothelioma. | journal=Cancer Res | year= 2011 | volume= 71 | issue= 3 | pages= 873-83 | pmid=21245096 | doi=10.1158/0008-5472.CAN-10-2164 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21245096 }} </ref> | ||
:*''[[BAP1]]'' | :*''[[BAP1]]'' | ||
:*''[[CDKN2A]]'' | :*''[[CDKN2A]]'' | ||
:*''[[WT1]]'' | |||
:*''[[NF2 gene|NF2]]'' | |||
:*''[[TP53]]'' | |||
:*''[[PTEN]]'' | |||
:*''MSLN'' | |||
:*''[[LATS2]]'' | |||
*[[Asbestos]] has also been shown to mediate the entry of foreign DNA into target cells. Incorporation of this foreign DNA may lead to [[mutation]]s and [[oncogenesis]], by several possible mechanisms: | |||
:*Inactivation of [[tumor suppressor gene]]s | |||
:*Activation of [[oncogene]]s | |||
:*Activation of [[oncogene#proto-oncogene|proto-oncogenes]] due to incorporation of foreign DNA containing a [[promoter]] region | |||
:*Activation of DNA repair enzymes, which may be prone to error | |||
:*Activation of [[telomerase]] | |||
:*Prevention of [[apoptosis]] | |||
==Gross Pathology== | |||
The following features are seen on gross pathology of mesothelioma:<ref name="epidemiologymesothelioma1">Mesothelioma. CGMH.ORG 2016. https://www1.cgmh.org.tw/intr/intr5/c6700/OBGYN/f/web/Mesothelioma/index.htm. Accessed on February 15, 2016</ref> | |||
*On gross pathology, [[Pleural cavity|pleural]] mesothelioma is characterized by discrete [[Plaque|plaques]] and nodules that coalesce to produce a sheet-like tumor, with the pleural surface seeding of [[malignant]] mesothelioma cells. | |||
*The growth usually starts at the inferior margins of the [[pleura]] and may invade the [[diaphragm]]. | |||
*The lung and interlobar [[Fissure|fissures]] may also be involved. | |||
* | |||
* | |||
* | |||
===Gallery=== | |||
<gallery> | |||
Image:Mesothelioma gross pathology image 1.jpg|<sub>Mesothelioma completely encasing the lung.<ref name=grosspictureofmesotheliomaimage1>Image courtesy of Dr. Yale Rosen. Radiopaedia (original file [http://radiopaedia.org/cases/mesothelioma-gross-pathology-1 here]). Creative Commons BY-SA-NC | |||
</ref></sub></gallery> | |||
==Microscopic Pathology== | |||
Based on the '''histology''', mesothelioma may be classified into 3 subtypes:<ref name="mesotheliomaclass1">Mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 8, 2016</ref> | |||
*Epithelial | |||
*Sarcomatoid | |||
*Biphasic (mixed) | |||
==Immunohistochemistry== | ==Immunohistochemistry== | ||
*The cytological and histological diagnosis can be difficult, with mesothelial hyperplasia and metastatic adenocarcinoma appearing similar. Specific markers may be helpful in the diagnosis of mesothelioma.<ref name=pathologymesotehelioma1>Pathology of mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 10, 2016</ref> | *The cytological and histological diagnosis can be difficult, with mesothelial hyperplasia and metastatic adenocarcinoma appearing similar. Specific markers may be helpful in the diagnosis of mesothelioma.<ref name="pathologymesotehelioma1">Pathology of mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 10, 2016</ref> | ||
*Mesothelioma is demonstrated by positivity to [[tumor marker]]s, such as:<ref name=pathologymesotehelioma1>Pathology of mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 10, 2016</ref><ref name=ihcmesothelioma1>Immunohistochemistry of mesothelioma. Wikipedia 2016. https://en.wikipedia.org/wiki/Mesothelioma. Accessed on February 12, 2016</ref> | *Mesothelioma is demonstrated by positivity to [[tumor marker]]s, such as:<ref name="pathologymesotehelioma1">Pathology of mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 10, 2016</ref><ref name="ihcmesothelioma1">Immunohistochemistry of mesothelioma. Wikipedia 2016. https://en.wikipedia.org/wiki/Mesothelioma. Accessed on February 12, 2016</ref> | ||
:*[[Calretinin]] | :*[[Calretinin]] | ||
:*Epithelial membrane antigen | :*Epithelial membrane antigen | ||
| Line 65: | Line 104: | ||
[[Category:Asbestos]] | [[Category:Asbestos]] | ||
[[Category:Occupational diseases]] | [[Category:Occupational diseases]] | ||
[[Category:Oncology]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
Latest revision as of 19:37, 9 March 2018
|
Mesothelioma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mesothelioma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Mesothelioma pathophysiology |
|
Risk calculators and risk factors for Mesothelioma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Feham Tariq, MD [2],Sujit Routray, M.D. [3]
Overview
Asbestos causes DNA damage directly by mechanically interfering with the segregation of chromosomes during mitosis and indirectly by inducing mesothelial cells and macrophages, to release mutagenic reactive oxygen and nitrogen species. Asbestos fibres have been shown to alter the function and secretory properties of macrophages, ultimately creating conditions which favor the development of mesothelioma. Following asbestos phagocytosis, macrophages generate increased amounts of hydroxyl radicals, which are normal by-products of cellular anaerobic metabolism. However, these free radicals are also known clastogenic and membrane-active agents thought to promote asbestos carcinogenicity. These oxidants can participate in the oncogenic process by directly and indirectly interacting with DNA, modifying membrane-associated cellular events, including oncogene activation and perturbation of cellular antioxidant defences. Genes involved in the pathogenesis of mesothelioma include BAP1, CDKN2A, WT1, NF2, and TP53. On gross pathology, pleural mesothelioma is characterized by discrete plaques and nodules that coalesce to produce a sheet-like tumor, with the pleural surface seeding of malignant mesothelioma cells. Based on the histology, mesothelioma may be classified into 3 subtypes: epithelial, sarcomatoid, and biphagic. Mesothelioma is demonstrated by positivity to tumor markers, such as calretinin, epithelial membrane antigen, cytokeratin, and mesothelin.
Pathogenesis
The pathogenesis of mesothelioma is influenced by the following factors:[1][2][3][4]
Types of Asbestos fibres
There are two types of asbestos fibers:
- Amphibole (sharp, rod-like)
- Serpentine
Role of Asbestos fibers
Asbestos fibers play the following role in the development of mesothelioma:
- The most common type of fibres found in US are the serpentine fibers, which are considered less carcinogenic than the amphibole ones.
- Following inhalation from the environment, these fibres are trapped in the lower third zone of the lung.
- They are phagocytosed by the mesothelial cells and initiate an oncogenic cascade of events which include:
- Asbestos causes DNA damage directly by mechanically interfering with the segregation of chromosomes during mitosis and indirectly by inducing mesothelial cells and macrophages, to release mutagenic reactive oxygen and nitrogen species.
- Asbestos fibres have been shown to alter the function and secretory properties of macrophages, ultimately creating conditions which favor the development of mesothelioma.
- Following asbestos phagocytosis, macrophages generate increased amounts of hydroxyl radicals, which are normal by-products of cellular anaerobic metabolism.
- However, these free radicals are also known clastogenic and membrane-active agents thought to promote asbestos carcinogenicity. These oxidants can participate in the oncogenic process by directly and indirectly interacting with DNA, modifying membrane-associated cellular events, including oncogene activation and perturbation of cellular antioxidant defences.
- The mesothelium consists of a single layer of flattened to cuboidal cells forming the epithelial lining of the serous cavities of the body including the peritoneal, pericardial and pleural cavities.
- Deposition of asbestos fibres in the parenchyma of the lung may result in the penetration of the visceral pleura from where the fibre can then be carried to the pleural surface, thus leading to the development of malignant mesothelial plaques.
- The processes leading to the development of peritoneal mesothelioma remain unresolved, although it has been proposed that asbestos fibres from the lung are transported to the abdomen and associated organs via the lymphatic system.
- Additionally, asbestos fibres may be deposited in the gut after ingestion of sputum contaminated with asbestos fibres.
- Asbestos also may possess immunosuppressive properties. For example, chrysotile fibres have been shown to depress the in-vitro proliferation of phytohemagglutinin-stimulated peripheral blood lymphocytes, suppress natural killer cell lysis, and significantly reduce lymphokine-activated killer cell viability and recovery.
- Furthermore, genetic alterations in asbestos-activated macrophages may result in the release of potent mesothelial cell mitogens such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) which in turn, may induce the chronic stimulation and proliferation of mesothelial cells after injury by asbestos fibres.
Radiation therapy
Survivors of radiation therapy have been found to develop mesothelioma according to several studies. Following cancers have been studied post-radiation therapy which are seen to develop mesothelioma:[5][6][7]
- Non-Hodgkins lymphoma[8]
- Testicular cancer
- Breast cancer
Smoking
- Smoking has a synergistic effect on asbestos fibre inhalation in the pathogenesis of mesothelioma.
Genetics
- Development of mesothelioma is the result of multiple genetic mutations.
- Genes involved in the pathogenesis of mesothelioma include:[9][10][11][12][13][14]
- Asbestos has also been shown to mediate the entry of foreign DNA into target cells. Incorporation of this foreign DNA may lead to mutations and oncogenesis, by several possible mechanisms:
- Inactivation of tumor suppressor genes
- Activation of oncogenes
- Activation of proto-oncogenes due to incorporation of foreign DNA containing a promoter region
- Activation of DNA repair enzymes, which may be prone to error
- Activation of telomerase
- Prevention of apoptosis
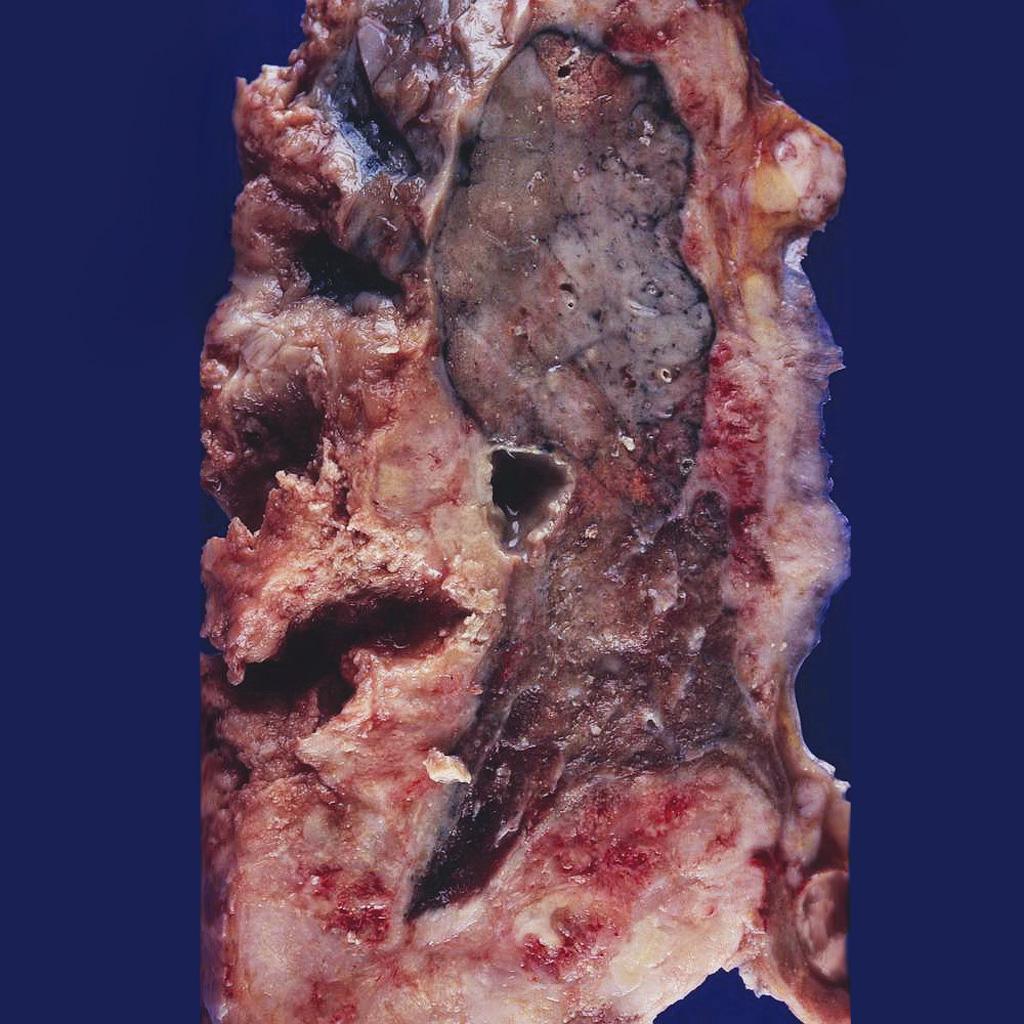
Gross Pathology
The following features are seen on gross pathology of mesothelioma:[15]
- On gross pathology, pleural mesothelioma is characterized by discrete plaques and nodules that coalesce to produce a sheet-like tumor, with the pleural surface seeding of malignant mesothelioma cells.
- The growth usually starts at the inferior margins of the pleura and may invade the diaphragm.
- The lung and interlobar fissures may also be involved.
Gallery
-
Mesothelioma completely encasing the lung.<ref name=grosspictureofmesotheliomaimage1>Image courtesy of Dr. Yale Rosen. Radiopaedia (original file here). Creative Commons BY-SA-NC
Microscopic Pathology
Based on the histology, mesothelioma may be classified into 3 subtypes:[16]
- Epithelial
- Sarcomatoid
- Biphasic (mixed)
Immunohistochemistry
- The cytological and histological diagnosis can be difficult, with mesothelial hyperplasia and metastatic adenocarcinoma appearing similar. Specific markers may be helpful in the diagnosis of mesothelioma.[17]
- Mesothelioma is demonstrated by positivity to tumor markers, such as:[17][18]
- Calretinin
- Epithelial membrane antigen
- Cytokeratin
- Mesothelin
- WT1
- Podoplanin
- Osteopontin
- HBME-1
References
- ↑ Selikoff IJ, Hammond EC, Seidman H (1980). "Latency of asbestos disease among insulation workers in the United States and Canada". Cancer. 46 (12): 2736–40. PMID 7448712.
- ↑ Heintz NH, Janssen-Heininger YM, Mossman BT (2010). "Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways". Am J Respir Cell Mol Biol. 42 (2): 133–9. doi:10.1165/rcmb.2009-0206TR. PMC 2822975. PMID 20068227.
- ↑ Thomas, Anish; Chen, Yuanbin; Yu, Tinghui; Gill, Ammara; Prasad, Vinay (2015). "Distinctive clinical characteristics of malignant mesothelioma in young patients". Oncotarget. 6 (18): 16766–16773. doi:10.18632/oncotarget.4414. ISSN 1949-2553.
- ↑ Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT (2003). "Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases". Free Radic Biol Med. 34 (9): 1117–29. PMID 12706492.
- ↑ Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H; et al. (2005). "Second cancers among 40,576 testicular cancer patients: focus on long-term survivors". J Natl Cancer Inst. 97 (18): 1354–65. doi:10.1093/jnci/dji278. PMID 16174857.
- ↑ Matesich SM, Shapiro CL (2003). "Second cancers after breast cancer treatment". Semin Oncol. 30 (6): 740–8. PMID 14663775.
- ↑ Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK (2006). "The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma". Cancer. 107 (1): 108–15. doi:10.1002/cncr.21971. PMID 16708354.
- ↑ Travis LB, Curtis RE, Glimelius B, Holowaty E, Van Leeuwen FE, Lynch CF; et al. (1993). "Second cancers among long-term survivors of non-Hodgkin's lymphoma". J Natl Cancer Inst. 85 (23): 1932–7. PMID 8230284.
- ↑ Ladanyi M (2005). "Implications of P16/CDKN2A deletion in pleural mesotheliomas". Lung Cancer. 49 Suppl 1: S95–8. doi:10.1016/j.lungcan.2005.03.017. PMID 15950811.
- ↑ Kumar-Singh S, Segers K, Rodeck U, Backhovens H, Bogers J, Weyler J; et al. (1997). "WT1 mutation in malignant mesothelioma and WT1 immunoreactivity in relation to p53 and growth factor receptor expression, cell-type transition, and prognosis". J Pathol. 181 (1): 67–74. doi:10.1002/(SICI)1096-9896(199701)181:1<67::AID-PATH723>3.0.CO;2-Z. PMID 9072005.
- ↑ Ladanyi M, Zauderer MG, Krug LM, Ito T, McMillan R, Bott M; et al. (2012). "New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment". Clin Cancer Res. 18 (17): 4485–90. doi:10.1158/1078-0432.CCR-11-2375. PMC 3432735. PMID 22825583.
- ↑ Andujar P, Pairon JC, Renier A, Descatha A, Hysi I, Abd-Alsamad I; et al. (2013). "Differential mutation profiles and similar intronic TP53 polymorphisms in asbestos-related lung cancer and pleural mesothelioma". Mutagenesis. 28 (3): 323–31. doi:10.1093/mutage/get008. PMID 23435014.
- ↑ Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst-Hensch N, Stahel R; et al. (2008). "PTEN expression is a strong predictor of survival in mesothelioma patients". Eur J Cardiothorac Surg. 33 (3): 502–6. doi:10.1016/j.ejcts.2007.09.045. PMID 18248818.
- ↑ Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T; et al. (2011). "LATS2 is a tumor suppressor gene of malignant mesothelioma". Cancer Res. 71 (3): 873–83. doi:10.1158/0008-5472.CAN-10-2164. PMID 21245096.
- ↑ Mesothelioma. CGMH.ORG 2016. https://www1.cgmh.org.tw/intr/intr5/c6700/OBGYN/f/web/Mesothelioma/index.htm. Accessed on February 15, 2016
- ↑ Mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 8, 2016
- ↑ 17.0 17.1 Pathology of mesothelioma. Dr Bruno Di Muzio and A.Prof Frank Gaillard et al. Radiopaedia 2016. http://radiopaedia.org/articles/mesothelioma. Accessed on February 10, 2016
- ↑ Immunohistochemistry of mesothelioma. Wikipedia 2016. https://en.wikipedia.org/wiki/Mesothelioma. Accessed on February 12, 2016