Porphyrin
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
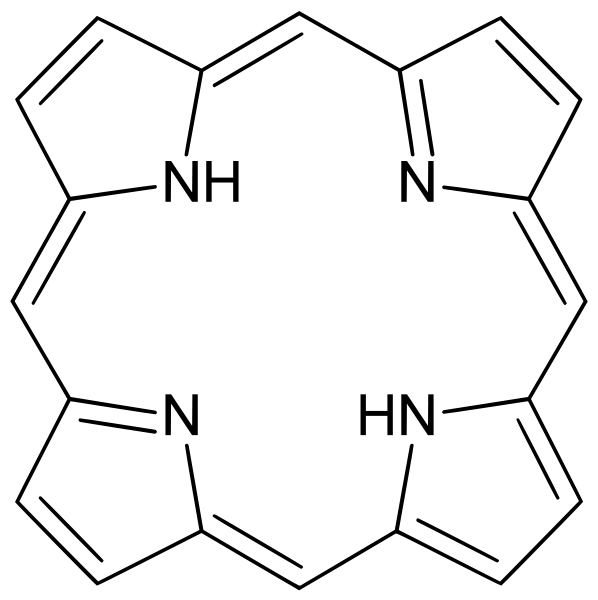
A porphyrin is a heterocyclic macrocycle derived from four pyrrole-like subunits interconnected via their α carbon atoms via methine bridges (=CH-). The macrocycle, therefore, is a highly conjugated system, and is consequently deeply coloured—the name porphyrin comes from a Greek word for purple. The macrocycle has 22 pi electrons. The parent porphyrin is porphine, and substituted porphines are called porphyrins. Many porphyrins occur in nature, such as in green leaves and red blood cells, and in bio-inspired synthetic catalysts and devices.
Complexes of porphyrins and related molecules
Porphyrins bind metals to form complexes. The metal ion, usually with a charge of 2+ or 3+, resides in the central N4 cavity formed by the loss of two protons. Most metals can be inserted. A schematic equation for these syntheses is shown:
- H2porphyrin + [MLn]2+ → M(porphyrinate)Ln-4 + 4 L + 2 H+
A porphyrin in which no metal is inserted in its cavity is sometimes called a free base. Some iron-containing porphyrins are called hemes; and heme-containing proteins, or hemoproteins, are found extensively in Nature. Hemoglobin and myoglobin are two O2-binding proteins that contain iron porphyrins.
Related to porphyrins are several other heterocycles, including corrins, chlorins, bacteriochlorophylls, and corphins. Chlorins (2,3-dihydroporphyrin) are more reduced, that contain more hydrogen, than porphyrins, featuring a pyrroline subunit. This structure occurs in chlorophyll. Replacement of two of the four pyrrolic subunits with pyrrolinic subunits results in either a bacteriochlorin (as found in some photosynthetic bacteria) or an isobacteriochlorin, depending on the relative positions of the reduced rings. Some porphyrin derivatives follow Hückel's rule, but most do not.
Laboratory synthesis
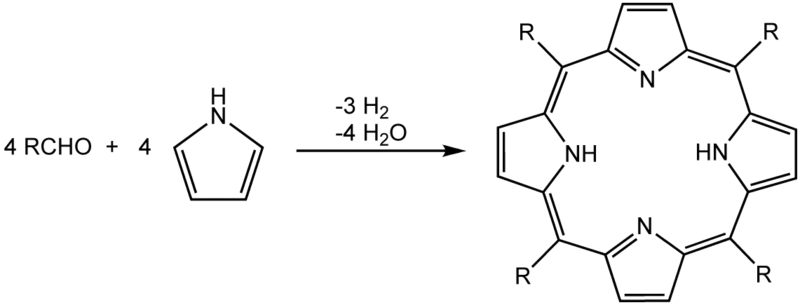
One of the more common syntheses for porphyrins is based on work by Paul Rothemund.[1][2] His techniques underpin more modern syntheses such as those described by Alder and Longo.[3] The synthesis of simple porphyrins such as meso-tetraphenylporphyrin is also commonly done in university teaching labs.[4]
In this method, porphyrins are assembled from pyrrole and substituted aldehydes. Acidic conditions are essential; formic acid, acetic acid, and propionic acid are typical reaction solvents, or p-toluenesulfonic acid can be used with a non-acidic solvent. Lewis acids such as boron trifluoride etherate and ytterbium triflate have also been known to catalyse porphyrin formation. A large amount of side product is formed and is removed, usually by chromatography.
Biosynthesis
The "committed step" for porphyrin biosynthesis is the formation of D-aminolevulinic acid (dALA) by the reaction of the amino acid glycine and succinyl-CoA, from the citric acid cycle. Two molecules of dALA combine to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM database. The porphyria associated with the deficiency of each enzyme is also shown:
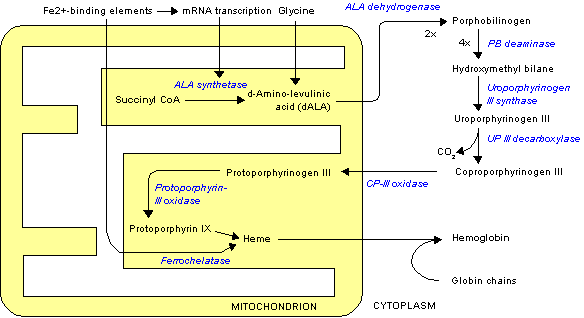
| Enzyme | substrate | Product | Chromosome | EC | OMIM | porphyria |
| ALA synthase | Glycine, succinyl CoA | D-Aminolevulinic acid | 3p21.1 | 2.3.1.37 | 125290 | none |
| ALA dehydratase | D-Aminolevulinic acid | Porphobilinogen | 9q34 | 4.2.1.24 | 125270 | ALA-Dehydratase deficiency |
| PBG deaminase | Porphobilinogen | Hydroxymethyl bilane | 11q23.3 | 2.5.1.61 | 176000 | acute intermittent porphyria |
| Uroporphyrinogen III synthase | Hydroxymethyl bilane | Uroporphyrinogen III | 10q25.2-q26.3 | 4.2.1.75 | 606938 | congenital erythropoietic porphyria |
| Uroporphyrinogen III decarboxylase | Uroporphyrinogen III | Coproporphyrinogen III | 1q34 | 4.1.1.37 | 176100 | porphyria cutanea tarda |
| Coproporphyrinogen III oxidase | Coproporphyrinogen III | Protoporphyrinogen IX | 3q12 | 1.3.3.3 | 121300 | coproporphyria |
| Protoporphyrinogen oxidase | Protoporphyrinogen IX | Protoporphyrin IX | 1q22 | 1.3.3.4 | 600923 | variegate porphyria |
| Ferrochelatase | Protoporphyrin IX | Heme | 18q21.3 | 4.99.1.1 | 177000 | erythropoietic protoporphyria |
Applications
Although natural porphyin complexes are essential for life, synthetic porphyrins and their complexes have limited utility. Complexes of meso-tetraphenylporphyrin, e.g. the iron-(III) chloride complex (TPPFeCl) catalyse a variety of reactions in organic chemistry, but none are of practical value. Porphyrin-based compounds are of interest in molecular electronics and supramolecular building blocks. Phthalocyanines, which are structurally related to porphyrins, are used in commerce as dyes and catalysts. Synthetic porphyrin dyes that are incorporated in the design of solar cells are the subject of ongoing research. See Dye-sensitized solar cells.
Supramolecular chemistry
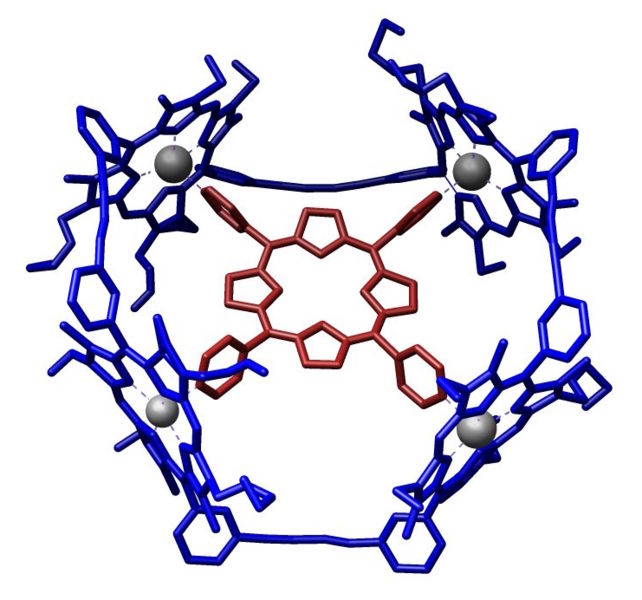
Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host-guest complex that was constructed from a macrocycle composed of four porphyrins.[5] A guest-free base porphyrin is bound to the center by coordination with its four pyridine sustituents.
See also
- A porphyrin-related disease: porphyria
- Porphyrin coordinated to iron: heme
- Porphyrin coordinated to magnesium: chlorophyll
- The one-carbon-shorter analogues: corroles
- Corphins, the highly reduced porphyrin that binds the F430 active site in methyl coenzyme M reductase (MCR)
References
- ↑ P. Rothemund (1936). "A New Porphyrin Synthesis. The Synthesis of Porphin". J. Am. Chem. Soc. 58 (4): 625–627. doi:10.1021/ja01295a027.
- ↑ P. Rothemund (1935). "Formation of Porphyrins from Pyrrole and Aldehydes". J. Am. Chem. Soc. 57 (10): 2010–2011. doi:10.1021/ja01313a510.
- ↑ A. D. Adler, F. R. Longo, J. D. Finarelli, J. Goldmacher, J. Assour and L. Korsakoff (1967). "A simplified synthesis for meso-tetraphenylporphine". J. Org. Chem. 32 (2): 476–476. doi:10.1021/jo01288a053.
- ↑ Falvo, RaeAnne E.; Mink, Larry M.; Marsh, Diane F. "Microscale Synthesis and 1H NMR Analysis of Tetraphenylporphyrins". J. Chem. Educ. 1999 (76): 237.
- ↑ Sanders and coworkers in Angew. Chem., Int. Ed. Engl. 1995, 34, 1096-1099.
External links
cs:Porfyriny de:Porphyrine io:Porfirino it:Porfirina nl:Porfyrine fi:Porfyriini sv:Porfyrin Template:Jb1 Template:WH Template:WS