Pazopanib hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
* Severe and fatal hepatotoxicity has been observed in clinical trials. Monitor hepatic function and interrupt, reduce, or discontinue dosing as recommended.
|
Overview
Pazopanib hydrochloride is an antineoplastic agent and tyrosine kinase inhibitor that is FDA approved for the treatment of advanced renal cell carcinoma, advanced soft tissue sarcoma,. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, hair color change, lymphocytopenia, musculoskeletal pain, headache and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Pazopanib hydrochloride® is indicated for the treatment of patients with advanced renal cell carcinoma (RCC).
- Pazopanib hydrochloride is indicated for the treatment of patients with advanced soft tissue sarcoma (STS) who have received prior chemotherapy.
Limitation of Use: The efficacy of Pazopanib hydrochloride for the treatment of patients with adipocytic STS or gastrointestinal stromal tumors has not been demonstrated.
Dosage
- The recommended starting dose of Pazopanib hydrochloride is 800 mg orally once daily without food (at least 1 hour before or 2 hours after a meal). The dose of Pazopanib hydrochloride should not exceed 800 mg.
- Do not crush tablets due to the potential for increased rate of absorption which may affect systemic exposure.
- If a dose is missed, it should not be taken if it is less than 12 hours until the next dose.
Dose Modification Guidelines
- In RCC, the initial dose reduction should be 400 mg, and additional dose decrease or increase should be in 200 mg steps based on individual tolerability.
- In STS, a decrease or increase should be in 200 mg steps based on individual tolerability.
- Hepatic Impairment: No dose adjustment is required in patients with mild hepatic impairment. In patients with moderate hepatic impairment, alternatives to Pazopanib hydrochloride should be considered. If Pazopanib hydrochloride is used in patients with moderate hepatic impairment, the dose should be reduced to 200 mg per day. Pazopanib hydrochloride is not recommended in patients with severe hepatic impairment.
- Concomitant Strong CYP3A4 Inhibitors: The concomitant use of strong CYP3A4 inhibitors (e.g., ketoconazole, ritonavir, clarithromycin) increases pazopanib concentrations and should be avoided. Consider an alternate concomitant medication with no or minimal potential to inhibit CYP3A4. If coadministration of a strong CYP3A4 inhibitor is warranted, reduce the dose of Pazopanib hydrochloride to 400 mg. Further dose reductions may be needed if adverse effects occur during therapy.
- Concomitant Strong CYP3A4 Inducer: The concomitant use of strong CYP3A4 inducers (e.g., rifampin) may decrease pazopanib concentrations and should be avoided. Consider an alternate concomitant medication with no or minimal enzyme induction potential. Pazopanib hydrochloride should not be used in patients who cannot avoid chronic use of strong CYP3A4 inducers.
DOSAGE FORMS AND STRENGTHS
- 200 mg tablets of Pazopanib hydrochloride — modified capsule-shaped, gray, film-coated with GS JT debossed on one side. Each tablet contains 216.7 mg of pazopanib hydrochloride equivalent to 200 mg of pazopanib.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pazopanib hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pazopanib hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Pazopanib hydrochloride in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pazopanib hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pazopanib hydrochloride in pediatric patients.
Contraindications
- None
Warnings
|
HEPATOTOXICITY
See full prescribing information for complete Boxed Warning.
* Severe and fatal hepatotoxicity has been observed in clinical trials. Monitor hepatic function and interrupt, reduce, or discontinue dosing as recommended.
|
Hepatic Toxicity and Hepatic Impairment
- In clinical trials with Pazopanib hydrochloride, hepatotoxicity, manifested as increases in serum transaminases (ALT, AST) and bilirubin, was observed. This hepatotoxicity can be severe and fatal. Transaminase elevations occur early in the course of treatment (92.5% of all transaminase elevations of any grade occurred in the first 18 weeks).
- In the randomized RCC trial, ALT >3 X ULN was reported in 18% and 3% of the Pazopanib hydrochloride and placebo groups, respectively. ALT >10 X ULN was reported in 4% of patients who received Pazopanib hydrochloride and in <1% of patients who received placebo. Concurrent elevation in ALT >3 X ULN and bilirubin >2 X ULN in the absence of significant alkaline phosphatase >3 X ULN occurred in 2% (5/290) of patients on Pazopanib hydrochloride and 1% (2/145) on placebo.
- In the randomized STS trial, ALT >3 X ULN was reported in 18% and 5% of the Pazopanib hydrochloride and placebo groups, respectively. ALT >8 X ULN was reported in 5% and 2% of the Pazopanib hydrochloride and placebo groups, respectively. Concurrent elevation in ALT >3 X ULN and bilirubin >2 X ULN in the absence of significant alkaline phosphatase >3 X ULN occurred in 2% (4/240) of patients on Pazopanib hydrochloride and <1% (1/123) on placebo.
- Two-tenths percent of the patients (2/977) from trials that supported the RCC indication died with disease progression and hepatic failure and 0.4% of patients (1/240) in the randomized STS trial died of hepatic failure.
- Monitor serum liver tests before initiation of treatment with Pazopanib hydrochloride and at Weeks 3, 5, 7, and 9. Thereafter, monitor at Month 3 and at Month 4, and as clinically indicated. Periodic monitoring should then continue after Month 4.
- Patients with isolated ALT elevations between 3 X ULN and 8 X ULN may be continued on Pazopanib hydrochloride with weekly monitoring of liver function until ALT return to Grade 1 or baseline.
- Patients with isolated ALT elevations of >8 X ULN should have Pazopanib hydrochloride interrupted until they return to Grade 1 or baseline. If the potential benefit for reinitiating treatment with Pazopanib hydrochloride is considered to outweigh the risk for hepatotoxicity, then reintroduce Pazopanib hydrochloride at a reduced dose of no more than 400 mg once daily and measure serum liver tests weekly for 8 weeks. Following reintroduction of Pazopanib hydrochloride, if ALT elevations >3 X ULN recur, then Pazopanib hydrochloride should be permanently discontinued.
- If ALT elevations >3 X ULN occur concurrently with bilirubin elevations >2 X ULN, Pazopanib hydrochloride should be permanently discontinued. Patients should be monitored until resolution. Pazopanib hydrochloride is a UGT1A1 inhibitor. Mild, indirect (unconjugated) hyperbilirubinemia may occur in patients with Gilbert’s syndrome. Patients with only a mild indirect hyperbilirubinemia, known Gilbert’s syndrome, and elevation in ALT >3 X ULN should be managed as per the recommendations outlined for isolated ALT elevations.
- Concomitant use of Pazopanib hydrochloride and simvastatin increases the risk of ALT elevations and should be undertaken with caution and close monitoring. Insufficient data are available to assess the risk of concomitant administration of alternative statins and Pazopanib hydrochloride.
- In patients with pre-existing moderate hepatic impairment, the starting dose of Pazopanib hydrochloride should be reduced or alternatives to Pazopanib hydrochloride should be considered. Treatment with Pazopanib hydrochloride is not recommended in patients with pre-existing severe hepatic impairment, defined as total bilirubin >3 X ULN with any level of ALT.
Prolongation and Torsades de Pointes
- In the RCC trials of Pazopanib hydrochloride, QT prolongation (≥500 msec) was identified on routine electrocardiogram monitoring in 2% (11/558) of patients. Torsades de pointes occurred in <1% (2/977) of patients who received Pazopanib hydrochloride in the monotherapy trials.
- In the randomized RCC and STS trials, 1% (3/290) of patients and 0.4% (1/240) of patients, respectively, who received Pazopanib hydrochloride had post-baseline values between 500 to 549 msec. Post-baseline QT data were only collected in the STS trial if ECG abnormalities were reported as an adverse reaction. None of the 268 patients who received placebo on the two trials had post-baseline QTc values ≥500 msec.
- Pazopanib hydrochloride should be used with caution in patients with a history of QT interval prolongation, in patients taking antiarrhythmics or other medications that may prolong QT interval, and those with relevant pre-existing cardiac disease. When using Pazopanib hydrochloride, baseline and periodic monitoring of electrocardiograms and maintenance of electrolytes (e.g., calcium, magnesium, potassium) within the normal range should be performed.
Cardiac Dysfunction
- In clinical trials with Pazopanib hydrochloride, events of cardiac dysfunction such as decreased left ventricular ejection fraction (LVEF) and congestive heart failure have occurred. In the overall safety population for RCC (N = 586), cardiac dysfunction was observed in 0.6% (4/586) of patients without routine on-study LVEF monitoring. In a randomized RCC trial of Pazopanib hydrochloride compared with sunitinib, myocardial dysfunction was defined as symptoms of cardiac dysfunction or ≥15% absolute decline in LVEF compared with baseline or a decline in LVEF of ≥10% compared with baseline that is also below the lower limit of normal. In patients who had baseline and follow up LVEF measurements, myocardial dysfunction occurred in 13% (47/362) of patients on Pazopanib hydrochloride compared with 11% (42/369) of patients on sunitinib. Congestive heart failure occurred in 0.5% of patients on each arm. In the randomized STS trial, myocardial dysfunction occurred in11% (16/142) of patients on Pazopanib hydrochloride compared with 5% (2/40) of patients on placebo. One percent (3/240) of patients on Pazopanib hydrochloride in the STS trial had congestive heart failure which did not resolve in one patient.
- Fourteen of the 16 patients with myocardial dysfunction treated with Pazopanib hydrochloride in the STS trial had concurrent hypertension which may have exacerbated cardiac dysfunction in patients at risk (e.g., those with prior anthracycline therapy) possibly by increasing cardiac afterload. Blood pressure should be monitored and managed promptly using a combination of anti-hypertensive therapy and dose modification of Pazopanib hydrochloride (interruption and re-initiation at a reduced dose based on clinical judgment). Patients should be carefully monitored for clinical signs or symptoms of congestive heart failure. Baseline and periodic evaluation of LVEF is recommended in patients at risk of cardiac dysfunction including previous anthracycline exposure.
Hemorrhagic Events
- Fatal hemorrhage occurred in 0.9% (5/586) in the RCC trials; there were no reports of fatal hemorrhage in the STS trials. In the randomized RCC trial, 13% (37/290) of patients treated with Pazopanib hydrochloride and 5% (7/145) of patients on placebo experienced at least 1 hemorrhagic event. The most common hemorrhagic events in the patients treated with Pazopanib hydrochloride were hematuria (4%), epistaxis (2%), hemoptysis (2%), and rectal hemorrhage (1%). Nine of 37 patients treated with Pazopanib hydrochloride who had hemorrhagic events experienced serious events including pulmonary, gastrointestinal, and genitourinary hemorrhage. One percent (4/290) of patients treated with Pazopanib hydrochloride died from hemorrhage compared with no (0/145) patients on placebo. In the overall safety population in RCC (N = 586), cerebral/intracranial hemorrhage was observed in <1% (2/586) of patients treated with Pazopanib hydrochloride.
- In the randomized STS trial, 22% (53/240) of patients treated with Pazopanib hydrochloride compared with 8% (10/123) treated with placebo experienced at least 1 hemorrhagic event. The most common hemorrhagic events were epistaxis (8%), mouth hemorrhage (3%), and anal hemorrhage (2%). Grade 4 hemorrhagic events in the STS population occurred in 1% (3/240) of patients and included intracranial hemorrhage, subarachnoid hemorrhage, and peritoneal hemorrhage.
- Pazopanib hydrochloride has not been studied in patients who have a history of hemoptysis, cerebral, or clinically significant gastrointestinal hemorrhage in the past 6 months and should not be used in those patients.
Arterial Thromboembolic Events
- Fatal arterial thromboembolic events were observed in 0.3% (2/586) of patients in the RCC trials and in no patients in the STS trials. In the randomized RCC trial, 2% (5/290) of patients receiving Pazopanib hydrochloride experienced myocardial infarction or ischemia, 0.3% (1/290) had a cerebrovascular accident and 1% (4/290) had an event of transient ischemic attack. In the randomized STS trial, 2% (4/240) of patients receiving Pazopanib hydrochloride experienced a myocardial infarction or ischemia, 0.4% (1/240) had a cerebrovascular accident and there were no incidents of transient ischemic attack. No arterial thromboembolic events were reported in patients who received placebo in either trial. Pazopanib hydrochloride should be used with caution in patients who are at increased risk for these events or who have had a history of these events. Pazopanib hydrochloride has not been studied in patients who have had an arterial thromboembolic event within the previous 6 months and should not be used in those patients.
Venous Thromboembolic Events
- In RCC and STS trials of Pazopanib hydrochloride, venous thromboembolic events (VTE) including venous thrombosis and fatal pulmonary embolus (PE) have occurred. In the randomized STS trial, venous thromboembolic events were reported in 5% of patients treated with Pazopanib hydrochloride compared with 2% with placebo. In the randomized RCC trial, the rate was 1% in both arms. Fatal pulmonary embolus occurred in 1% (2/240) of STS patients receiving Pazopanib hydrochloride and in no patients receiving placebo. There were no fatal pulmonary emboli in the RCC trial. Monitor for signs and symptoms of VTE and PE.
Thrombotic Microangiopathy
- Thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) has been reported in clinical trials of Pazopanib hydrochloride as monotherapy, in combination with bevacizumab, and in combination with topotecan. Pazopanib hydrochloride is not indicated for use in combination with other agents. Six of the 7 TMA cases occurred within 90 days of the initiation of Pazopanib hydrochloride. Improvement of TMA was observed after treatment was discontinued. Monitor for signs and symptoms of TMA. Permanently discontinue Pazopanib hydrochloride in patients developing TMA. Manage as clinically indicated.
Gastrointestinal Perforation and Fistula
- In the RCC and STS trials, gastrointestinal perforation or fistula occurred in 0.9% (5/586) of patients and 1% (4/382) of patients receiving Pazopanib hydrochloride, respectively. Fatal perforations occurred in 0.3% (2/586) of these patients in the RCC trials and in 0.3% (1/382) of these patients in the STS trials. Monitor for signs and symptoms of gastrointestinal perforation or fistula.
Reversible Posterior Leukoencephalopathy Syndrome
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS) has been reported in patients receiving Pazopanib hydrochloride and may be fatal.
- RPLS is a neurological disorder which can present with headache, seizure, lethargy, confusion, blindness, and other visual and neurologic disturbances. Mild to severe hypertension may be present. The diagnosis of RPLS is optimally confirmed by magnetic resonance imaging. Permanently discontinue Pazopanib hydrochloride in patients developing RPLS.
Hypertension
- In clinical trials, hypertension (systolic blood pressure ≥150 or diastolic blood pressure ≥100 mm Hg) and hypertensive crisis were observed in patients treated with Pazopanib hydrochloride. Blood pressure should be well controlled prior to initiating Pazopanib hydrochloride. Hypertension occurs early in the course of treatment (40% of cases occurred by Day 9 and 90% of cases occurred in the first 18 weeks). Blood pressure should be monitored early after starting treatment (no longer than one week) and frequently thereafter to ensure blood pressure control. Approximately 40% of patients who received Pazopanib hydrochloride experienced hypertension. Grade 3 hypertension was reported in 4% to 7% of patients receiving Pazopanib hydrochloride.
- Increased blood pressure should be treated promptly with standard anti-hypertensive therapy and dose reduction or interruption of Pazopanib hydrochloride as clinically warranted. Pazopanib hydrochloride should be discontinued if there is evidence of hypertensive crisis or if hypertension is severe and persistent despite anti-hypertensive therapy and dose reduction. Approximately 1% of patients required permanent discontinuation of Pazopanib hydrochloride because of hypertension.
Wound Healing
- No formal trials on the effect of Pazopanib hydrochloride on wound healing have been conducted. Since vascular endothelial growth factor receptor (VEGFR) inhibitors such as pazopanib may impair wound healing, treatment with Pazopanib hydrochloride should be stopped at least 7 days prior to scheduled surgery. The decision to resume Pazopanib hydrochloride after surgery should be based on clinical judgment of adequate wound healing. Pazopanib hydrochloride should be discontinued in patients with wound dehiscence.
Hypothyroidism
- Hypothyroidism, confirmed based on a simultaneous rise of TSH and decline of T4, was reported in 7% (19/290) of patients treated with Pazopanib hydrochloride in the randomized RCC trial and in 5% (11/240) of patients treated with Pazopanib hydrochloride in the randomized STS trial. No patients on the placebo arm of either trial had hypothyroidism. In RCC and STS trials of Pazopanib hydrochloride, hypothyroidism was reported as an adverse reaction in 4% (26/586) and 5% (20/382) of patients, respectively. Proactive monitoring of thyroid function tests is recommended.
Proteinuria
- In the randomized RCC trial, proteinuria was reported as an adverse reaction in 9% (27/290) of patients receiving Pazopanib hydrochloride and in no patients receiving placebo. In 2 patients, proteinuria led to discontinuation of treatment with Pazopanib hydrochloride. In the randomized STS trial, proteinuria was reported as an adverse reaction in 1% (2/240) of patients, and nephrotic syndrome was reported in 1 patient treated with Pazopanib hydrochloride compared with none in patients receiving placebo. Treatment was withdrawn in the patient with nephrotic syndrome.
- Baseline and periodic urinalysis during treatment is recommended with follow up measurement of 24-hour urine protein as clinically indicated. Interrupt Pazopanib hydrochloride and dose reduce for 24-hour urine protein ≥3 grams; discontinue Pazopanib hydrochloride for repeat episodes despite dose reductions.
Infection
- Serious infections (with or without neutropenia), including some with fatal outcome, have been reported. Monitor patients for signs and symptoms of infection. Institute appropriate anti-infective therapy promptly and consider interruption or discontinuation of Pazopanib hydrochloride for serious infections.
Increased Toxicity With Other Cancer Therapy
- Pazopanib hydrochloride is not indicated for use in combination with other agents. Clinical trials of Pazopanib hydrochloride in combination with pemetrexed and lapatinib were terminated early due to concerns over increased toxicity and mortality. The fatal toxicities observed included pulmonary hemorrhage, gastrointestinal hemorrhage, and sudden death. A safe and effective combination dose has not been established with these regimens.
Increased Toxicity in Developing Organs
- The safety and effectiveness of Pazopanib hydrochloride in pediatric patients have not been established. Pazopanib hydrochloride is not indicated for use in pediatric patients. Based on its mechanism of action, pazopanib may have severe effects on organ growth and maturation during early post-natal development. Administration of pazopanib to juvenile rats less than 21 days old resulted in toxicity to the lungs, liver, heart, and kidney and in death at doses significantly lower than the clinically recommended dose or doses tolerated in older animals. Pazopanib hydrochloride may potentially cause serious adverse effects on organ development in pediatric patients, particularly in patients younger than 2 years of age.
Pregnancy
- Pazopanib hydrochloride can cause fetal harm when administered to a pregnant woman. Based on its mechanism of action, Pazopanib hydrochloride is expected to result in adverse reproductive effects. In pre-clinical studies in rats and rabbits, pazopanib was teratogenic, embryotoxic, fetotoxic, and abortifacient.
- There are no adequate and well-controlled studies of Pazopanib hydrochloride in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while taking Pazopanib hydrochloride
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Potentially serious adverse reactions with Pazopanib hydrochloride included:
- Hepatotoxicity
- QT prolongation and torsades de pointes
- Cardiac dysfunction
- Hemorrhagic events
- Arterial and venous thromboembolic events
- Thrombotic microangiopathy
- Gastrointestinal perforation and fistula
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
- Hypertension
- Infection
- Increased toxicity with other cancer therapies
- Renal Cell Carcinoma: The safety of Pazopanib hydrochloride has been evaluated in 977 patients in the monotherapy trials which included 586 patients with RCC at the time of NDA submission. With a median duration of treatment of 7.4 months , the most commonly observed adverse reactions (≥20%) in the 586 patients were diarrhea, hypertension, hair color change, nausea, fatigue, anorexia, and vomiting.
- The data described below reflect the safety profile of Pazopanib hydrochloride in 290 RCC patients who participated in a randomized, double-blind, placebo-controlled trial . The median duration of treatment was 7.4 months (range 0 to 23) for patients who received Pazopanib hydrochloride and 3.8 months (range 0 to 22) for the placebo arm. Forty-two percent of patients on Pazopanib hydrochloride required a dose interruption. Thirty-six percent of patients on Pazopanib hydrochloride were dose reduced. Table 1 presents the most common adverse reactions occurring in ≥10% of patients who received Pazopanib hydrochloride.
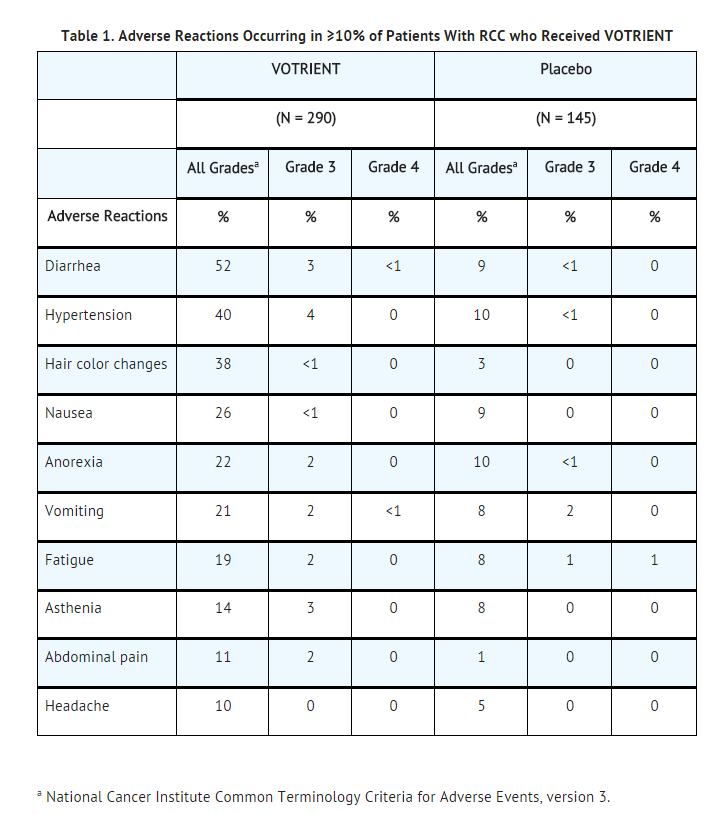
- Other adverse reactions observed more commonly in patients treated with Pazopanib hydrochloride than placebo and that occurred in <10% (any grade) were alopecia (8% versus <1%), chest pain (5% versus 1%), dysgeusia (altered taste) (8% versus <1%), dyspepsia (5% versus <1%), dysphonia (4% versus <1%), facial edema (1% versus 0%), palmar-plantar erythrodysesthesia (hand-foot syndrome) (6% versus <1%), proteinuria (9% versus 0%), rash (8% versus 3%), skin depigmentation (3% versus 0%), and weight decreased (9% versus 3%).
- Additional adverse reactions from other clinical trials in RCC patients treated with Pazopanib hydrochloride are listed below:
- Musculoskeletal and Connective Tissue Disorders: Arthralgia, muscle spasms.
- Table 2 presents the most common laboratory abnormalities occurring in >10% of patients who received Pazopanib hydrochloride and more commonly (≥5%) in patients who received Pazopanib hydrochloride versus placebo.
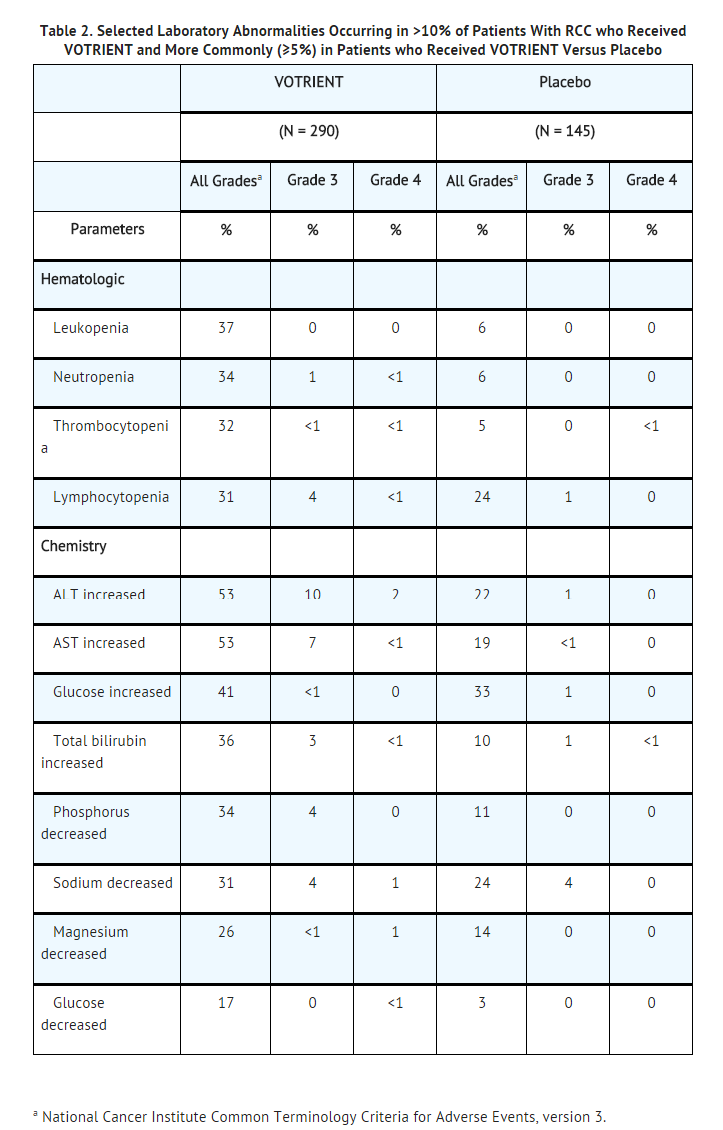
- Soft Tissue Sarcoma: The safety of Pazopanib hydrochloride has been evaluated in 382 patients with advanced soft tissue sarcoma, with a median duration of treatment of 3.6 months (range 0 to 53). The most commonly observed adverse reactions (≥20%) in the 382 patients were fatigue, diarrhea, nausea, decreased weight, hypertension, decreased appetite, vomiting, tumor pain, hair color changes, musculoskeletal pain, headache, dysgeusia, dyspnea, and skin hypopigmentation.
- The data described below reflect the safety profile of Pazopanib hydrochloride in 240 patients who participated in a randomized, double-blind, placebo-controlled trial. The median duration of treatment was 4.5 months (range 0 to 24) for patients who received Pazopanib hydrochloride and 1.9 months (range 0 to 24) for the placebo arm. Fifty-eight percent of patients on Pazopanib hydrochloride required a dose interruption. Thirty-eight percent of patients on Pazopanib hydrochloride had their dose reduced. Seventeen percent of patients who received Pazopanib hydrochloride discontinued therapy due to adverse reactions. Table 3 presents the most common adverse reactions occurring in ≥10% of patients who received Pazopanib hydrochloride.

- Other adverse reactions observed more commonly in patients treated with Pazopanib hydrochloride that occurred in ≥5% of patients and at an incidence of more than 2% difference from placebo included insomnia (9% versus 6%), hypothyroidism (8% versus 0%), dysphonia (8% versus 2%), epistaxis (8% versus 2%), left ventricular dysfunction (8% versus 4%), dyspepsia (7% versus 2%), dry skin (6% versus <1%), chills (5% versus 1%), vision blurred (5% versus 2%), and nail disorder (5% versus 0%).
- Table 4 presents the most common laboratory abnormalities occurring in >10% of patients who received Pazopanib hydrochloride and more commonly (≥5%) in patients who received Pazopanib hydrochloride versus placebo.

- Diarrhea: Diarrhea occurred frequently and was predominantly mild to moderate in severity in both the RCC and STS clinical trials. Patients should be advised how to manage mild diarrhea and to notify their healthcare provider if moderate to severe diarrhea occurs so appropriate management can be implemented to minimize its impact.
- Lipase Elevations: In a single-arm RCC trial, increases in lipase values were observed for 27% (48/181) of patients. Elevations in lipase as an adverse reaction were reported for 4% (10/225) of patients and were Grade 3 for 6 patients and Grade 4 for 1 patient. In the RCC trials of Pazopanib hydrochloride, clinical pancreatitis was observed in <1% (4/586) of patients.
- Pneumothorax: Two of 290 patients treated with Pazopanib hydrochloride and no patient on the placebo arm in the randomized RCC trial developed a pneumothorax. In the randomized trial of Pazopanib hydrochloride for the treatment of STS, pneumothorax occurred in 3% (8/240) of patients treated with Pazopanib hydrochloride and in no patients on the placebo arm.
- Bradycardia: In the randomized trial of Pazopanib hydrochloride for the treatment of RCC, bradycardia based on vital signs (<60 beats per minute) was observed in 19% (52/280) of patients treated with Pazopanib hydrochloride and in 11% (16/144) of patients on the placebo arm. Bradycardia was reported as an adverse reaction in 2% (7/290) of patients treated with Pazopanib hydrochloride compared with <1% (1/145) of patients treated with placebo. In the randomized trial of Pazopanib hydrochloride for the treatment of STS, bradycardia based on vital signs (<60 beats per minute) was observed in 19% (45/238) of patients treated with Pazopanib hydrochloride and in 4% (5/121) of patients on the placebo arm. Bradycardia was reported as an adverse reaction in 2% (4/240) of patients treated with Pazopanib hydrochloride compared with <1% (1/123) of patients treated with placebo.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of Pazopanib hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
- Gastrointestinal Disorders: Pancreatitis
Drug Interactions
Drugs That Inhibit or Induce Cytochrome P450 3A4 Enzymes
- In vitro studies suggested that the oxidative metabolism of pazopanib in human liver microsomes is mediated primarily by CYP3A4, with minor contributions from CYP1A2 and CYP2C8. Therefore, inhibitors and inducers of CYP3A4 may alter the metabolism of pazopanib.
- CYP3A4 Inhibitors: Coadministration of pazopanib with strong inhibitors of CYP3A4 (e.g., ketoconazole, ritonavir, clarithromycin) increases pazopanib concentrations and should be avoided. Consider an alternate concomitant medication with no or minimal potential to inhibit CYP3A4. If coadministration of a strong CYP3A4 inhibitor is warranted, reduce the dose of Pazopanib hydrochloride to 400 mg. Grapefruit or grapefruit juice should be avoided as it inhibits CYP3A4 activity and may also increase plasma concentrations of pazopanib.
- CYP3A4 Inducers: CYP3A4 inducers such as rifampin may decrease plasma pazopanib concentrations. Consider an alternate concomitant medication with no or minimal enzyme induction potential. Pazopanib hydrochloride should not be used if chronic use of strong CYP3A4 inducers cannot be avoided .
Drugs That Inhibit Transporters
- In vitro studies suggested that pazopanib is a substrate of P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP). Therefore, absorption and subsequent elimination of pazopanib may be influenced by products that affect Pgp and BCRP.
- Concomitant treatment with strong inhibitors of Pgp or breast cancer resistance protein (BCRP) should be avoided due to risk of increased exposure to pazopanib. Selection of alternative concomitant medicinal products with no or minimal potential to inhibit Pgp or BCRP should be considered.
Effects of Pazopanib on CYP Substrates
- Results from drug-drug interaction trials conducted in cancer patients suggest that pazopanib is a weak inhibitor of CYP3A4, CYP2C8, and CYP2D6 in vivo, but had no effect on CYP1A2, CYP2C9, or CYP2C19.
- Concomitant use of Pazopanib hydrochloride with agents with narrow therapeutic windows that are metabolized by CYP3A4, CYP2D6, or CYP2C8 is not recommended. Coadministration may result in inhibition of the metabolism of these products and create the potential for serious adverse events.
Effect of Concomitant use of Pazopanib hydrochloride and Simvastatin
- Concomitant use of Pazopanib hydrochloride and simvastatin increases the incidence of ALT elevations. Across monotherapy studies with Pazopanib hydrochloride, ALT >3 X ULN was reported in 126/895 (14%) of patients who did not use statins, compared with 11/41 (27%) of patients who had concomitant use of simvastatin. If a patient receiving concomitant simvastatin develops ALT elevations, follow dosing guidelines for Pazopanib hydrochloride or consider alternatives to Pazopanib hydrochloride. Alternatively, consider discontinuing simvastatin . Insufficient data are available to assess the risk of concomitant administration of alternative statins and Pazopanib hydrochloride.
Drugs That Raise Gastric pH
- In a drug interaction trial in patients with solid tumors, concomitant administration of pazopanib with esomeprazole, a proton pump inhibitor (PPI), decreased the exposure of pazopanib by approximately 40% (AUC and Cmax). Therefore, concomitant use of Pazopanib hydrochloride with drugs that raise gastric pH should be avoided. If such drugs are needed, short-acting antacids should be considered in place of PPIs and H2 receptor antagonists. Separate antacid and pazopanib dosing by several hours to avoid a reduction in pazopanib exposure.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category D
- Pazopanib hydrochloride can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Pazopanib hydrochloride in pregnant women.
- In pre-clinical studies in rats and rabbits, pazopanib was teratogenic, embryotoxic, fetotoxic, and abortifacient. Administration of pazopanib to pregnant rats during organogenesis at a dose level of ≥3 mg/kg/day (approximately 0.1 times the human clinical exposure based on AUC) resulted in teratogenic effects including cardiovascular malformations (retroesophageal subclavian artery, missing innominate artery, changes in the aortic arch) and incomplete or absent ossification. In addition, there was reduced fetal body weight, and pre- and post-implantation embryolethality in rats administered pazopanib at doses ≥3 mg/kg/day. In rabbits, maternal toxicity (reduced food consumption, increased post-implantation loss, and abortion) was observed at doses ≥30 mg/kg/day (approximately 0.007 times the human clinical exposure). In addition, severe maternal body weight loss and 100% litter loss were observed at doses ≥100 mg/kg/day (0.02 times the human clinical exposure), while fetal weight was reduced at doses ≥3 mg/kg/day (AUC not calculated).
- If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while taking Pazopanib hydrochloride.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pazopanib hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pazopanib hydrochloride during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Pazopanib hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Pazopanib hydrochloride in pediatric patients have not been established.
- In rats, weaning occurs at day 21 postpartum which approximately equates to a human pediatric age of 2 years. In a juvenile animal toxicology study performed in rats, when animals were dosed from day 9 through day 14 postpartum (pre-weaning), pazopanib caused abnormal organ growth/maturation in the kidney, lung, liver, and heart at approximately 0.1 times the clinical exposure, based on AUC in adult patients receiving Pazopanib hydrochloride. At approximately 0.4 times the clinical exposure (based on the AUC in adult patients), pazopanib administration resulted in mortality.
- In repeat-dose toxicology studies in rats including 4-week, 13-week, and 26-week administration, toxicities in bone, teeth, and nail beds were observed at doses ≥3 mg/kg/day (approximately 0.07 times the human clinical exposure based on AUC). Doses of 300 mg/kg/day (approximately 0.8 times the human clinical exposure based on AUC) were not tolerated in 13- and 26-week studies and animals required dose reductions due to body weight loss and morbidity. Hypertrophy of epiphyseal growth plates, nail abnormalities (including broken, overgrown, or absent nails) and tooth abnormalities in growing incisor teeth (including excessively long, brittle, broken and missing teeth, and dentine and enamel degeneration and thinning) were observed in rats at doses ≥30 mg/kg/day (approximately 0.35 times the human clinical exposure based on AUC) at 26 weeks, with the onset of tooth and nail bed alterations noted clinically after 4 to 6 weeks. Similar findings were noted in repeat-dose studies in juvenile rats dosed with pazopanib beginning day 21 postpartum (post-weaning). In the post-weaning animals, the occurrence of changes in teeth and bones occurred earlier and with greater severity than in older animals. There was evidence of tooth degeneration and decreased bone growth at doses ≥30 mg/kg (approximately 0.1 to 0.2 times the AUC in human adults at the clinically recommended dose). Pazopanib exposure in juvenile rats was lower than that seen at the same dose levels in adult animals, based on comparative AUC values. At pazopanib doses approximately 0.5 to 0.7 times the exposure in adult patients at the clinically recommended dose, decreased bone growth in juvenile rats persisted even after the end of the dosing period. Finally, despite lower pazopanib exposures than those reported in adult animals or adult humans, juvenile animals administered 300 mg/kg/dose pazopanib required dose reduction within 4 weeks of dosing initiation due to significant toxicity, although adult animals could tolerate this same dose for at least 3 times as long .
Geriatic Use
- In clinical trials with Pazopanib hydrochloride for the treatment of RCC, 33% (196/582) of patients were aged ≥65 years. No overall differences in safety or effectiveness of Pazopanib hydrochloride were observed between these patients and younger patients. However, patients >60 years of age may be at greater risk for an ALT >3 X ULN. In the STS trials, 24% (93/382) of patients were aged ≥65 years. Patients ≥65 years had increased Grade 3 or 4 fatigue (19% versus 12% for <65), hypertension (10% versus 6%), decreased appetite (11% versus 2%), and ALT (3% versus 2%) or AST elevations (4% versus 1%). Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Pazopanib hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pazopanib hydrochloride with respect to specific racial populations.
Renal Impairment
- Patients with renal cell cancer and mild/moderate renal impairment (creatinine clearance ≥30 mL/min) were included in clinical trials for Pazopanib hydrochloride.
- There are no clinical or pharmacokinetic data in patients with severe renal impairment or in patients undergoing peritoneal dialysis or hemodialysis. However, renal impairment is unlikely to significantly affect the pharmacokinetics of pazopanib since <4% of a radiolabeled oral dose was recovered in the urine. In a population pharmacokinetic analysis using 408 patients with various cancers, creatinine clearance (30-150 mL/min) did not influence clearance of pazopanib. Therefore, renal impairment is not expected to influence pazopanib exposure, and dose adjustment is not necessary.
Hepatic Impairment
- In clinical studies for Pazopanib hydrochloride, patients with total bilirubin ≤1.5 X ULN and AST and ALT ≤2 X ULN were included.
- An analysis of data from a pharmacokinetic study of pazopanib in patients with varying degrees of hepatic dysfunction suggested that no dose adjustment is required in patients with mild hepatic impairment [either total bilirubin within normal limit (WNL) with ALT >ULN or bilirubin >1 X to 1.5 X ULN regardless of the ALT value]. The maximum tolerated dose in patients with moderate hepatic impairment (total bilirubin >1.5 X to 3 X ULN regardless of the ALT value) was 200 mg per day (N = 11). The median steady-state Cmax and AUC(0-24) achieved at this dose was approximately 40% and 29%, respectively, of that seen in patients with normal hepatic function at the recommended daily dose of 800 mg. The maximum dose explored in patients with severe hepatic impairment (total bilirubin >3 X ULN regardless of the ALT value) was 200 mg per day (N = 14). This dose was not well tolerated. Median exposures achieved at this dose were approximately 18% and 15% of those seen in patients with normal liver function at the recommended daily dose of 800 mg. Therefore, Pazopanib hydrochloride is not recommended in these patients.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pazopanib hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pazopanib hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Pazopanib hydrochloride in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Pazopanib hydrochloride in the drug label.
Overdosage
- Pazopanib doses up to 2,000 mg have been evaluated in clinical trials. Dose-limiting toxicity (Grade 3 fatigue) and Grade 3 hypertension were each observed in 1 of 3 patients dosed at 2,000 mg daily and 1,000 mg daily, respectively.
- Treatment of overdose with Pazopanib hydrochloride should consist of general supportive measures. There is no specific antidote for overdosage of Pazopanib hydrochloride.
- Hemodialysis is not expected to enhance the elimination of Pazopanib hydrochloride because pazopanib is not significantly renally excreted and is highly bound to plasma proteins.
Pharmacology
There is limited information regarding Pazopanib hydrochloride Pharmacology in the drug label.
Mechanism of Action
- Pazopanib is a multi-tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-α and -β, fibroblast growth factor receptor (FGFR)-1 and -3, cytokine receptor (Kit), interleukin-2 receptor inducible T-cell kinase (Itk), leukocyte-specific protein tyrosine kinase (Lck), and transmembrane glycoprotein receptor tyrosine kinase (c-Fms). In vitro, pazopanib inhibited ligand-induced autophosphorylation of VEGFR-2, Kit and PDGFR-β receptors. In vivo, pazopanib inhibited VEGF-induced VEGFR-2 phosphorylation in mouse lungs, angiogenesis in a mouse model, and the growth of some human tumor xenografts in mice.
Structure
- Pazopanib hydrochloride (pazopanib) is a tyrosine kinase inhibitor (TKI). Pazopanib is presented as the hydrochloride salt, with the chemical name 5-4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide monohydrochloride. It has the molecular formula C21H23N7O2S•HCl and a molecular weight of 473.99. Pazopanib hydrochloride has the following chemical structure:
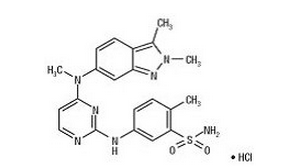
- Pazopanib hydrochloride is a white to slightly yellow solid. It is very slightly soluble at pH 1 and practically insoluble above pH 4 in aqueous media.
- Tablets of Pazopanib hydrochloride are for oral administration. Each 200 mg tablet of Pazopanib hydrochloride contains 216.7 mg of pazopanib hydrochloride, equivalent to 200 mg of pazopanib free base.
- The inactive ingredients of Pazopanib hydrochloride are: Tablet Core: Magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate. Coating: Gray film-coat: Hypromellose, iron oxide black, macrogol/polyethylene glycol 400 (PEG 400), polysorbate 80, titanium dioxide.
Pharmacodynamics
- Increases in blood pressure have been observed and are related to steady-state trough plasma pazopanib concentrations.
- The QT prolongation potential of pazopanib was assessed in a randomized, blinded, parallel trial (N = 96) using moxifloxacin as a positive control. Pazopanib 800 mg was dosed under fasting conditions on Days 2 to 8 and 1,600 mg was dosed on Day 9 after a meal in order to increase exposure to pazopanib and its metabolites. No large changes (i.e., >20 msec) in QTc interval following the treatment of pazopanib were detected in this QT trial. The trial was not able to exclude small changes (<10 msec) in QTc interval, because assay sensitivity below this threshold (<10 msec) was not established in this trial.
Pharmacokinetics
Absorption: Pazopanib is absorbed orally with median time to achieve peak concentrations of 2 to 4 hours after the dose. Daily dosing at 800 mg results in geometric mean AUC and Cmax of 1,037 mcg•hr/mL and 58.1 mcg/mL (equivalent to 132 μM), respectively. There was no consistent increase in AUC or Cmax at pazopanib doses above 800 mg.
- Administration of a single pazopanib 400 mg crushed tablet increased AUC(0-72) by 46% and Cmax by approximately 2 fold and decreased tmax by approximately 2 hours compared with administration of the whole tablet. These results indicate that the bioavailability and the rate of pazopanib oral absorption are increased after administration of the crushed tablet relative to administration of the whole tablet. Therefore, due to this potential for increased exposure, tablets of Pazopanib hydrochloride should not be crushed.
- Systemic exposure to pazopanib is increased when administered with food. Administration of pazopanib with a high-fat or low-fat meal results in an approximately 2 fold increase in AUC and Cmax. Therefore, pazopanib should be administered at least 1 hour before or 2 hours after a meal.
Distribution: Binding of pazopanib to human plasma protein in vivo was greater than 99% with no concentration dependence over the range of 10 to 100 mcg/mL. In vitro studies suggest that pazopanib is a substrate for P-glycoprotein (Pgp) and breast cancer resistant protein (BCRP).
Metabolism: In vitro studies demonstrated that pazopanib is metabolized by CYP3A4 with a minor contribution from CYP1A2 and CYP2C8.
Elimination: Pazopanib has a mean half-life of 30.9 hours after administration of the recommended dose of 800 mg. Elimination is primarily via feces with renal elimination accounting for <4% of the administered dose.
Hepatic Impairment: Mild hepatic impairment was defined as either total bilirubin WNL with ALT >ULN or bilirubin >1 X to 1.5 X ULN regardless of the ALT value. The median steady-state pazopanib Cmax and AUC(0-24) after a once daily dose of 800 mg/day in patients (N = 12) with mild impairment were 34 mcg/mL (range 11 to 104) and 774 mcg•hr/mL (range 215 to 2,034), respectively. These were in a similar range as the median steady-state pazopanib Cmax and AUC(0-24) in patients (N = 18) with no hepatic impairment (52 mcg/mL, range 17 to 86 and 888 mcg•hr/mL, range 346 to 1,482, respectively).
- Moderate hepatic impairment was defined as total bilirubin >1.5 X to 3 X ULN regardless of the ALT value. The maximum tolerated pazopanib dose in patients with moderate impairment was 200 mg once daily. The median (N = 11) steady-state Cmax with that regimen was 22 mcg/mL (range 4.2 to 33), and the median AUC(0-24) was 257 mcg•hr/mL (range 66 to 488). These values were approximately 43% and 29% of the corresponding median values after administration of 800 mg once daily in patients with normal hepatic function (N = 18).
- Severe hepatic impairment was defined as total bilirubin >3 X ULN regardless of the ALT value. Median exposures in patients with severe hepatic impairment receiving 200 mg once daily (N = 14) were unexpectedly lower than those observed in patients with moderate hepatic impairment receiving 200 mg once daily. The median steady-state Cmax was 9.4 mcg/mL (range 2.4 to 24), and the median AUC(0-24) was 131 mcg•hr/mL (range 47 to 473). These values were approximately 18% and 15% of the corresponding median values after administration of 800 mg once daily in patients with normal hepatic function. Despite the observed concentrations, the dose of 200 mg was not well tolerated in patients with severe hepatic impairment. Use of Pazopanib hydrochloride is not recommended in patients with severe hepatic impairment.
Drug Interactions: Coadministration of multiple doses of oral pazopanib 400 mg with multiple doses of oral ketoconazole 400 mg (strong CYP3A4/P-gp inhibitor) resulted in a 1.7 fold increase in the AUC(0-24) and a 1.5 fold increase in the Cmax of pazopanib compared with when pazopanib was administered alone. Concurrent administration of a single dose of pazopanib eye drops with ketoconazole in healthy volunteers resulted in a 2 fold and 1.5 fold increase in mean AUC(0-t) and Cmax values, respectively.
- Administration of 1,500 mg lapatinib, a substrate and weak inhibitor of CYP3A4, Pgp, and BCRP, with 800 mg pazopanib resulted in an approximately 50% to 60% increase in mean pazopanib AUC(0-24) and Cmax compared with administration of 800 mg pazopanib alone.
- In vitro studies with human liver microsomes showed that pazopanib inhibited the activities of CYP enzymes 1A2, 3A4, 2B6, 2C8, 2C9, 2C19, 2D6, and 2E1. Potential induction of human CYP3A4 was demonstrated in an in vitro human PXR assay. Clinical pharmacology studies, using pazopanib 800 mg once daily, have demonstrated that pazopanib does not have a clinically relevant effect on the pharmacokinetics of caffeine (CYP1A2 probe substrate), warfarin (CYP2C9 probe substrate), or omeprazole (CYP2C19 probe substrate) in cancer patients. Pazopanib resulted in an increase of approximately 30% in the mean AUC and Cmax of midazolam (CYP3A4 probe substrate) and increases of 33% to 64% in the ratio of dextromethorphan to dextrorphan concentrations in the urine after oral administration of dextromethorphan (CYP2D6 probe substrate). Coadministration of pazopanib 800 mg once daily and paclitaxel 80 mg/m2 (CYP3A4 and CYP2C8 substrate) once weekly resulted in a mean increase of 26% and 31% in paclitaxel AUC and Cmax, respectively.
- Pazopanib exhibits pH dependent solubility. In a drug interaction trial in patients with solid tumors, concomitant administration of pazopanib with esomeprazole, a PPI, decreased the exposure of pazopanib by approximately 40% (AUC and Cmax).
- In vitro studies also showed that pazopanib inhibits UGT1A1 and OATP1B1 with IC50s of 1.2 and 0.79 μM, respectively. Pazopanib may increase concentrations of drugs eliminated by UGT1A1 and OATP1B1.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies with pazopanib have not been conducted. However, in a 13-week study in mice, proliferative lesions in the liver including eosinophilic foci in 2 females and a single case of adenoma in another female was observed at doses of 1,000 mg/kg/day (approximately 2.5 times the human clinical exposure based on AUC).
- Pazopanib did not induce mutations in the microbial mutagenesis (Ames) assay and was not clastogenic in both the in vitro cytogenetic assay using primary human lymphocytes and in the in vivo rat micronucleus assay.
- Pazopanib may impair fertility in humans. In female rats, reduced fertility including increased pre-implantation loss and early resorptions were noted at dosages ≥30 mg/kg/day (approximately 0.4 times the human clinical exposure based on AUC). Total litter resorption was seen at 300 mg/kg/day (approximately 0.8 times the human clinical exposure based on AUC). Post-implantation loss, embryolethality, and decreased fetal body weight were noted in females administered doses ≥10 mg/kg/day (approximately 0.3 times the human clinical exposure based on AUC). Decreased corpora lutea and increased cysts were noted in mice given ≥100 mg/kg/day for 13 weeks and ovarian atrophy was noted in rats given ≥300 mg/kg/day for 26 weeks (approximately 1.3 and 0.85 times the human clinical exposure based on AUC, respectively). Decreased corpora lutea was also noted in monkeys given 500 mg/kg/day for up to 34 weeks (approximately 0.4 times the human clinical exposure based on AUC).
- Pazopanib did not affect mating or fertility in male rats. However, there were reductions in sperm production rates and testicular sperm concentrations at doses ≥3 mg/kg/day, epididymal sperm concentrations at doses ≥30 mg/kg/day, and sperm motility at ≥100 mg/kg/day following 15 weeks of dosing. Following 15 and 26 weeks of dosing, there were decreased testicular and epididymal weights at doses of ≥30 mg/kg/day (approximately 0.35 times the human clinical exposure based on AUC); atrophy and degeneration of the testes with aspermia, hypospermia and cribiform change in the epididymis was also observed at this dose in the 6-month toxicity studies in male rats.
Clinical Studies
Renal Cell Carcinoma
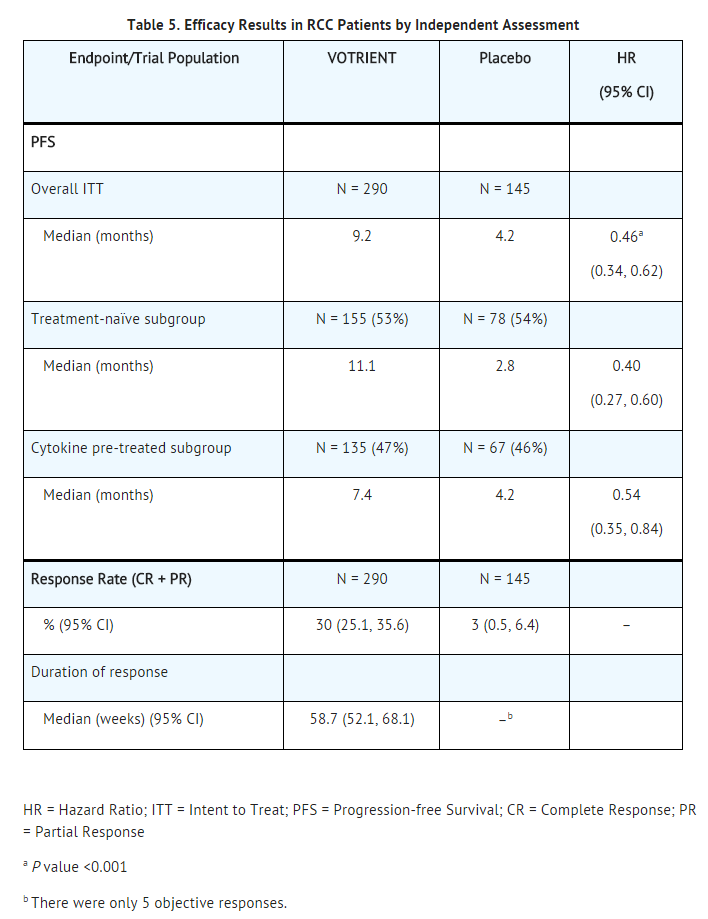
- The safety and efficacy of Pazopanib hydrochloride in renal cell carcinoma (RCC) were evaluated in a randomized, double-blind, placebo-controlled, multicenter, Phase 3 trial. Patients (N = 435) with locally advanced and/or metastatic RCC who had received either no prior therapy or one prior cytokine-based systemic therapy were randomized (2:1) to receive Pazopanib hydrochloride 800 mg once daily or placebo once daily. The primary objective of the trial was to evaluate and compare the 2 treatment arms for progression-free survival (PFS); the secondary endpoints included overall survival (OS), overall response rate (RR), and duration of response.
- Of the total of 435 patients enrolled in this trial, 233 patients had no prior systemic therapy (treatment-naïve subgroup) and 202 patients received one prior IL-2 or INFα-based therapy (cytokine-pretreated subgroup). The baseline demographic and disease characteristics were balanced between the Pazopanib hydrochloride and placebo arms. The majority of patients were male (71%) with a median age of 59 years. Eighty-six percent of patients were Caucasian, 14% were Asian, and less than 1% were other. Forty-two percent were ECOG performance status 0 and 58% were ECOG performance status 1. All patients had clear cell histology (90%) or predominantly clear cell histology (10%). Approximately 50% of all patients had 3 or more organs involved with metastatic disease. The most common metastatic sites at baseline were lung (74%), lymph nodes (56%), bone (27%), and liver (25%).
- A similar proportion of patients in each arm were treatment-naïve and cytokine-pretreated (see Table 5). In the cytokine-pretreated subgroup, the majority (75%) had received interferon-based treatment. Similar proportions of patients in each arm had prior nephrectomy (89% and 88% for Pazopanib hydrochloride and placebo, respectively).
- The analysis of the primary endpoint PFS was based on disease assessment by independent radiological review in the entire trial population. Efficacy results are presented in Table 5 and Figure 1.
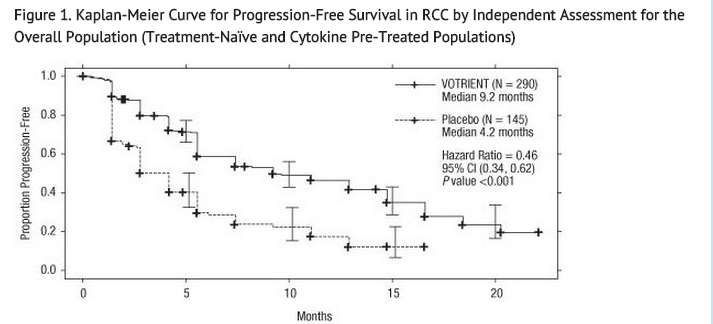
- At the protocol-specified final analysis of OS, the median OS was 22.9 months for patients randomized to Pazopanib hydrochloride and 20.5 months for the placebo arm [HR = 0.91 (95% CI: 0.71, 1.16)]. The median OS for the placebo arm includes 79 patients (54%) who discontinued placebo treatment because of disease progression and crossed over to treatment with Pazopanib hydrochloride. In the placebo arm, 95 (66%) patients received at least one systemic anti-cancer treatment after progression compared with 88 (30%) patients randomized to Pazopanib hydrochloride.
Soft Tissue Sarcoma
- The safety and efficacy of Pazopanib hydrochloride in patients with STS were evaluated in a randomized, double-blind, placebo-controlled, multicenter trial. Patients (N = 369) with metastatic STS who had received prior chemotherapy, including anthracycline treatment, or were unsuited for such therapy, were randomized (2:1) to receive Pazopanib hydrochloride 800 mg once daily or placebo. Patients with gastrointestinal stromal tumors (GIST) or adipocytic sarcoma were excluded from the trial. Randomization was stratified by the factors of WHO performance status (WHO PS) 0 or 1 at baseline and the number of lines of prior systemic therapy for advanced disease (0 or 1 versus 2+). Progression-free survival (PFS) was assessed by independent radiological review. Other efficacy endpoints included overall survival (OS), overall response rate, and duration of response.
- The majority of patients were female (59%) with a median age of 55 years. Seventy-two percent of patients were Caucasian, 22% were Asian, and 6% were other. Forty-three percent of patients had leiomyosarcoma, 10% had synovial sarcoma, and 47% had other soft tissue sarcomas. Fifty-six percent of patients had received 2 or more lines of prior systemic therapy and 44% had received 0 or 1 lines of prior systemic therapy. The median duration of treatment was 4.5 months for patients on the pazopanib arm and 1.9 months for patients on the placebo arm.
- Efficacy results are presented in Table 6 and Figure 2.
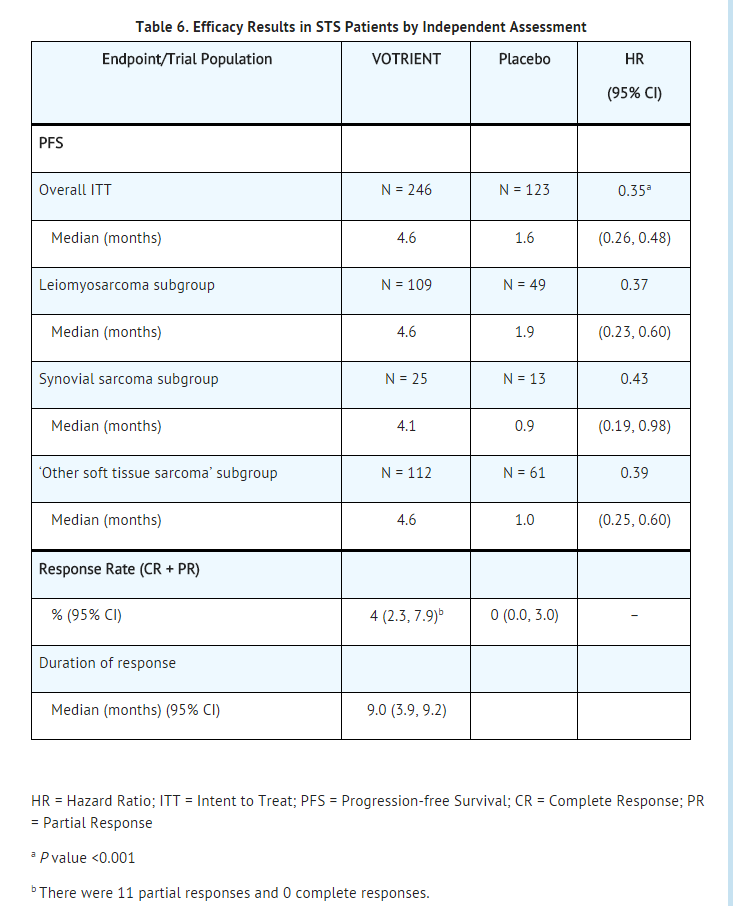
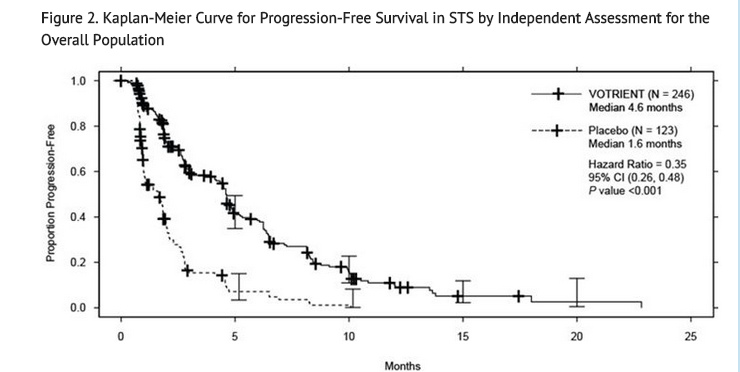
- At the protocol-specified final analysis of OS, the median OS was 12.6 months for patients randomized to Pazopanib hydrochloride and 10.7 months for the placebo arm [HR = 0.87 (95% CI: 0.67, 1.12)].
How Supplied
- The 200 mg tablets of Pazopanib hydrochloride are modified capsule-shaped, gray, film-coated with GS JT debossed on one side and are available in:
- Bottles of 120 tablets: NDC 0173-0804-09
Storage
- Store at room temperature between 20°C and 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Pazopanib hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pazopanib hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
The Medication Guide is contained in a separate leaflet that accompanies the product.
However, inform patients of the following:
- Therapy with Pazopanib hydrochloride may result in hepatobiliary laboratory abnormalities. Monitor serum liver tests (ALT, AST, and bilirubin) prior to initiation of Pazopanib hydrochloride and at Weeks 3, 5, 7, and 9. Thereafter, monitor at Month 3 and at Month 4, and as clinically indicated. Inform patients that they should report signs and symptoms of liver dysfunction to their healthcare provider right away.
- Prolonged QT intervals and torsades de pointes have been observed. Patients should be advised that ECG monitoring may be performed. Patients should be advised to inform their physicians of concomitant medications.
- Cardiac dysfunction (such as CHF and LVEF decrease) has been observed in patients at risk (e.g., prior anthracycline therapy) particularly in association with development or worsening of hypertension. Patients should be advised to report hypertension or signs and symptoms of congestive heart failure.
- Serious hemorrhagic events have been reported. Patients should be advised to report unusual bleeding.
- Arterial thrombotic events have been reported. Patients should be advised to report signs or symptoms of an arterial thrombosis.
- Reports of pneumothorax and venous thromboembolic events including pulmonary embolus have been reported. Patients should be advised to report if new onset of dyspnea, chest pain, or localized limb edema occurs.
- Advise patients to inform their doctor if they have worsening of neurological function consistent with RPLS (headache, seizure, lethargy, confusion, blindness, and other visual and neurologic disturbances).
- Hypertension and hypertensive crisis have been reported. Patients should be advised to monitor blood pressure early in the course of therapy and frequently thereafter and report increases of blood pressure or symptoms such as blurred vision, confusion, severe headache, or nausea and vomiting.
- GI perforation or fistula has occurred. Advise patients to report signs and symptoms of a GI perforation or fistula.
- VEGFR inhibitors such as Pazopanib hydrochloride may impair wound healing. Advise patients to stop Pazopanib hydrochloride at least 7 days prior to a scheduled surgery.
- Hypothyroidism and proteinuria have been reported. Advise patients that thyroid function testing and urinalysis will be performed during treatment.
- Serious infections including some with fatal outcomes have been reported. Advise patients to promptly report any signs or symptoms of infection.
- Women of childbearing potential should be advised of the potential hazard to the fetus and to avoid becoming pregnant.
- Gastrointestinal adverse reactions such as diarrhea, nausea, and vomiting have been reported with Pazopanib hydrochloride. Patients should be advised how to manage diarrhea and to notify their healthcare provider if moderate to severe diarrhea occurs.
- Patients should be advised to inform their healthcare providers of all concomitant medications, vitamins, or dietary and herbal supplements.
- Patients should be advised that depigmentation of the hair or skin may occur during treatment with Pazopanib hydrochloride.
- Patients should be advised to take Pazopanib hydrochloride without food (at least 1 hour before or 2 hours after a meal).
Precautions with Alcohol
- Alcohol-Pazopanib hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Pazopanib hydrochloride
Look-Alike Drug Names
- A® — B®[1]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Pazopanib hydrochloride |Label Name=Votrient image.jpg
}}
{{#subobject:
|Label Page=Pazopanib hydrochloride |Label Name=Pazopanib ingredients and appearance.png
}}