Ioxilan
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
* NOT FOR INTRATHECAL USE
|
Overview
Ioxilan is a diagnostic agent that is FDA approved for the procedure of cerebral arteriography, ventriculography, visceral angiography, aortography, and peripheral arteriography. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, fever, chills, hypertension, bradycardia,nausea, diarrhea,vomiting and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
INTRAARTERIAL:
- OXILAN® Injection (300 mgI/mL) is indicated for cerebral arteriography.OXILAN® Injection (350 mgI/mL) is indicated for coronary arteriography and left ventriculography, visceral angiography, aortography, and peripheral arteriography.
INTRAVENOUS:
- OXILAN® Injection (300 mgI/mL) and OXILAN® Injection (350 mgI/mL) are indicated for excretory urography and contrast enhanced computed tomographic (CECT) imaging of the head and body.
Dosage
ADULT DOSAGE AND ADMINISTRATION - General
- The combination of volume and OXILAN® concentration to be used should be carefully individualized accounting for factors such as age, body weight, size of the vessel and the rate of blood flow within the vessel. Specific dose adjustments for age, gender, weight, and renal function have not been studied for OXILAN®. As with all iodinated contrast agents, lower doses of OXILAN® Injection may have less risk. The efficacy of OXILAN® Injection below doses recommended has not been studied. Other factors such as anticipated pathology, degree and extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed should also be considered.
- The maximum recommended total dose of iodine is 86 grams.
- If during administration a reaction occurs, the injection should be immediately stopped.
- Patients should be adequately hydrated prior to and following intravascular administration of OXILAN® Injection.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ioxilan in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioxilan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Ioxilan in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ioxilan in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioxilan in pediatric patients.
Contraindications
- OXILAN® Injection is not indicated for intrathecal use.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
* NOT FOR INTRATHECAL USE
|
SEVERE ADVERSE EVENTS-INADVERTENT INTRATHECAL ADMINISTRATION:
- Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not administered intrathecally.
- Nonionic iodinated contrast media inhibit blood coagulation, in vitro, less than ionic contrast media. Clotting has been reported when blood remains in contact with syringes containing nonionic contrast media. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
- Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state, and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three way stopcocks, frequent catheter flushing with heparinized saline solutions, and minimizing the length of the procedure.
- Serious or fatal reactions have been associated with the administration of iodine-containing radiopaque media. It is of utmost importance to be completely prepared to treat any contrast agent reaction.
- Caution must be exercised in patients with severely impaired renal function, combined renal and hepatic disease, combined renal and cardiac disease, severe thyrotoxicosis, myelomatosis, or anuria, particularly when large doses are administered.
- Intravascularly administered iodine-containing radiopaque media are potentially hazardous in patients with multiple myeloma or other paraproteinacious diseases, who are prone to disease-induced renal insufficiency and/or failure. Although neither the contrast agent nor dehydration has been proven to be the cause of renal insufficiency (or worsening renal insufficiency) in myelomatous patients, it has been speculated that the combination of both may be causative. Special precautions, including maintenance of normal hydration and close monitoring, are required. Partial dehydration in the preparation of these patients prior to injection is not recommended since this may predispose the patient to precipitation of the myeloma protein.
- Reports of thyroid storm following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism, or with an autonomously functioning thyroid nodule, suggest that this additional risk be evaluated in such patients before use of any contrast agent.
- Administration of radiopaque materials to patients with known or suspected pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available. These patients should be monitored very closely during contrast enhanced procedures.
- Contrast agents may promote sickling in individuals who are homozygous for sickle cell disease when administered intravascularly.
Adverse Reactions
Clinical Trials Experience
- For demographics, see clinical trials section. The following table of incidence of reactions is based upon controlled clinical studies in which OXILAN® was compared with a nonionic contrast agent (iohexol) in 531 patients. It includes all reported adverse events, regardless of attribution. Adverse reactions are listed by body system and in decreasing order of occurrence greater than 0.5% in the OXILAN® group.
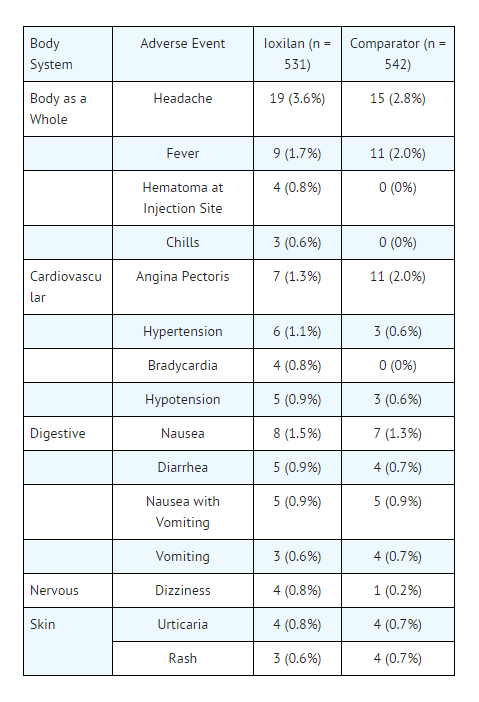
- One or more adverse reactions were reported in 76 of 531 (14.3%) of patients in the clinical trials, coincidental with the administration of OXILAN® or within the study follow-up period of 24 to 72 hours. The incidence and type of adverse reactions were similar to those associated with the nonionic comparator (iohexol) used in the clinical trials. OXILAN®, as do other iodinated contrast agents, often causes warmth and/or pain on injection. The rates are similar to that of the iohexol comparator.
- Serious, life threatening and fatal reactions have been associated with the administration of iodine-containing contrast media. In all clinical trials 3/835 (0.3%) patients given OXILAN® and 3/542 (0.6%) given iohexol died 4 days or later after drug administration. In the controlled trials 8/531 (1.5%) patients given OXILAN® and 6/542 (1.1%) given iohexol had serious adverse events.
- The following adverse reactions were observed ≤ 0.5% of patients receiving OXILAN® Injection:
- BODY: allergic reaction, asthenia, chest and back pain, edema of the neck, facial edema, pain, peripheral edema;
- CARDIOVASCULAR: atrial fibrillation, syncope, tachycardia, vasodilation, ventricular extrasystole;
- DIGESTIVE: anorexia, constipation, dyspepsia, dysphagia, GI hemorrhage, ileus, liver failure;
- NERVOUS: hypotonia, nystagmus, paresthesia, somnolence, vertigo;
- RESPIRATORY: dyspnea, pharyngitis, rhinitis;
- SPECIAL SENSES: amblyopia, conjunctivitis, taste perversion, vision abnormality;
- UROGENITAL: anuria, dysuria, hematuria, infection of urinary tract, impairment of urination, kidney failure.
Postmarketing Experience
- Additional adverse events reported in postmarketing surveillance with the use of OXILAN® Injection include: bronchospasm.
Drug Interactions
- Renal toxicity has been reported in a few patients with liver dysfunction who were given an oral cholecystographic agent followed by intravascular contrast agents. Administration of any intravascular contrast agent should therefore be postponed in any patient with a known or suspected hepatic or biliary disorder who has recently received a cholecystographic contrast agent.
- Other drugs should not be admixed with OXILAN® (Ioxilan Injection).
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects: Pregnancy Category B
- Reproduction studies performed with ioxilan injection in rats at doses up to 6.5 gI/kg (3.7 times the recommended dose for a 50 kg human, or approximately 0.7 times the human dose following normalization of the data to body surface area estimates) and rabbits at doses up to 3.5 gI/kg (2 times the recommended dose for a 50 kg human, or approximately the same as the human dose following normalization of the data to body surface area estimates) did not reveal evidence of direct harm to the fetus. Embryolethality was not detected. Adequate and well-controlled studies in pregnant women have not been conducted. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ioxilan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ioxilan during labor and delivery.
Nursing Mothers
- It is not known whether ioxilan is excreted in human milk. However, many injectable contrast agents are excreted unchanged in human milk. Although it has not been established that serious adverse reactions occur in nursing infants, caution should be exercised when intravascular contrast media are administered to nursing women because of potential adverse reactions, and consideration should be given to temporarily discontinuing nursing.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Ioxilan with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Ioxilan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ioxilan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ioxilan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ioxilan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ioxilan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ioxilan in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
INTRAARTERIAL PROCEDURES
Coronary Arteriography and Left Ventriculography
- OXILAN® Injection (350 mgI/mL) is indicated for intraarterial injection in the radiographic contrast evaluation of coronary arteries and the left ventricle. Injection rates should be approximately equal to flow rate in the vessel being injected.
- The usual individual injection volumes for visualization of the coronary arteries and the left ventricle are as follows:
- Left and Right Coronary: 2 mL to 10 mL (0.7 to 3.5 gI) of OXILAN® Injection - 350 (350 mgI/mL)
- Left Ventricle: 25 mL to 50 mL (8.75 to 17.5 gI) of OXILAN® Injection - 350 (350 mgI/mL)
- Total dose for the procedure should not usually exceed 250 mL.
- When large individual volumes are administered, as in ventriculography and aortography, it is recommended that sufficient time be permitted to elapse between each injection to allow for subsidence of possible hemodynamic disturbances.
- Mandatory prerequisites to the procedure are specialized personnel, ECG monitoring apparatus and adequate facilities for immediate resuscitation and cardioversion. Electrocardiograms and vital signs should be routinely monitored throughout the procedure.
Aortography and Selective Visceral Arteriography
- OXILAN® Injection (350 mgI/mL) is indicated for intraarterial injection in the radiographic contrast evaluation of the aorta and major visceral arterial branches. The volume and rate of contrast injection should be proportional to the blood flow through the vessels of interest, and related to the vascular and pathological characteristics of the specific vessels being studied.
- Total dose for the procedure should not usually exceed 250 mL.
Peripheral Arteriography
- OXILAN® Injection (350 mgI/mL) is indicated for intraarterial injection in the radiographic contrast evaluation of peripheral arteries. Injection rates should be approximately equal to flow rate in the vessel being injected. The usual individual injection volumes for visualization of various peripheral arteries are as follows:
- Aortic bifurcation for distal runoff: 45 mL to 100 mL (26 to 70 gI) of OXILAN® Injection - 350 (350 mgI/mL)
- Subclavian or femoral artery: 10 mL to 40 mL (4 to 14 gI) of OXILAN® Injection - 350 (350 mgI/mL)
- Total dose for the procedure should not usually exceed 250 mL.
- Pulsation should be present in the artery to be injected.
Cerebral Arteriography
- OXILAN® Injection (300 mgI/mL) is indicated for intraarterial injection in the radiographic contrast evaluation of arterial lesions of the brain. The usual individual volumes per injection are 8 mL to 12 mL (2.4 to 3.6 gI) of OXILAN® Injection - 300 (300 mgI/mL).
- Total dose for the procedure should not usually exceed 150 mL.
INTRAVENOUS PROCEDURES
Intravenous Excretory Urography
- OXILAN® Injection (300 mgI/mL or 350 mgI/mL) is indicated for intravenous injection for routine excretory urography. A volume of contrast which gives a dose of approximately 250 to 390 mgI/kg of body weight is recommended as suitable for adults with normal renal function.
- Total dose for the procedure should not usually exceed 100 mL.
Contrast Enhanced Computed Tomography
- OXILAN® Injection (300 mgI/mL or 350 mgI/mL) is indicated for intravenous injection for contrast-enhancement in the evaluation of neoplastic and non-neoplastic lesions of the head and body (intrathoracic, intraabdominal, and retroperitoneal regions).
CECT of the Head:
- The usual dose is 100 mL to 200 mL (30 to 60 gI) of OXILAN® Injection (300 mgI/mL) or 86 mL to 172 mL of OXILAN® Injection (350 mgI/mL). Scanning may be performed immediately after completion of the intravenous administration.
- Total dose for the procedure should not usually exceed 200 mL.
CECT of the Body:
- OXILAN® Injection (300 mgI/mL or 350 mgI/mL) may be administered intravenously by bolus, by rapid infusion, or by a combination of both. The usual dose is 50 mL to 200 mL (15 to 60 gI) of OXILAN(300 mgI/mL) or 43 mL to 172 mL of OXILAN® (350 mgI/mL).
- Total dose for the procedure should not usually exceed 200 mL.
DRUG HANDLING:
- As with all contrast media, because of the potential for chemical incompatibility, OXILAN® Injection should not be mixed with, or injected in, intravenous administration lines containing other drugs, solutions, or total nutritional admixtures.
- Sterile technique must be used in all vascular injections involving contrast media.
- It is desirable that intravascularly administered iodinated contrast agents be at or close to body temperature when injected.
- If non-disposable equipment is used, scrupulous care should be taken to prevent residual contamination with traces of cleaning agents.
- Withdrawal of contrast agents from their containers should be accomplished under aseptic conditions using only sterile syringes and transfer devices. Contrast agents which have been transferred into other delivery systems should be used immediately.
- discoloration prior to administration, whenever solution and container permit. Ioxilan solutions should be used only if clear and within the normal colorless to pale yellow range.
- OXILAN® formulations are supplied in single dose containers. Discard unused portion.
Direction for proper use of OXILAN®, Pharmacy Bulk Package
- The transfer of OXILAN® (ioxilan injection) from the Pharmacy Bulk Package is restricted to a suitable work area, such as a laminar flow hood.
- The container closure may be penetrated only one time, utilizing a suitable transfer device and aseptic technique.
- The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of [4] hours from initial closure entry is permitted to complete fluid transfer operations. The container should not be removed from the aseptic area during the entire 4 hour period.
- The temperature of the container should not exceed 30°C, after the closure has been entered.
Monitoring
There is limited information regarding Monitoring of Ioxilan in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ioxilan in the drug label.
Overdosage
- The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. Treatment of overdosage is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
- OXILAN® Injection binds negligibly to plasma or serum protein and can, therefore, be dialyzed.
Pharmacology
| |
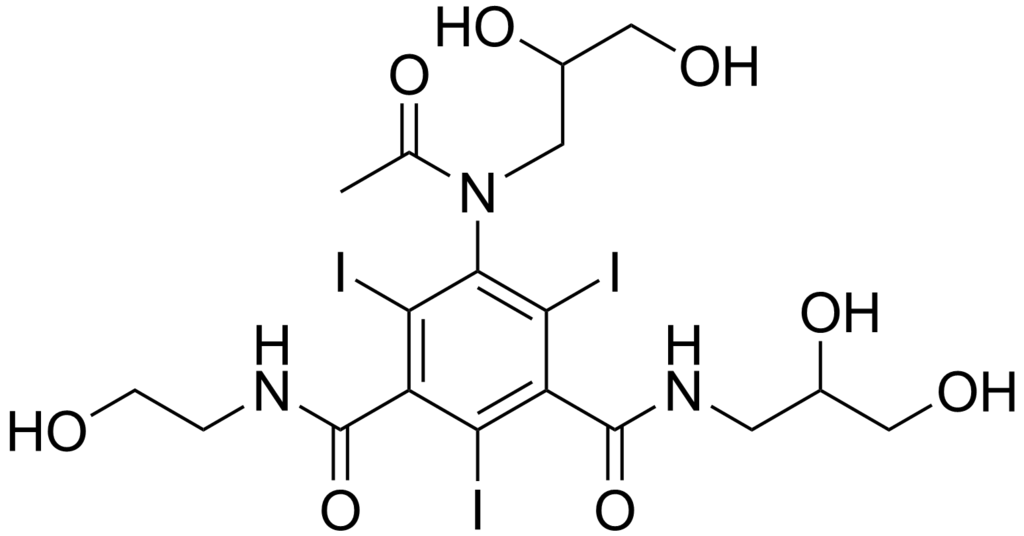
Ioxilan
| |
| Systematic (IUPAC) name | |
| 1-N-(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)acetamido]-3-N-(2-hydroxyethyl)-2,4,6-triiodobenzene-1,3-dicarboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | V08 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 791.112 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | negligible |
| Metabolism | ? |
| Half life | 2 hours |
| Excretion | Mostly renal |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | intravenously |
Mechanism of Action
There is limited information regarding Ioxilan Mechanism of Action in the drug label.
Structure
- OXILAN® (Ioxilan Injection) formulations are stable, aqueous, sterile, and non-pyrogenic solutions for intravascular administration as diagnostic radiopaque media. Ioxilan is designated chemically as N-(2,3-dihydroxypropyl)-N’-(2-hydroxyethyl)-5-[N-(2,3-dihydroxypropyl) acetamido]-2,4,6-triiodoisophthalamide and has the following structural formula:
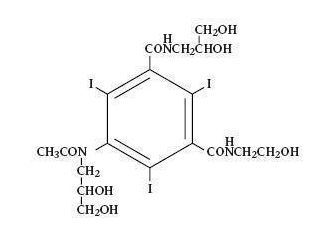
- The molecular weight of ioxilan is 791.12 and the organically bound iodine content is 48.1%. Ioxilan is nonionic and does not dissociate in solution.
- OXILAN® (Ioxilan Injection) Pharmacy Bulk Package is available in two strengths:
- OXILAN® (Ioxilan Injection) 300 mgI/mL and OXILAN® (Ioxilan Injection) 350 mgI/mL.
- Each mL of OXILAN® (Ioxilan Injection) 300 mgI/mL provides 623 mg ioxilan.
- Each mL of OXILAN® (Ioxilan Injection) 350 mgI/mL provides 727 mg ioxilan.
- Each mL of OXILAN® (Ioxilan Injection) 300 mgI/mL Pharmacy Bulk Package provides 623 mg ioxilan.
- Each mL of OXILAN® (Ioxilan Injection) 350 mgI/mL Pharmacy Bulk Package provides 727 mg ioxilan.
- Each mL of OXILAN® solution contains 0.1 mg edetate calcium disodium (anhydrous basis), 1.0 mg tromethamine, 0.5 mg sodium chloride and a minimum of 0.2 mg (0.01 mEq) sodium. The pH is adjusted to 6.8 (5.5 to 7.5) with hydrochloric acid and sodium hydroxide. The solutions contain no preservative.
- Pertinent physicochemical data are below. OXILAN® (Ioxilan Injection) is hypertonic compared to plasma (approximately 285 mOsm/kg water).
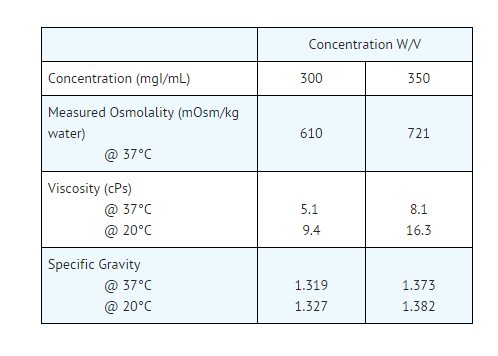
- The OXILAN® formulations are clear, colorless to pale yellow solutions containing no undissolved solids. Crystallization does not occur at room temperature. OXILAN® solutions have osmolalities of 2.1 and 2.5 times that of plasma (285 mOsm/kg water) and are hypertonic under conditions of use.
- Each bottle of OXILAN® Pharmacy Bulk Package is to be used for dispensing multiple single dose preparations utilizing a suitable transfer device.
Pharmacodynamics
- As with other iodinated contrast agents, following OXILAN® Injection, the degree of contrast enhancement is directly related to the iodine content in the administered dose; peak iodine plasma levels occur immediately following rapid intravenous injection. Iodine plasma levels fall rapidly within 5 to 10 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments.
- Intravascular Contrast: Contrast enhancement appears to be greatest immediately after bolus injections (15 seconds to 120 seconds). Thus, greatest enhancement may be detected by a series of consecutive two-to-three second scans performed within 30 to 90 seconds after injection (i.e., dynamic computed tomographic imaging).
- OXILAN® Injection may be visualized in the renal parenchyma within 30-60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1-3 minutes, with optimum contrast occurring within 5-15 minutes.
- Contrast Enhanced Computerized Tomography (CECT): AS WITH OTHER IODINATED CONTRAST AGENTS, THE USE OF OXILAN® INJECTION CONTRAST ENHANCEMENT MAY OBSCURE SOME LESIONS WHICH WERE SEEN ON PREVIOUSLY UNENHANCED CT SCANS.
- In CECT some performance characteristics are different in the brain and body. In CECT of the body, iodinated contrast agents diffuse rapidly from the vascular into the extravascular space. Following the administration of iodinated contrast agents, the increase in tissue density to x-rays is related to blood flow, the concentration of the contrast agent, and the extraction of the contrast agent by various interstitial tissues. Contrast enhancement is thus due to any relative differences in extravascular diffusion between adjacent tissues.
- In the normal brain with an intact blood-brain barrier, contrast is generally due to the presence of iodinated contrast agent within the intravascular space. The radiographic enhancement of vascular lesions, such as arteriovenous malformations and aneurysms, depends on the iodine content of the circulating blood pool.
- In tissues with a break in the blood-brain barrier, contrast agent accumulates within interstitial brain tissue. The time to maximum contrast enhancement can vary from the time that peak blood iodine levels are reached to 1 hour after intravenous bolus administration. This delay suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine containing medium within the lesion and outside the blood pool. The mechanism by which this occurs is not clear.
- IN PATIENTS WITH NORMAL BLOOD BRAIN BARRIERS and RENAL FAILURE, iodinated contrast agents have been associated with blood brain barrier DISRUPTION and ACCUMULATION OF CONTRAST IN THE BRAIN.
- The usefulness of contrast enhancement for the investigation of the retrobulbar space and of low grade or infiltrative glioma has not been demonstrated. Calcified lesions are less likely to enhance. The enhancement of tumors after therapy may decrease. The opacification of the inferior vermis following contrast agent administration has resulted in false-positive diagnosis. Cerebral infarctions of recent onset may be better visualized with contrast enhancement. Older infarctions are obscured by the contrast agent.
- For information on coagulation parameters, fibrinolysis and complement system, please refer to the Laboratory Test Findings section.
Pharmacokinetics
- In healthy young (21-27 years) male (n = 4) and female volunteers (n = 4) who each received OXILAN® Injection, 72.8 g ioxilan (35.0 g iodine), the drug showed biphasic and first order pharmacokinetics. Ioxilan is distributed mainly in the blood as suggested by the apparent volume of distribution (central compartment), 7.2 ± 1.0 L in women and 10.0 ± 2.4 L in men (mean ± sd). The total clearance values were 95.4 ± 11.1 mL·min-1 and 101.0 ± 14.7 mL·min-1 and the renal clearance values were 89.4 ± 13.3 mL·min-1 and 94.9 ± 16.6 mL·min-1 for women and men, respectively. An initial fast distribution phase with a half-life of 13.1 ± 4.2 minutes (women) or 23.5 ± 15.3 minutes (men) was followed by an elimination phase with a half-life of 102.0 ± 16.9 minutes (women) and 137 ± 35.4 minutes (men). Binding of ioxilan to plasma protein is negligible.
- The average amount of ioxilan excreted unchanged in urine at 24 hours represents 93.7% of the dose in young healthy subjects (21-27 years) after intravenous administration of OXILAN® Injection. This finding suggests that, compared to the renal excretion, biliary and/or gastrointestinal excretion are not important for OXILAN®.
- The pharmacokinetics profile and total clearance of ioxilan in patients with significantly impaired renal function have not been studied. In pooled data from 80 subjects with abnormal baseline BUNS or creatinines, who received either ioxilan (n = 44) or iohexol (n = 36), there was a higher occurrence of post-procedure increased creatinine levels (p = 0.008); also, the systolic pressure was lower at 2-6 hours (p = 0.043). Dose adjustment in patients with renal failure and blood brain barrier interaction has not been studied. OXILAN® Injection binds negligibly to plasma or serum protein and can be dialyzed.
Metabolism
- There is no evidence for metabolism of OXILAN® Injection.
Nonclinical Toxicology
- Long-term animal studies have not been performed with ioxilan to evaluate carcinogenic potential or effects on fertility. Ioxilan was not genotoxic in a series of studies including the Ames test, an in vitro human lymphocytes analysis of chromosomal aberrations, an in vivo mouse micronucleus assay, and in an in vivo mouse dominant lethal assay.
Clinical Studies
- OXILAN® Injection was administered to 834 patients in controlled and uncontrolled studies. Of these 679 patients were between 18 and 69 years of age, and 155 patients were 70 years of age or older; the mean age was 56.4 years (range 18-88). Of the 834 patients, 579 (69%) were male and 255 (31%) were female. The racial distribution was: Caucasian 668 (80%), Black 84 (10%), Hispanic 58 (7%), Asian 14 (2%), and other or unknown 10 (1%). The demographic information for patients who received the comparator (iohexol) was similar.
- In the controlled studies, 530 patients given OXILAN® Injection and 540 patients given the comparator (iohexol) were evaluated for efficacy. Efficacy assessment was based on the global evaluation of the quality of the radiographs by rating visualization as either excellent, good, poor, or no image, and on the ability to make a diagnosis. Results were compared to those with the active control (iohexol injection) at concentrations which were identical to those of OXILAN® Injection.
- Four (4) intraarterial and three (3) intravenous procedures were studied with 1 of 2 concentrations (350 mgI/mL and 300 mgI/mL). These procedures were: aortography/visceral angiography, coronary arteriography and left ventriculography, cerebral arteriography, peripheral arteriography, contrast-enhanced computed tomography (CECT) of head and body, and excretory urography.
- Cerebral arteriography was evaluated in 3 randomized, double-blind clinical trials of OXILAN® Injection 300 mgI/mL in patients with conditions such as altered cerebrovascular perfusion and/or permeability occurring in central nervous system diseases due to various CNS disorders. Results were assessed in 78 patients with OXILAN® (Ioxilan Injection) and 83 with iohexol injection 300 mgI/mL. Visualization ratings were good or excellent in 95% of the patients with OXILAN® Injection; a radiologic diagnosis was made in the majority of the patients. The results were similar to those with iohexol injection. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
- Coronary arteriography/left ventriculography was evaluated in 4 randomized, double-blind clinical trials of OXILAN® Injection 350 mgI/mL in patients with conditions such as altered coronary artery perfusion due to metabolic causes and in patients with conditions such as altered ventricular function. Results were assessed in 139 patients with OXILAN® Injection and 142 with iohexol injection 350 mgI/mL. Visualization ratings were good or excellent in 99% or more of the patients with OXILAN® Injection; a radiologic diagnosis was made in the majority of the patients. The results were similar to those with iohexol injection. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
- Aortography/visceral angiography was evaluated in 3 randomized, double-blind clinical trials of OXILAN® Injection 350 mgI/mL in patients with conditions such as altered aortic blood flow and/or visceral vascular disorders. The results were assessed in 51 patients with OXILAN® Injection 350 mgI/mL and 47 with iohexol injection 350 mgI/mL. Visualization ratings were good or excellent in the majority of the patients; a radiologic diagnosis was made in 90% of the patients with OXILAN® Injection. The results were similar to those with iohexol injection. Confirmation of radiologic findings by other diagnostic methods was not obtained.
- Intravenous excretory urography was evaluated in 2 randomized, double-blind clinical trials of OXILAN® Injection 350 mgI/mL. The results were assessed in 61 patients with OXILAN® Injection 350 mgI/mL and 62 with iohexol injection 350 mgI/mL. Visualization ratings were good or excellent in all of the patients; a radiologic diagnosis was made in 100% of the patients with OXILAN® Injection. The results were similar to those with iohexol injection. Confirmation of radiologic findings by other diagnostic methods was not obtained.
- CECT of head and body was evaluated in 5 randomized, double-blind clinical trials in patients with vascular disorders. A total of 146 patients received OXILAN® Injection 300 mgI/mL and 149 received iohexol injection 300 mgI/mL. Visualization ratings were good or excellent in 98% of the patients with OXILAN® Injection; a radiologic diagnosis was made in the majority of the patients. The results were similar to those with iohexol injection.
How Supplied
- OXILAN®(Ioxilan Injection) 300 mgI/mL
- Ten 50 mL single dose bottles, NDC 67684-1000-1
- Ten 100 mL single dose bottles, NDC 67684-1000-2
- Ten 150 mL single dose bottles, NDC 67684-1000-3
- OXILAN®(Ioxilan Injection) 350 mgI/mL
- Ten 50 mL single dose bottles, NDC 67684-1001-1
- Ten 100 mL single dose bottles, NDC 67684-1001-2
- Ten 150 mL single dose bottles, NDC 67684-1001-3
- Ten 200 mL single dose bottles, NDC 67684-1001-4
- OXILAN® (Ioxilan Injection) 300 mgI/mL Pharmacy Bulk Package - Six 500 mL bottles, NDC 67684-1000-5
- OXILAN® (Ioxilan Injection) 350 mgI/mL Pharmacy Bulk Package - Six 500 mL bottles, NDC 67684-1001-5
Storage
- Store at room temperature between 15° and 30°C (59° and 86°F) and protect from light. Do not freeze.
Images
Drug Images
{{#ask: Page Name::Ioxilan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




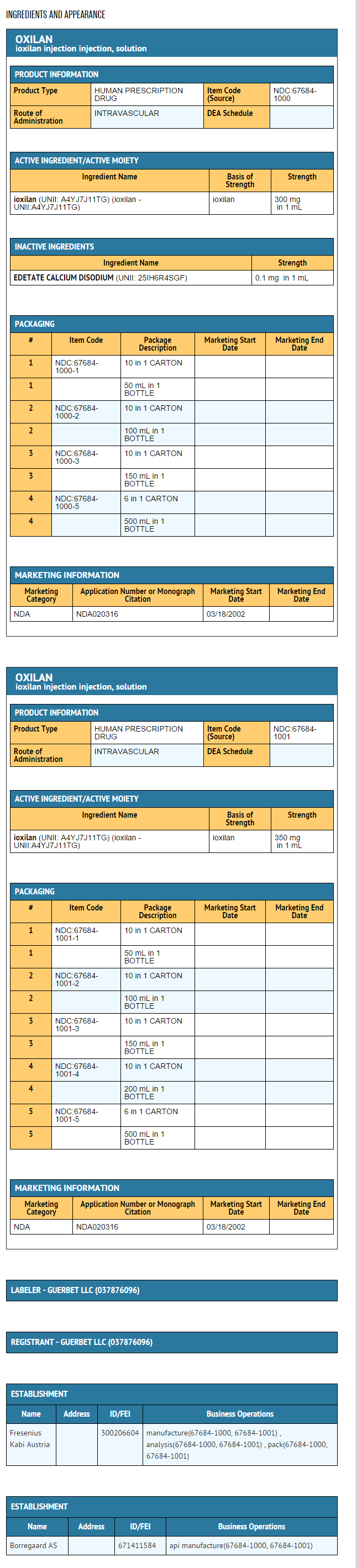
{{#ask: Label Page::Ioxilan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients:
- Patients receiving iodinated intravascular contrast agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease, or known thyroid disorder.
- Inform your physician if you are allergic to any drugs, or food, or if you have immune, autoimmune or immune deficiency disorders. Also inform your physician if you had any reactions to previous injections of dyes used for x-ray procedures.
- Inform your physician about all medications you are currently taking, including non-prescription drugs (over-the-counter) drugs, before you have this procedure.
Precautions with Alcohol
- Alcohol-Ioxilan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- OXILAN®[1]
Look-Alike Drug Names
- A® — B®[2]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "ioxilan injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ioxilan
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ioxilan |Label Name=Ioxilan11.png
}}
{{#subobject:
|Label Page=Ioxilan |Label Name=Ioxilan11.png
}}