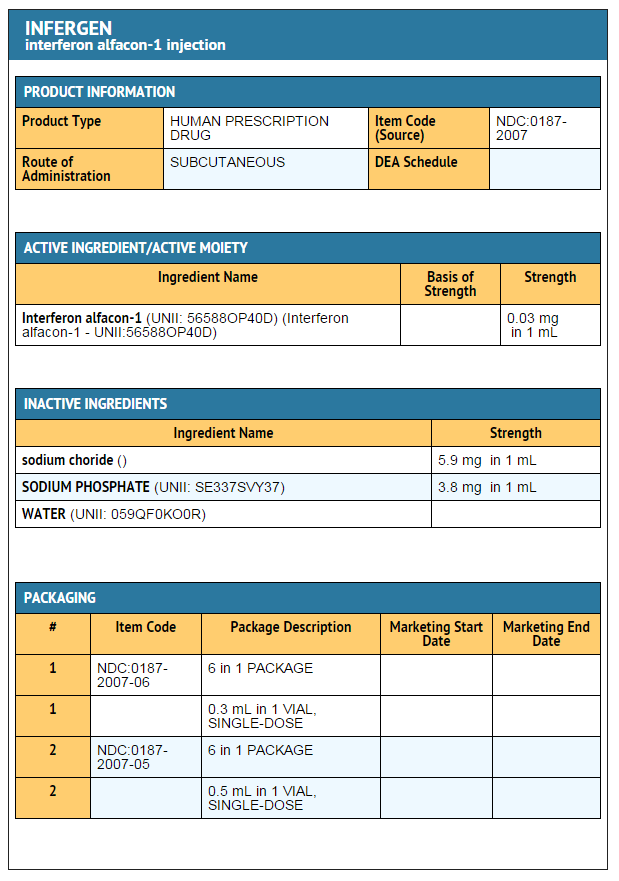
Interferon alfacon-1
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Interferon alfacon-1 is a Immunological Agent that is FDA approved for the treatment of INFERGEN is indicated for the treatment of chronic HCV infection in patients 18 years of age or older with compensated liver disease who have anti-HCV serum antibodies and/or the presence of HCV RNA. Other causes of hepatitis, such as viral hepatitis B or autoimmune hepatitis, should be ruled out prior to initiation of therapy with INFERGEN. In some patients with chronic HCV infection, INFERGEN normalizes serum ALT, reduces serum HCV RNA concentrations to undetectable quantities (< 100 copies/mL), and improves liver histology.. Common adverse reactions include Injection site reactions, fatigue, fever, rigors, body pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- INFERGEN is indicated for the treatment of chronic HCV infection in patients 18 years of age or older with compensated liver disease who have anti-HCV serum antibodies and/or the presence of HCV RNA. Other causes of hepatitis, such as viral hepatitis B or autoimmune hepatitis, should be ruled out prior to initiation of therapy with INFERGEN. In some patients with chronic HCV infection, INFERGEN normalizes serum ALT, reduces serum HCV RNA concentrations to undetectable quantities (< 100 copies/mL), and improves liver histology.
Dosage
- The recommended dose of INFERGEN for treatment of chronic HCV infection is 9 mcg TIW administered SC as a single injection for 24 weeks. At least 48 hours should elapse between doses of INFERGEN.
- Patients who tolerated previous interferon therapy and did not respond or relapsed following its discontinuation may be subsequently treated with 15 mcg of INFERGEN TIW administered SC as a single injection for up to 48 weeks.
- There are significant differences in specific activities among interferons. Healthcare providers should be aware that changes in interferon brand may require adjustments of dosage and/or change in route of administration. Patients should be warned not to change brands of interferon without medical consultation. Patients should also be instructed by their physician not to reduce the dosage of INFERGEN prior to medical consultation.
Dose Reduction
- For patients who experience a severe adverse reaction on INFERGEN, dosage should be withheld temporarily. If the adverse reaction does not become tolerable, therapy should be discontinued. Dose reduction to 7.5 mcg may be necessary following an intolerable adverse event. In the pivotal study, 11% of patients (26/231) who initially received INFERGEN at a dose of 9 mcg (0.3 mL) were dose-reduced to 7.5 mcg (0.25 mL).
- If adverse reactions continue to occur at the reduced dosage, the physician may discontinue treatment or reduce dosage further. However, decreased efficacy may result from continued treatment at dosages below 7.5 mcg.
- During subsequent treatment for 48 weeks with 15 mcg of INFERGEN, up to 36% of patients required dose reductions in 3 mcg increments.
Administration of INFERGEN
- If home use is determined to be desirable by the physician, instructions on appropriate use should be given by a healthcare professional. After administration of INFERGEN, it is essential to follow the procedure for proper disposal of syringes and needles.
Storage
- Just prior to injection, INFERGEN may be allowed to reach room temperature.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration; if particulates or discoloration are observed, the container should not be used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Interferon alfacon-1 in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Interferon alfacon-1 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness has not been established on pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Interferon alfacon-1 in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Interferon alfacon-1 in pediatric patients.
Contraindications
- known hypersensitivity to alpha interferons or to any component of the product
Warnings
- Treatment with INFERGEN should be administered under the guidance of a qualified physician, and may lead to moderate-to-severe adverse experiences requiring dose reduction, temporary dose cessation, or discontinuation of further therapy.
- Withdrawal from study for adverse events occurred in 7% of patients initially treated with 9 mcg INFERGEN (including 4% due to psychiatric events). Withdrawal from study due to adverse events occurred in 5% of patients subsequently treated with 15 mcg INFERGEN for 24 weeks and 11% of patients subsequently treated with 15 mcg INFERGEN for 48 weeks.
Neuropsychiatric Disorders
- Severe psychiatric adverse events may manifest in patients receiving therapy with alpha interferons, including INFERGEN. Depression, suicidal ideation, suicide attempt, and suicide may occur. Other prominent psychiatric adverse events may also occur, including psychosis, aggressive behavior, nervousness, anxiety, emotional liability, abnormal thinking, agitation, apathy and relapse of drug addiction. INFERGEN should be used with extreme caution in patients who report a history of depression. Physicians should monitor all patients for evidence of depression and other psychiatric symptoms. Prior to initiation of INFERGEN therapy, physicians should inform patients of the possible development of depression and patients should be advised to immediately report any sign or symptom of depression and/or suicidal ideation. In severe cases, therapy should be stopped immediately and psychiatric intervention instituted.
Bone Marrow Toxicity
- Alpha interferons suppress bone marrow function and may result in severe cytopenias including very rare events of aplastic anemia. It is advised that complete blood counts be obtained pretreatment and monitored routinely during therapy. Alpha interferon therapy should be discontinued in patients who develop severe decreases in neutrophil (<0.5 x 109/L) or platelet counts (<50 x 109/L).
Cardiovascular Disorders
- Hypertension, tachycardia, palpitation, and tachyarrhythmias have been reported in patients treated with INFERGEN. INFERGEN should be administered with caution to patients with preexisting cardiac disease. Supraventricular arrhythmias, chest pain, and myocardial infarction have been associated with alpha interferon therapies.8
Hypersensitivity
- Serious acute hypersensitivity reactions have been reported in rare instances following treatment with alpha interferons. If hypersensitivity reactions occur (e.g., urticaria, angioedema, bronchoconstriction, anaphylaxis), INFERGEN should be discontinued immediately and appropriate medical treatment instituted.
Endocrine Disorders
- INFERGEN should be administered with caution to patients with a history of endocrine disorders. Occurrence or aggravation of hyperthyroidism or hypothyroidism have been reported with INFERGEN. Hyperglycemia and diabetes mellitus have also been observed in patients treated with INFERGEN. Patients who develop these conditions during treatment that cannot be controlled with medication should not continue INFERGEN therapy.
Autoimmune Disorders
- Development of or exacerbation of autoimmune disorders (e.g., autoimmune thrombocytopenia, idiopathic thrombocytopenic purpura, psoriasis, rheumatoid arthritis) have been reported in patients receiving alpha interferon therapies, including INFERGEN. INFERGEN should not be used in patients with autoimmune hepatitis and should be used with caution in patients with other autoimmune disorders.
Pulmonary Disorder
- Pneumonia and interstitial pneumonitis, some resulting in respiratory failure and/or patient deaths, have been induced or aggravated by alpha interferon therapy, including INFERGEN. Patients who develop persistent or unexplained pulmonary infiltrates or pulmonary function impairment should discontinue treatment with INFERGEN.
Colitis
- Hemorrhagic/ischemic colitis, sometimes fatal, has been observed within 12 weeks of alpha interferon therapies and has been reported in patients treated with INFERGEN. INFERGEN treatment should be discontinued immediately in patients who develop signs and symptoms of colitis.
Pancreatitis
- Pancreatitis, sometimes fatal, has been observed in patients treated with alpha interferons, including INFERGEN. INFERGEN should be suspended in patients with signs and symptoms suggestive of pancreatitis and discontinued in patients diagnosed with pancreatitis.
Hepatic Exacerbations and Decompensated Hepatic Disease
- Chronic hepatitis C patients with cirrhosis may be at risk of hepatic decompensation when treated with alpha interferons, including INFERGEN. During treatment, patients’ clinical status and hepatic function should be closely monitored, and INFERGEN treatment should be immediately discontinued if symptoms of hepatic decompensation, such as jaundice, ascites, coagulopathy, or decreased serum albumin, are observed.
Ophthalmologic Disorders
- Decrease or loss of vision, retinopathy including macular edema, retinal artery or vein thrombosis, retinal hemorrhages and cotton wool spots; optic neuritis, and papilledema are induced or aggravated by treatment with INFERGEN or other alpha interferons. All patients should receive an eye examination at baseline. Patients with preexisting ophthalmologic disorders (e.g., diabetic or hypertensive retinopathy) should receive periodic ophthalmologic exams during interferon alpha treatment. Any patient who develops ocular symptoms should receive a prompt and complete eye examination. INFERGEN therapy should be discontinued in patients who develop new or worsening ophthalmologic disorders.
Cerebrovascular Disorders
- Ischemic and hemorrhagic cerebrovascular events have been observed in patients treated with interferon alfa-based therapies, including INFERGEN. Events occurred in patients with few or no reported risk factors for stroke, including patients less than 45 years of age. Because these are spontaneous reports, estimates of frequency cannot be made and a causal relationship between interferon alfa-based therapies and these events is difficult to establish.
Precautions
General
- While fever may be related to the flu-like symptoms reported in patients treated with INFERGEN, when fever occurs, other possible causes of persistent fever should be ruled out.
Bone Marrow Toxicity
- INFERGEN should be used cautiously in patients with abnormally low peripheral blood cell counts or who are receiving agents that are known to cause myelosuppression. Transplantation patients or other chronically immunosuppressed patients should receive alpha interferon therapy with caution.
Renal Impairment
- Increases in serum creatinine levels, and rarely renal failure, have been observed in patients receiving INFERGEN. INFERGEN has not been studied in patients with renal insufficiency. Patients with impaired renal function should be closely monitored and INFERGEN should be used with caution in patients with renal insufficiency.
Laboratory Tests
- Laboratory tests are recommended for all patients on INFERGEN therapy, prior to beginning treatment (baseline), 2 weeks after initiation of therapy, and periodically thereafter during the 24 or 48 weeks of therapy at the discretion of the physician. Following completion of INFERGEN therapy, any abnormal test values should be monitored periodically. The entrance criteria that were used for the clinical study of INFERGEN may be considered as a guideline to acceptable baseline values for initiation of treatment:
Platelet count ≥ 75 x 109/L
Hemoglobin concentration ≥ 100 g/L
ANC ≥ 1500 x 106/L
Serum creatinine concentration < 180 μmol/L (< 2.0 mg/dL) or creatinine clearance > 0.83 mL/second (> 50 mL/minute)
Serum albumin concentration ≥ 25 g/L
Bilirubin within normal limits
TSH and T4 within normal limits
Neutropenia, thrombocytopenia, hypertriglyceridemia, and thyroid disorders have been reported with administration of INFERGEN. Therefore, these laboratory parameters should be monitored closely.
Adverse Reactions
Clinical Trials Experience
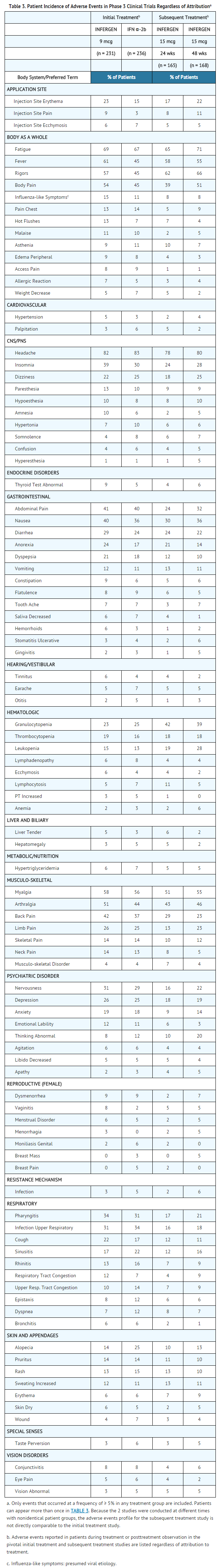
- Adverse experiences that were reported, regardless of attribution to treatment, in at least 5% of patients in the 9 mcg INFERGEN or 3 MIU IFN α-2b groups of the pivotal study are presented in TABLE 3, listed in decreasing order by the 9 mcg INFERGEN group. The incidence of adverse events is expressed based on the number of patients experiencing each event at least once during treatment or during posttreatment observation.
- Most adverse events were mild-to-moderate in severity and abated with cessation of therapy. Flu-like symptoms (i.e., headache, fatigue, fever, rigors, myalgia, sweating increased, and arthralgia) were the most frequently reported treatment-related adverse reactions. Most were short-lived and could be treated symptomatically.
- Depression, usually mild-to-moderate in severity, was reported in 26% of patients who received 9 mcg INFERGEN and was the most common adverse event resulting in study drug discontinuation.
- In patients who had tolerated previous interferon therapy (9 mcg INFERGEN or 3 MIU IFN α-2b) and failed to normalize ALT, or who had achieved normalization of ALT during the treatment period but who relapsed during the posttreatment observation period, subsequent treatment with 15 mcg TIW of INFERGEN for 24 or 48 weeks was generally tolerated. Adverse experiences of patients receiving subsequent treatment, regardless of attribution to treatment, are reported in TABLE 3. The higher dose of INFERGEN used in these patients was associated with a greater incidence of leukopenia and granulocytopenia. One or more dose reductions for all causes were required in up to 36% of patients. Patients who do not tolerate initial standard interferon therapy should not receive therapy with 15 mcg TIW of INFERGEN.
Laboratory Values
- The following laboratory values were found to be affected by therapy with INFERGEN in the 231 patients who received treatment with 9 mcg INFERGEN.
- Hemoglobin and Hematocrit: Treatment with INFERGEN was associated with gradual decreases in mean values for hemoglobin and hematocrit, which were 4% and 5% below baseline at the end of treatment. Decreases from baseline of 20% or more in hemoglobin or hematocrit were seen in 1% of patients or less.
- White Blood Cells: INFERGEN treatment was associated with decreases in mean values for both total white blood cell (WBC) count and ANC within the first 2 weeks of treatment. By the end of treatment, mean decreases from baseline of 19% for WBCs and 23% for ANC were observed. These effects reversed during the posttreatment observation period. In 2 INFERGEN-treated patients in the phase 3 trial, decreases in ANC to levels below 500 x 106 cells/L were seen. In both cases, the ANC returned to clinically acceptable levels with reduction of the dose of INFERGEN, and these transient decreases in neutrophils were not associated with infections.
- Platelets: INFERGEN treatment was associated with alterations in platelet count. Decreases in mean platelet count of 16% compared to baseline were seen by the end of treatment. These decreases were reversed during the posttreatment observation period. Values below normal were common during treatment with 3% of patients developing values less than 50 x 109 cells/L, usually necessitating dose reduction.
- Triglycerides: Mean values for serum triglyceride increased shortly after the start of administration of INFERGEN, with increases of 41%, compared with baseline, at the end of the treatment period. Seven percent of the patients developed values which were at least 3 times above pretreatment levels during treatment. This effect was promptly reversed after discontinuation of treatment.
- Thyroid Function: INFERGEN treatment was associated with biochemical changes consistent with hypothyroidism including increases in TSH and decreases in T4 mean values. Increases in TSH to greater than 7 mU/L were seen in 10% of 9 mcg INFERGEN-treated patients either during the treatment period or the 24-week posttreatment observation period. Thyroid supplements were instituted in approximately one-third of these patients.
- Laboratory Values for Subsequent Treatment: From a database of 165 patients receiving subsequent treatment with 15 mcg of INFERGEN for 24 weeks, and 168 patients receiving subsequent treatment with 15 mcg of INFERGEN for 48 weeks after failing initial interferon therapy, similar changes in the laboratory values as outlined above were observed. Mean decreases from baseline up to 23% for WBCs and up to 27% for ANC were observed for patients subsequently treated with interferon, which was greater than during initial treatment. Two patients in the 24-week group experienced reversible reductions in ANC to less than 500 x 106 cells/L, which were not associated with infectious complications. No patients discontinued as a result of hematologic toxicity.
Postmarketing Experience
- In addition, the following potential adverse reactions have been reported during post-approval use of INFERGEN. Because the reports of these adverse events are voluntary and the population of uncertain size, it is not possible to reliably estimate the frequency of the reaction or establish a causal relationship to drug exposure.
- Application site: injection site reaction, including injection site necrosis ulcer, and bruising; Ear and Labyrinth: hearing loss, hearing impairment; Gastrointestinal: abdominal distention, gastrointestinal bleeding, gastritis; Hepatobiliary: hepatic enzyme elevations, including ALT and AST elevation, abnormal hepatic function, hyperbilirubinemia, jaundice, ascites, hepatic encephalopathy; Infections: sepsis; Metabolism and Nutritional: dehydration; Musculoskeletal: rhabdomyolysis, arthritis, bone pain; Nervous: speech disorder, ataxia, gait abnormal, convulsions, loss of consciousness, memory impairment, tremors, visual field defect; Psychiatric: delusions, hallucinations; Skin and Subcutaneous: bruising, pyoderma gangrenosum, toxic epidermal necrolysis; Vascular Disorders: hemorrhage
Drug Interactions
- No formal drug interaction studies have been conducted with INFERGEN. INFERGEN should be used cautiously in patients who are receiving agents that are known to cause myelosuppression or with agents known to be metabolized via the cytochrome P-450 pathway.9 Patients taking drugs that are metabolized by this pathway should be monitored closely for changes in the therapeutic and/or toxic levels of concomitant drugs.
Use in Specific Populations
Pregnancy
- INFERGEN has been shown to have embryolethal or abortifacient effects in golden Syrian hamsters when given at 135 times the human dose and in cynomolgus and rhesus monkeys when given at 9 to 81 times (based on body surface area) the human dose. There are no adequate and well-controlled studies in pregnant women. INFERGEN should not be used during pregnancy. If a woman becomes pregnant or plans to become pregnant while taking INFERGEN, she should be informed of the potential hazards to the fetus. Males and females treated with INFERGEN should be advised to use effective contraception.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Interferon alfacon-1 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Interferon alfacon-1 during labor and delivery.
Nursing Mothers
- It is not known whether INFERGEN is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if INFERGEN is administered to a nursing woman. The effect on the nursing neonate of orallyingested INFERGEN in breast milk has not been evaluated.
Pediatric Use
The safety and effectiveness of INFERGEN have not been established in patients below the age of 18 years. INFERGEN therapy is not recommended in pediatric patients.
Geriatic Use
- Clinical studies of INFERGEN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, treatment with Interferons, including INFERGEN, is associated with psychiatric, cardiac, and systemic (flu-like) adverse effects. Since decreased hepatic, renal or cardiac function, concomitant disease and the use of other drug therapies in elderly patients may produce adverse reactions of greater severity, caution should be exercised in the use of INFERGEN in this population.
Gender
There is no FDA guidance on the use of Interferon alfacon-1 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Interferon alfacon-1 with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Interferon alfacon-1 in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Interferon alfacon-1 in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Interferon alfacon-1 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Interferon alfacon-1 in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- It is advised that complete blood counts be obtained pretreatment and monitored routinely during therapy
- During treatment, patients’ clinical status and hepatic function should be closely monitored, and INFERGEN treatment should be immediately discontinued if symptoms of hepatic decompensation, such as jaundice, ascites, coagulopathy, or decreased serum albumin, are observed
- Patients with impaired renal function should be closely monitored and INFERGEN should be used with caution in patients with renal insufficiency.
IV Compatibility
There is limited information regarding IV Compatibility of Interferon alfacon-1 in the drug label.
Overdosage
- In INFERGEN trials, the maximum overdose reported was a dose of 150 mcg INFERGEN administered SC in a patient enrolled in a phase 1 advanced malignancy trial. The patient received 10 times the prescribed dosage for 3 days. The patient experienced a mild increase in anorexia, chills, fever, and myalgia. Increases in ALT (15 to 127 IU/L), aspartate transaminase (AST) (15 to 164 IU/L), and lactic dehydrogenase (LDH) (183 to 281 IU/L) were reported. These laboratory values returned to normal or to the patient’s baseline values within 30 days.
Pharmacology
Interferon alfacon-1
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | L03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 19343 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Parenteral |
Mechanism of Action
- Interferons are a family of naturally occurring, small protein molecules with molecular weights of 15,000 to 21,000 daltons that are produced and secreted by cells in response to viral infections or to various synthetic and biological inducers. Two major classes of interferons have been identified (i.e., type-I and type-II). Type-I interferons include a family of more than 25 alpha interferons as well as beta interferon and omega interferon. While all alpha interferons have similar biological effects, not all the activities are shared by each alpha interferon and, in many cases, the extent of activity varies substantially for each interferon subtype.
- All type-I interferons share common biological activities generated by binding of interferon to the cell-surface receptor, leading to the production of several interferon-stimulated gene products. Type-I interferons induce pleiotropic biologic responses which include antiviral, antiproliferative, and immunomodulatory effects, regulation of cell surface major histocompatibility antigen (HLA class I and class II) expression and regulation of cytokine expression. Examples of interferon-stimulated gene products include 2'5' oligoadenylate synthetase (2'5'OAS) and β-2 microglobulin.
- The antiviral, antiproliferative, natural killer (NK) cell activation, and gene-induction activities of INFERGEN have been compared with other recombinant alpha interfereons in in vitro assays and have demonstrated similar ranges of activity. INFERGEN exhibited at least 5 times higher specific activity in vitro than Interferon alfa-2a and Interferon alfa-2b.2 Comparison of INFERGEN with a WHO international potency standard for recombinant alpha interferon (83/514) revealed that the specific activity of INFERGEN in both an in vitro antiviral cytopathic effect assay and an antiproliferative assay was 1 x 109 U/mg. However, correlation between in vitro activity and clinical activity of any interferon is unknown.
Structure
There is limited information regarding Interferon alfacon-1 Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Interferon alfacon-1 in the drug label.
Pharmacokinetics
- The pharmacokinetic properties of INFERGEN have not been evaluated in patients with chronic hepatitis C. Pharmacokinetic profiles were evaluated in normal, healthy volunteer subjects after SC injection of 1, 3, or 9 mcg INFERGEN. Plasma levels of INFERGEN after SC administration of any dose were too low to be detected by either enzyme-linked immunosorbent assay (ELISA) or by inhibition of viral cytopathic effect. However, analysis of INFERGEN-induced cellular products (induction of 2'5' OAS and β-2 microglobulin) after treatment in these subjects revealed a statistically significant, dose-related increase in the area under the curve (AUC) for the levels of 2'5' OAS or β-2 microglobulin induced over time (p < 0.001 for all comparisons). Concentrations of 2'5' OAS were maximal at 24 hours after dosing, while serum levels of β-2 microglobulin appeared to reach a maximum 24 to 36 hours after dosing. The dose-response relationships observed for 2'5' OAS and β-2 microglobulin were indicative of biological activity after SC administration of 1 to 9 mcg INFERGEN.
Preclinical Experience
- All interferons have been shown to be highly species-specific. Antiviral activity of INFERGEN was observed in the rhesus monkey LLC cell line and golden Syrian hamster BHK cell line. Antiviral activity of INFERGEN in the golden Syrian hamster was confirmed further in vivo.3 Pharmacokinetic studies of INFERGEN in golden Syrian hamsters and rhesus monkeys demonstrated rapid absorption following SC injection. Peak serum concentrations of INFERGEN were observed at 1 hour and 4 hours in golden Syrian hamsters and in rhesus monkeys, respectively. Subcutaneous bioavailability was high in both species, averaging 99% in golden Syrian hamsters and 83% to 104% in rhesus monkeys. Clearance of INFERGEN, averaging 1.99 mL/minute/kg in golden Syrian hamsters and 0.71 to 0.92 mL/minute/kg in rhesus monkeys, was due predominantly to catabolism and excretion by the kidneys. The terminal half-life of INFERGEN following SC dosing was 1.3 hours in golden Syrian hamsters and 3.4 hours in rhesus monkeys. Upon 7-day multiple SC dosing, no accumulation of serum levels was observed in golden Syrian hamsters.
- In preclinical toxicology studies in golden Syrian hamsters and rhesus monkeys, administration of INFERGEN at doses of up to 100 mcg/kg/day was associated with decreased body weight, decreased food consumption, and bone marrow suppression. High-dose chronic exposure at doses of 10 to 100 mcg/kg/day (50- to 500-fold higher than the maximum clinical dose given daily) in rhesus monkeys was not tolerated for greater than 1 month, due to the development of vascular leak syndrome.
- Reproductive toxicity studies in pregnant rhesus monkeys and golden Syrian hamsters demonstrated an increase in fetal loss in hamsters treated with INFERGEN at doses of > 150 mcg/kg/day and in rhesus monkeys at doses of 3 and 10 mcg/kg/day. The INFERGEN toxicity profile described is consistent with the known toxicity profile of other alpha interferons.4
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis: No carcinogenicity data for INFERGEN are available in animals or humans.
- Mutagenesis: INFERGEN was not mutagenic when tested in several in vitro assays, including the Ames bacterial mutagenicity assay and an in vitro cytogenetic assay in human lymphocytes, either in the presence or absence of metabolic activation.
- Impairment of Fertility: INFERGEN at doses as high as 100 mcg/kg did not selectively affect reproductive performance or the development of the offspring when administered SC to male and female golden Syrian hamsters for 70 and 14 days before mating, respectively, and then through mating and to day 7 of pregnancy.
Clinical Studies
There is limited information regarding Clinical Studies of Interferon alfacon-1 in the drug label.
How Supplied
- Use only 1 dose per vial; do not re-enter the vial. Discard unused portions. Do not save unused drug for later administration.
- Single-dose, preservative-free vials containing 9 mcg (0.3 mL) of Interferon alfacon-1 are available in dispensing packs of 6 vials (NDC 0187-2007-06).
- Single-dose, preservative-free vials containing 15 mcg (0.5 mL) of Interferon alfacon-1 are available in dispensing packs of 6 vials (NDC 0187-2006-05).
Storage
- INFERGEN should be stored in the refrigerator at 2° to 8°C (36° to 46°F). Do not freeze. Avoid vigorous shaking and exposure to direct sunlight.
Images
Drug Images
{{#ask: Page Name::Interferon alfacon-1 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
INGREDIENTS AND APPEARANCE
{{#ask: Label Page::Interferon alfacon-1 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- If home use is determined to be desirable by the physician, instructions on appropriate use should be given by a healthcare professional. The patient must be instructed as to the proper dosage and administration. Information included in the MEDICATION GUIDE should be fully reviewed with the patient; it is not a disclosure of all, or possible, adverse effects. The most common adverse reactions occurring with INFERGEN therapy are flu-like symptoms including fatigue, fever, rigors, headache, arthralgia, myalgia, and increased sweating. Non-narcotic analgesics and bedtime administration of INFERGEN may be used to prevent or lessen some of these symptoms.
- Additionally, patients must be thoroughly instructed in the importance of proper disposal procedures and cautioned against the reuse of needles, syringes, or re-entry of the drug product. A puncture-resistant container for the disposal of used syringes and needles should be used by the patient and should be disposed of according to the directions provided by the health care provider
MEDICATION GUIDE
Precautions with Alcohol
- Alcohol-Interferon alfacon-1 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- INFERGEN®[1]
Look-Alike Drug Names
There is limited information regarding the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.