Intravascular ultrasound
| Intravascular ultrasound | |
 | |
|---|---|
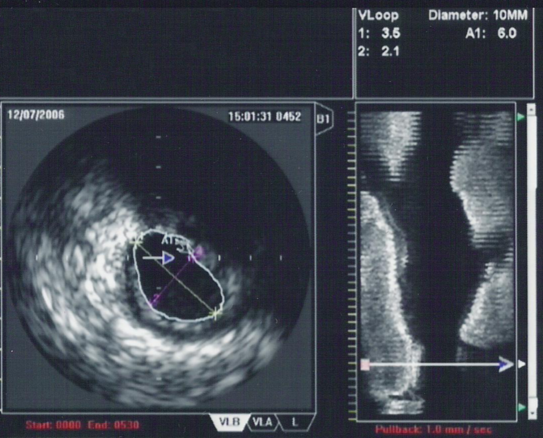
| An IVUS image of the ostial left main coronary artery (left). The blue outline delineates the cross-sectional area of the lumen of the artery (A1 in the upper right corner), measuring 6.0 mm2. A 2-dimensional mapping of the proximal LAD and left main coronary arteries is shown on the right |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Intravascular ultrasound (IVUS) is a medical imaging methodology using a specially designed catheter with a miniaturized ultrasound probe attached to the distal end the catheter. The proximal end of the catheter is attached to computerized ultrasound equipment. It allows the application of ultrasound technology to see from inside blood vessels out through the surrounding blood column, visualizing the endothelium (inner wall) of blood vessels in living individuals.
Assessment of Atherosclerosis
The arteries of the heart (the coronary arteries) are the most frequent imaging target for IVUS. IVUS is used in the coronary arteries to determine the amount of atheromatous plaque built up at any particular point in the epicardial coronary artery. The progressive accumulation of plaque within the artery wall over decades is the setup for vulnerable plaque which, in turn, leads to myocardial infarction and stenosis (narrowing) of the artery (known as coronary artery lesions). IVUS is of use to determine both plaque volume within the wall of the artery and/or the degree of stenosis of the artery lumen. It can be especially useful in situations in which angiographic imaging is considered unreliable; such as for the lumen of ostial lesions or where angiographic images do not visualize lumen segments adequately, such as regions with multiple overlapping arterial segments. It is also used to assess the effects of treatments of stenosis such as with hydraulic angioplasty expansion of the artery, with or without stents, and the results of medical therapy over time.
Arteries can either "positively remodel" or "negatively remodel". If there is an outward expansion of the artery to accommodate the plaque, this is referred to as positive or outward, or expansive modeling. Until the plaque occupies 40 to 50% of the volume of the artery there is no luminal encroachment.in contrast, if the lumen is encroached upon this is called negative, and word or constrictive remodeling.
Advantages over Angiography
Arguably the most valuable use of IVUS is to visualize plaque, which cannot be seen by angiography. It has been increasingly used in research to better understand the behavior of the atherosclerosis process in living people. Based on the angiographic view and long popular medical beliefs, it had long been assumed that areas of high grade stenosis (narrowing) of the lumen (opening) within the coronary arteries, visible by angiography, were the likely points at which most myocardial infarctions would occur. Research using IVUS has helped to reveal the fallacy (in most instances) of this belief.
IVUS enables accurately visualizing not only the lumen of the coronary arteries but also the atheroma (membrane/cholesterol loaded white blood cells) "hidden" within the wall. IVUS has thus enabled advances in clinical research providing a more thorough perspective and better understanding.
In the early 1990s, IVUS research on the re-stenosis problem after angioplasty lead to recognition that most of the re-stenosis problem (as visualized by an angiography examination) was not true re-stenosis. Instead it was simply a remodeling of the atheromatous plaque, which was still protruding into the lumen of the artery after completion of angioplasty; the stenosis only appearing to be reduced because blood and contrast could now flow around and through some of the plaque. The angiographic dye column appeared widened adequately; yet considerable plaque was within the newly widened lumen and the lumen remained partially obstructed. This recognition promoted more frequent use of stents to hold the plaque outward against the inner artery walls, out of the lumen.
Additionally, IVUS examinations, as they were done more frequently, served to reveal and confirm the autopsy research findings of the late 1980s, showing that atheromatous plaque tends to cause expansion of the internal elastic lamina, causing the degree of plaque burden to be greatly underestimated by angiography.[1] Angiography only reveals the edge of the atheroma that protrudes into the lumen.[2]
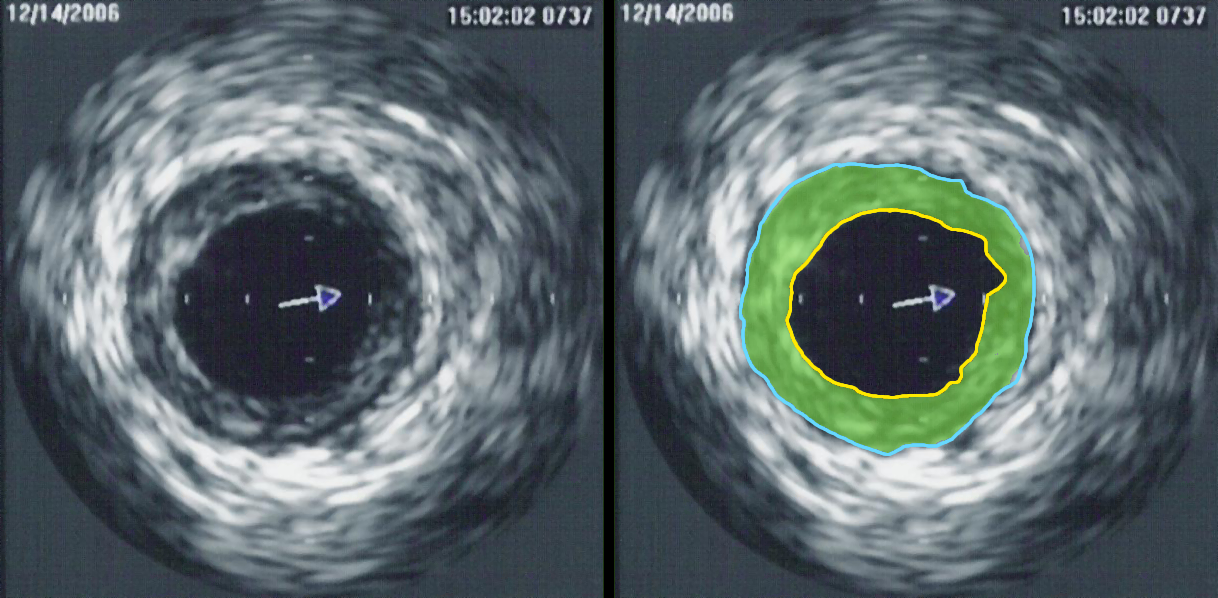
Perhaps the greatest contribution to understanding, so far, was achieved by clinical research trials completed in the United States in the late 1990s, using combined angiography and IVUS examination, to study which coronary lesions most commonly result in a myocardial infarction. The studies revealed that most myocardial infarctions occur at areas with extensive atheroma within the artery wall, however very little stenosis of the artery opening. The range of lumen stenosis locations at which myocardial infarctions occurred ranged from areas of mild dilatation all the way to areas of greater than 95% stenosis. However the average or typical stenosis at which myocardial infarctions occurred were found to be less than 50%, describing plaques long considered insignificant by many. Only 14% of myocardial infarction occurred at locations with 75% or more stenosis, the severe stenoses previously thought by many to present the greatest danger to the individual. This research has changed the primary focus for myocardial infarction prevention from severe narrowing to vulnerable plaque.
Current clinical uses of IVUS technology include checking how to treat complex lesions before angioplasty and checking how well an intracoronary stent has been deployed within a coronary artery after angioplasty. If a stent is not expanded flush against the wall of the vessel, turbulent flow may occur between the stent and the wall of the vessel; some fear this might create a nidus for acute thrombosis of the artery.
Disadvantages of Intravascular Ultrasound
- Expense
- The computerized IVUS echocardiographic recording and display equipment generally costs over $200,000, US, 2004. The disposable catheters used to do each examination typically cost ~$700-950, US, 2004. In many hospitals, the IVUS recording and display equipment is on loan to the catheterization lab from the manufacturing company, with the understanding that the lab has to purchase the specialized IVUS catheters. Because no standard exists, IVUS catheters cannot be interchanged between different manufacturers.
- Increase in the time of the procedure
- It is considered an interventional procedure, and should only be performed by angiographers that are trained in interventional cardiology techniques
- Risk imposed by the use of the IVUS catheter including coronary thrombosis
Indications
Intravascular ultrasound is used in the following ways:
To Assess the Severity of Lesions
- Angiography often underestimates the severity of lesions. The angiogram only evaluates the lumen, and does not evaluate the plaque burden in an artery. If a lesion is present ultrasound will generally done is demonstrate that 50 to 60% of the volume of the artery is made up of plaque both proximal and distal to the lesion.
- Intravascular ultrasound has been related to defects on nuclear imaging and Doppler flow wire measurements of coronary flow reserve (CFR) and fractional flow reserve (FFR) to validate its accuracy.
- The consensus view is that any minimum lumen area (MLA) < 4 mm² in an artery that is > 3 mm on angiography (excluding the left main) is a significant stenosis.
To Assess the Underlying Morphology of Lesions
Intravascular ultrasound is more sensitive in the assessment of calcium, particularly the presence of calcium deep in the wall of the artery. In one study-this detective calcium and 73% of patients where this angiography detective calcium and only 30% of patients. The sensitivity of angiography is approximately 25% if there is one quadrant of calcium present, the sensitivity is 50% if there are two quadrants of calcium, the sensitivity is 60% if there are three quadrants of calcium and finally the sensitivity is 85% if there are for quadrants calcium. Intravascular ultrasound demonstrates that the majority of plaque is located at the hips of bifurcation lesions and not at the Carina or flow divider. Intravascular ultrasound also indicates that the majority of lesions are more eccentric than we appreciate on angiography.
To Assess Ostial Lesions Such As An Ostial Left Main Lesion
Assessment of the left main is associated with the greatest amount of inter and intraobserver variability in angiography. The left main is short, and is often diseased with asymmetric lesions making its assessment on angiography difficult. There may be diffuse disease which may cause an underestimation of the extent of involvement on angiography. While luminal encroachment is defined as a minimum lumen area less than 4 mm² in the epicardial arteries, a minimum lumen area less than 6 mm² in the left main is considered to be significant. A minimum lumen area less than 6 mm² in the left main corresponds with a fractional flow reserve less than 0.75. A minimum lumen area less than 6 mm² also corresponds to a minimum lumen area less than 4 mm² in either the LAD or the circumflex arteries. In interrogating ostial lesions, it is critical to disengage the guide so that it is not mistaken for the lumen of the artery.
To Assess The Diameter And Length Of Lesions
- Assessment of the diameter of the vessel is particularly useful in research studies such as those evaluating lipid lowering agents. Care must be taken to identify reproducible start and end points for the mechanical pullback to ensure that the same area of the segment is being interrogated.
To Identify Complications Such As Dissection
While a dissection may appear to be hazy on an angiogram, intravascular ultrasound may be helpful in more definitively defining the presence of an edge dissection either proximal or distal to freshly deployed stent.
To Guide Stent Implantation
Intravascular ultrasound can be used to assess the size of the proximal and distal reference segment to aid in the selection of the stent diameter. Intravascular ultrasound can also be used to determine the length of the narrowing to select the length of the stent. It should be noted that angiography may underestimate the required link the stent due to foreshortening. Intravascular ultrasound can also be used to document that the stent is well opposed to the vessel wall. Intravascular ultrasound can also be used to ensure that the minimum stent area (MSA) is adequate. An inadequate minimum stent area is associated with a higher risk of stent thrombosis. intravascular ultrasound can also sure that the stent is symmetrically expanded and can exclude the presence of plaque prolapse. Finally intravascular ultrasound can ensure that there are no edge dissections. Data from the CRUISE trial suggested that a minimum stent area of 6.5 mm² is the optimal bare-metal stent minimum surface area. In the SIRIUS trial, a minimum stent area that best predicts restenosis in sirolimus-eluting stents is between 5 and 5.5 mm². Intravascular ultrasound may detect expansion of stent struts associated with high-pressure inflation that are not demonstrable on coronary angiography. Therefore intravascular ultrasound me the better tool that fine tuning stent expansion. Intravascular ultrasound data also demonstrates that the compliance charts that accompany balloons and stents cannot be used to predict final stent expansion.
Stent apposition refers to the stent touching the vessel wall while stent expansion refers to the size of the stent. Poor stent apposition is of a greater concern in an artery with a small minimum stent area than in a larger artery. Complete and position obviously may not be possible in aneurysmal segments or ectatic segments.
To Reduce The Risk Of Stent Restenosis
Insofar as intravascular ultrasound optimizes the minimum stented area, several studies have shown that the use of intravascular ultrasound reduces the risk of stent restenosis. There is also data to suggest that IVUS reduces the risk of arc defined stent thrombosis. These studies were based upon propensity matched analyses rather than randomized trial data.
Method
To visualize an artery or vein, angiographic techniques are used and the physician positions the tip of a guidewire, usually 0.014" diameter with a very soft and pliable tip and about 200 cm long. The physician steers the guidewire from outside the body, through angiography catheters and into the blood vessel branch to be imaged.
The ultrasound catheter tip is slid in over the guidewire and positioned, using angiography techniques so that the tip is at the farthest away position to be imaged. The sound waves are emitted from the catheter tip, are usually in the 10-20 MHz range, and the catheter also receives and conducts the return echo information out to the external computerized ultrasound equipment which constructs and displays a real time ultrasound image of a thin section of the blood vessel currently surrounding the catheter tip, usually displayed at 30 frames/second image.
The guide wire is kept stationary and the ultrasound catheter tip is slid backwards, usually under motorized control at a pullback speed of 0.5 mm/s. (The motorized pullback tends to be smoother than hand movement by the physician.)
The (a) blood vessel wall inner lining, (b) atheromatous disease within the wall and (c) connective tissues covering the outer surface of the blood vessel are echogenic, i.e. they return echoes making them visible on the ultrasound display.
By contrast, the blood itself and the healthy muscular tissue portion of the blood vessel wall is relatively echolucent, just black circular spaces, in the images.
Heavy calcium deposits in the blood vessel wall both heavily reflect sound, i.e. are very echogenic, but are also distinguishable by shadowing. Heavy calcification blocks sound transmission beyond and so, in the echo images, are seen as both very bright areas but with black shadows behind (from the vantage point of the catheter tip emitting the ultrasound waves).
Intravascular ultrasound in the coronary anatomy
While the routine use of IVUS during percutaneous coronary intervention does not improve short term outcomes[3], there are a number of situations in which IVUS is of particular use in the treatment of coronary artery disease of the heart. In particular in cases when the degree of stenosis of a coronary artery is unclear, IVUS can directly quantify the percentage of stenosis and give insight into the anatomy of the plaque.
One particular use of IVUS in the coronary anatomy is in the quantification of left main disease in cases where routine coronary angiography gives equivocal results. Many studies in the past have shown that significant left main disease can increase mortality[4], and that intervention (either coronary artery bypass graft surgery or percutaneous coronary intervention) to reduce mortality is necessary when the left main stenosis is significant.
When using IVUS to determine whether an individual's left main disease is clinically significant, in terms of the desirability of physical intervention, the two most widely used parameters are the degree of stenosis and the minimal lumen area.[5] A cross sectional area of ≤7 mm² in a symptomatic individual or ≤6 mm² in an asymptomatic individual[6] is considered to be clinically significant and warrants intervention to improve one-year mortality. However, these exact cutoffs are up for debate and different cutoff cross-sectional areas may be used in practice depending on differing interpretations of the trial data.

Validating the efficacy of new treatments
Because IVUS is widely available in coronary catheterization labs woldwide and can accurately quantify arterial plaque, especially within the coronary arteries, it is increasingly being used to evaluate newer and evolving strategies for the treatment of coronary artery disease, including the statins[7], torcetrapib and other approaches.[8][9]
2011 and 2005 ACCF/AHA/SCAI Guidelines for Percutaneous Coronary Intervention (DO NOT EDIT)[10][11]
Intravascular Ultrasound (DO NOT EDIT)[10][11]
| Class I |
| "'1.Before implantation of DES, the interventional cardiologist should discuss with the patient the need for and duration of DAPT and the ability of the patient to comply with and tolerate DAPT. [12] (Level of Evidence: C) " |
| "'2.DES are useful as an alternative to BMS to reduce the risk of restenosis in cases in which the risk of restenosis is increased and the patient is likely to be able to tolerate and comply with prolonged DAPT ((Level of Evidence: A) " for elective PCI[13][14][15][16][17][18]; (Level of Evidence: C) " for UA/NSTEMI [19]; (Level of Evidence: A) " for STEMI[20][21][22][23][24]. |
| "'3.Balloon angioplasty or BMS should be used in patients with high bleeding risk, inability to comply with 12 months of DAPT, or anticipated invasive or surgical procedures within the next 12 months, during which time DAPT may be interrupted.[25][26][27][28](Level of Evidence: B) " |
| Class III (Harm) |
| "1.PCI with coronary stenting should not be performed if the patient is not likely to be able to tolerate and comply with DAPT.[29][30][31][32](Level of Evidence: B) " |
| "2.DES should not be implanted if the patient is not likely to be able to tolerate and comply with prolonged DAPT or this cannot be determined before stent implantation.[33][34][35][36](Level of Evidence: B)" |
| "3. IVUS for routine lesion assessment is not recommended when revascularization with PCI or CABG is not being contemplated. (Level of Evidence: C)" |
| Class IIa |
| "1. Intravascular ultrasound (IVUS) is reasonable for the assessment of angiographically indeterminant left main CAD.[37][38][39] (Level of Evidence: B)" |
| "2. IVUS and coronary angiography are reasonable 4 to 6 weeks and 1 year after cardiac transplantation to exclude donor CAD, detect rapidly progressive cardiac allograftvasculopathy, and provide prognostic information.[40][41][42] (Level of Evidence: B)" |
| "3. IVUS is reasonable to determine the mechanism of stent restenosis. [43] (Level of Evidence: C)" |
| Class IIb |
| "1. IVUS may be reasonable for the assessment of non–left main coronary arteries with angiographically intermediate coronary stenoses (50% to 70% diameter stenosis).[37][44][45] (Level of Evidence: B)" |
| "2. IVUS may be considered for guidance of coronary stent implantation, particularly in cases of left main coronary artery stenting.[38][43][46] (Level of Evidence: B)" |
| "3. IVUS may be reasonable to determine the mechanism of stent thrombosis.[43] (Level of Evidence: C)" |
References
- ↑ Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. (1987). "Compensatory enlargement of human atherosclerotic coronary arteries". N Engl J Med. 316 (22): 1371–5. PMID 3574413.
- ↑ Zarins CK, Weisenberg E, Kolettis G, Stankunavicius R, Glagov S. (1988). "Differential enlargement of artery segments in response to enlarging atherosclerotic plaques". J Vasc Surg. 7 (3): 386–94. PMID 3346952.
- ↑ Schiele F, Meneveau N, Vuillemenot A, Zhang DD, Gupta S, Mercier M, Danchin N, Bertrand B, Bassand JP. (1998). "Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies--with and without intravascular ultrasound guidance". J Am Coll Cardiol. 32 (2): 320–8. PMID 9708456.
- ↑ Abizaid AS, Mintz GS, Abizaid A, Mehran R, Lansky AJ, Pichard AD, Satler LF, Wu H, Kent KM, Leon MB (1999). "One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms". J Am Coll Cardiol. 34 (3): 707–15. PMID 10483951.
- ↑ Robert D. Safian, MD, Mark S. Freed, MD, ed. (2002). "Intravascular Ultrasound". The Manual Of Interventional Cardiology (Third Edition ed.). Royal Oak, Michigan: Physicians' Press. p. 712. ISBN 1-890114-39-1.
- ↑ Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. (2004). "Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis". Circulation. 110 (18): 2831–6. PMID 15492302.
- ↑ Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. url=http://jama.ama-assn.org/cgi/reprint/jama;295/13/1556.pdf?ijkey=Md42dlk7z9TzyL8&keytype=finite JAMA 2006;295:1556-65. PMID 16533939.
- ↑ Nissen SE (2002). "Who is at risk for atherosclerotic disease? Lessons from intravascular ultrasound". Am J Med. 112 (Suppl 8a): 27S–33S. PMID 12049992.
- ↑ Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. (2003). "Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients With Acute Coronary Syndromes". JAMA. 290 (17): 2292–2300. PMID 14600188.
- ↑ 10.0 10.1 Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH (2011). "2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions" (PDF). Journal of the American College of Cardiology. 58 (24): 2550–83. doi:10.1016/j.jacc.2011.08.006. PMID 22070837. Retrieved 2011-12-08. Text "PDF" ignored (help); Unknown parameter
|month=ignored (help) - ↑ 11.0 11.1 Smith SC, Feldman TE, Hirshfeld JW, Jacobs AK, Kern MJ, King SB; et al. (2006). "ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention)". Circulation. 113 (7): e166–286. doi:10.1161/CIRCULATIONAHA.106.173220. PMID 16490830.
- ↑ Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007; 297: 159– 68.
- ↑ Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349: 1315–23.
- ↑ Stone GW, Ellis SG, Cox DA, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004; 109: 1942– 7.
- ↑ Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008; 359: 1330– 42.
- ↑ Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360: 1946– 59.
- ↑ Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360: 1946– 59.
- ↑ Mehilli J, Pache J, Abdel-Wahab M, et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet, published online before print August 28, 2011, doi:10.1016/S0140-6736(11)61255-5.
- ↑ Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008; 359: 1330– 42.
- ↑ Mauri L, Silbaugh TS, Garg P, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. 2008; 359: 1330– 42.
- ↑ Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009; 360: 1946– 59.
- ↑ Pan XH, Chen YX, Xiang MX, et al. A meta-analysis of randomized trials on clinical outcomes of paclitaxel-eluting stents versus bare-metal stents in ST-segment elevation myocardial infarction patients. J Zhejiang Univ Sci B. 2010; 11: 754– 61.
- ↑ Hao PP, Chen YG, Wang XL, et al. Efficacy and safety of drug-eluting stents in patients with acute ST-segment-elevation myocardial infarction: a meta-analysis of randomized controlled trials. Tex Heart Inst J. 2010; 37: 516– 24.
- ↑ Suh HS, Song HJ, Choi JE, et al. Drug-eluting stents versus bare-metal stents in acute myocardial infarction: a systematic review and meta-analysis. Int J Technol Assess Health Care. 2011; 27: 11–22.
- ↑ Grines CL, Bonow RO, Casey DE Jr., et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007; 49: 734– 9.
- ↑ Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006; 98: 352–6.
- ↑ Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006; 113: 2803– 9.
- ↑ Nasser M, Kapeliovich M, Markiewicz W. Late thrombosis of sirolimus-eluting stents following noncardiac surgery. Catheter Cardiovasc Interv. 2005; 65: 516– 9.
- ↑ Grines CL, Bonow RO, Casey DE Jr., et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007; 49: 734– 9.
- ↑ Leon MB, Baim DS, Popma JJ, et al., Stent Anticoagulation Restenosis Study Investigators. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998; 339: 1665– 71.
- ↑ Mauri L, Hsieh WH, Massaro JM, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356: 1020– 9.
- ↑ McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004; 364: 1519– 21.
- ↑ Grines CL, Bonow RO, Casey DE Jr., et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007; 49: 734– 9.
- ↑ Park DW, Park SW, Park KH, et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006; 98: 352– 6.
- ↑ Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006; 113: 2803– 9.
- ↑ Nasser M, Kapeliovich M, Markiewicz W. Late thrombosis of sirolimus-eluting stents following noncardiac surgery. Catheter Cardiovasc Interv. 2005; 65: 516– 9.
- ↑ 37.0 37.1 Briguori C, Anzuini A, Airoldi F, Gimelli G, Nishida T, Adamian M, Corvaja N, Di Mario C, Colombo A (2001). "Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve". The American Journal of Cardiology. 87 (2): 136–41. PMID 11152827. Retrieved 2011-12-09. Unknown parameter
|month=ignored (help) - ↑ 38.0 38.1 Fassa AA, Wagatsuma K, Higano ST, Mathew V, Barsness GW, Lennon RJ, Holmes DR, Lerman A (2005). "Intravascular ultrasound-guided treatment for angiographically indeterminate left main coronary artery disease: a long-term follow-up study". Journal of the American College of Cardiology. 45 (2): 204–11. doi:10.1016/j.jacc.2004.09.066. PMID 15653016. Retrieved 2011-12-09. Unknown parameter
|month=ignored (help) - ↑ Kang SJ, Lee JY, Ahn JM, Mintz GS, Kim WJ, Park DW, Yun SC, Lee SW, Kim YH, Lee CW, Park SW, Park SJ (2011). "Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity". Circulation. Cardiovascular Interventions. 4 (1): 65–71. doi:10.1161/CIRCINTERVENTIONS.110.959148. PMID 21266708. Retrieved 2011-12-09. Unknown parameter
|month=ignored (help) - ↑ Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L, McGiffin D, Smith J, Taylor D, Meiser B, Webber S, Baran D, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, Kim D, Kobashigawa J, Shullo M, Stehlik J, Teuteberg J, Uber P, Zuckermann A, Hunt S, Burch M, Bhat G, Canter C, Chinnock R, Crespo-Leiro M, Delgado R, Dobbels F, Grady K, Kao W, Lamour J, Parry G, Patel J, Pini D, Towbin J, Wolfel G, Delgado D, Eisen H, Goldberg L, Hosenpud J, Johnson M, Keogh A, Lewis C, O'Connell J, Rogers J, Ross H, Russell S, Vanhaecke J (2010). "The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients". The Journal of Heart and Lung Transplantation : the Official Publication of the International Society for Heart Transplantation. 29 (8): 914–56. doi:10.1016/j.healun.2010.05.034. PMID 20643330. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT, Abeywickrama KH, Murphy J, Cretin N (2005). "Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years". Journal of the American College of Cardiology. 45 (9): 1532–7. doi:10.1016/j.jacc.2005.02.035. PMID 15862430. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ Kapadia SR, Nissen SE, Ziada KM, Guetta V, Crowe TD, Hobbs RE, Starling RC, Young JB, Tuzcu EM (1998). "Development of transplantation vasculopathy and progression of donor-transmitted atherosclerosis: comparison by serial intravascular ultrasound imaging". Circulation. 98 (24): 2672–8. PMID 9851952. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ 43.0 43.1 43.2 Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R (2010). "In-stent restenosis in the drug-eluting stent era". Journal of the American College of Cardiology. 56 (23): 1897–907. doi:10.1016/j.jacc.2010.07.028. PMID 21109112. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ Takagi A, Tsurumi Y, Ishii Y, Suzuki K, Kawana M, Kasanuki H (1999). "Clinical potential of intravascular ultrasound for physiological assessment of coronary stenosis: relationship between quantitative ultrasound tomography and pressure-derived fractional flow reserve". Circulation. 100 (3): 250–5. PMID 10411848. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ Magni V, Chieffo A, Colombo A (2009). "Evaluation of intermediate coronary stenosis with intravascular ultrasound and fractional flow reserve: Its use and abuse". Catheterization and Cardiovascular Interventions : Official Journal of the Society for Cardiac Angiography & Interventions. 73 (4): 441–8. doi:10.1002/ccd.21812. PMID 19133668. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help) - ↑ Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, Yun SC, Lee CW, Hong MK, Lee JH, Park SW (2009). "Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis". Circulation. Cardiovascular Interventions. 2 (3): 167–77. doi:10.1161/CIRCINTERVENTIONS.108.799494. PMID 20031713. Retrieved 2011-12-10. Unknown parameter
|month=ignored (help)
External links
- Intravascular Ultrasound Center from Angioplasty.Org
- Pages with reference errors
- Pages using duplicate arguments in template calls
- CS1 maint: Multiple names: authors list
- CS1 maint: Multiple names: editors list
- CS1 maint: Extra text
- Pages with citations using unnamed parameters
- Pages with citations using unsupported parameters
- CS1 maint: Explicit use of et al.
- Cardiology
- Up-To-Date
- Up-To-Date cardiology