Ezogabine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RETINAL ABNORMALITIES AND POTENTIAL VISION LOSS
See full prescribing information for complete Boxed Warning.
|
Overview
Ezogabine is a potassium channel opener that is FDA approved for the treatment of partial-onset seizures in patients aged 18 years and older who have responded inadequately to several alternative treatments and for whom the benefits outweigh the risk of retinal abnormalities and potential decline in visual acuity. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dizziness, somnolence, fatigue, confusional state, vertigo, tremor, abnormal coordination, diplopia, disturbance in attention, memory impairment, asthenia, blurred vision, gait disturbance, aphasia, dysarthria, and balance disorder.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Ezogabine is indicated as adjunctive treatment of partial-onset seizures in patients aged 18 years and older who have responded inadequately to several alternative treatments and for whom the benefits outweigh the risk of retinal abnormalities and potential decline in visual acuity.
Dosage
Dosing Information
- The initial dosage should be 100 mg 3 times daily (300 mg per day). The dosage should be increased gradually at weekly intervals by no more than 50 mg 3 times daily (increase in the daily dose of no more than 150 mg per day) up to a maintenance dosage of 200 mg to 400 mg 3 times daily (600 mg to 1,200 mg per day), based on individual patient response and tolerability. This information is summarized in Table 1 under Dosing in Specific Populations. In the controlled clinical trials, 400 mg 3 times daily showed limited evidence of additional improvement in seizure reduction, but an increase in adverse events and discontinuations, compared to the 300 mg 3 times daily dosage. The safety and efficacy of doses greater than 400 mg 3 times daily (1,200 mg per day) have not been examined in controlled trials.
- Ezogabine should be given orally in 3 equally divided doses daily, with or without food.
- Ezogabine Tablets should be swallowed whole.
- If ezogabine is discontinued, the dosage should be gradually reduced over a period of at least 3 weeks, unless safety concerns require abrupt withdrawal.
Dosing Considerations to Mitigate the Risk of Visual Adverse Reactions
- Because ezogabine may cause retinal abnormalities with long-term use, patients who fail to show substantial clinical benefit after adequate titration should be discontinued from ezogabine. Testing of visual function should be done at baseline and every 6 months during therapy with ezogabine. Patients who cannot be monitored should usually not be treated with ezogabine. If retinal pigmentary abnormalities or vision changes are detected, ezogabine should be discontinued unless no other suitable treatment options are available and the benefits of treatment outweigh the potential risk of vision loss.
Dosing in Specific Populations
- No adjustment in dosage is required for patients with mild renal or hepatic impairment (see Table 1). Dosage adjustment is required in geriatric and patients with moderate and greater renal or hepatic impairment (see Table 1).

Dosage Forms and Strength
- 50 mg, purple, round, film-coated tablets debossed with “RTG 50” on one side.
- 200 mg, yellow, oblong, film-coated tablets debossed with “RTG-200” on one side.
- 300 mg, green, oblong, film-coated tablets debossed with “RTG-300” on one side.
- 400 mg, purple, oblong, film-coated tablets debossed with “RTG-400” on one side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ezogabine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ezogabine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of ezogabine in patients under 18 years of age have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ezogabine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ezogabine in pediatric patients.
Contraindications
- None.
Warnings
|
RETINAL ABNORMALITIES AND POTENTIAL VISION LOSS
See full prescribing information for complete Boxed Warning.
|
Retinal Abnormalities and Potential Vision Loss
- Ezogabine can cause abnormalities of the retina. The abnormalities seen in patients treated with ezogabine have funduscopic features similar to those seen in retinal pigment dystrophies that are known to result in damage to photoreceptors and vision loss.
- The retinal abnormalities observed with ezogabine have been reported in patients who were originally enrolled in clinical trials with ezogabine and who have generally taken the drug for a long period of time in 2 ongoing extension studies. Approximately one third of the patients who had eye examinations performed after approximately 4 years of treatment were found to have retinal pigmentary abnormalities. However, an earlier onset cannot be ruled out, and it is possible that retinal abnormalities were present earlier in the course of exposure to ezogabine. Ezogabine causes skin, scleral, nail, and mucous membrane discoloration and it is not clear whether this discoloration is related to retinal abnormalities. Approximately 15% of patients with retinal pigmentary abnormalities had no such discoloration.
- Funduscopic abnormalities have most commonly been described as perivascular pigmentation (bone spicule pattern) in the retinal periphery and/or as areas of focal retinal pigment epithelium clumping. Although some of the patients with retinal abnormalities have been found to have abnormal visual acuity, it is not possible to assess whether ezogabine caused their decreased visual acuity, as baseline assessments are not available for these patients. Two patients with retinal abnormalities have had more extensive diagnostic retinal evaluations. The results of these evaluations were consistent with a retinal dystrophy, including abnormalities in the electroretinogram and electrooculogram of both patients, with abnormal fluorescein angiography and diminished sensitivity on visual field testing in one patient.
- The rate of progression of retinal abnormalities and the reversibility after drug discontinuation are unknown.
- Because of the observed ophthalmologic adverse reactions, ezogabine should only be used in patients who have responded inadequately to several alternative treatments and for whom the benefits outweigh the risk of retinal abnormalities and potential vision loss. Patients who fail to show substantial clinical benefit after adequate titration should be discontinued from ezogabine.
- Patients should have baseline ophthalmologic testing by an ophthalmic professional and follow-up testing every 6 months. The best method of detection of these abnormalities and the optimal frequency of periodic ophthalmologic monitoring are unknown. Patients who cannot be monitored should usually not be treated with ezogabine. The ophthalmologic monitoring program should include visual acuity testing and dilated fundus photography. Additional testing may include fluorescein angiograms (FA), ocular coherence tomography (OCT), perimetry, and electroretinograms (ERG). If retinal pigmentary abnormalities or vision changes are detected, ezogabine should be discontinued unless no other suitable treatment options are available and the benefits of treatment outweigh the potential risk of vision loss.
Urinary Retention
- Ezogabine caused Urinary Retention in clinical trials. Urinary retention was generally reported within the first 6 months of treatment, but was also observed later. Urinary retention was reported as an adverse event in 29 of 1,365 (approximately 2%) patients treated with ezogabine in the open-label and placebo-controlled epilepsy database. Of these 29 patients, 5 (17%) required catheterization, with post-voiding residuals of up to 1,500 mL. ezogabine was discontinued in 4 patients who required catheterization. Following discontinuation, these 4 patients were able to void spontaneously; however, 1 of the 4 patients continued intermittent self-catheterization. A fifth patient continued treatment with ezogabine and was able to void spontaneously after catheter removal. Hydronephrosis occurred in 2 patients, one of whom had associated renal function impairment that resolved upon discontinuation of ezogabine. Hydronephrosis was not reported in placebo patients.
- In the placebo-controlled epilepsy trials, “Urinary Retention,” “urinary hesitation,” and “dysuria” were reported in 0.9%, 2.2%, and 2.3% of patients on ezogabine, respectively, and in 0.5%, 0.9%, and 0.7% of patients on placebo, respectively.
- Because of the increased risk of Urinary Retention on ezogabine, urologic symptoms should be carefully monitored. Closer monitoring is recommended for patients who have other risk factors for urinary retention (e.g., benign prostatic hyperplasia [BPH]), patients who are unable to communicate clinical symptoms (e.g., cognitively impaired patients), or patients who use concomitant medications that may affect voiding (e.g., anticholinergics). In these patients, a comprehensive evaluation of urologic symptoms prior to and during treatment with ezogabine may be appropriate.
Skin Discoloration
- Ezogabine can cause skin discoloration. The skin discoloration is generally described as blue, but has also been described as grey-blue or brown. It is predominantly on or around the lips or in the nail beds of the fingers or toes, but more widespread involvement of the face and legs has also been reported. Discoloration of the palate, sclera, and conjunctiva has also been reported.
- Approximately 10% of patients in long-term clinical trials developed skin discoloration, generally after 2 or more years of treatment and at higher doses (900 mg or greater) of ezogabine. Among patients in whom the status of both skin, nail, lip, or mucous membrane discoloration and retinal pigmentary abnormalities are reported, approximately a quarter of those with skin, nail, lip, or mucous membrane discoloration had concurrent retinal pigmentary abnormalities.
- Information on the consequences, reversibility, time to onset, and pathophysiology of the skin abnormalities remains incomplete. The possibility of more extensive systemic involvement has not been excluded. If a patient develops skin discoloration, serious consideration should be given to changing to an alternate medication.
Neuro-Psychiatric Symptoms
- confusional state, psychotic symptoms, and Hallucinations were reported more frequently as adverse reactions in patients treated with ezogabine than in those treated with placebo in placebo-controlled epilepsy trials (see Table 2). Discontinuations resulting from these reactions were more common in the drug-treated group (see Table 2). These effects were dose-related and generally appeared within the first 8 weeks of treatment. Half of the patients in the controlled trials who discontinued ezogabine due to hallucinations or psychosis required hospitalization. Approximately two-thirds of patients with psychosis in controlled trials had no prior psychiatric history. The psychiatric symptoms in the vast majority of patients in both controlled and open-label trials resolved within 7 days of discontinuation of ezogabine. Rapid titration at greater than the recommended doses appeared to increase the risk of psychosis and hallucinations.

Dizziness and Somnolence
- Ezogabine causes dose-related increases in dizziness and somnolence. In placebo-controlled trials in patients with epilepsy, dizziness was reported in 23% of patients treated with ezogabine and 9% of patients treated with placebo. Somnolence was reported in 22% of patients treated with ezogabine and 12% of patients treated with placebo. In these trials 6% of patients on ezogabine and 1.2% on placebo discontinued treatment because of dizziness; 3% of patients on ezogabine and <1.0% on placebo discontinued because of somnolence.
- Most of these adverse reactions were mild to moderate in intensity and occurred during the titration phase. For those patients continued on ezogabine, dizziness and somnolence appeared to diminish with continued use.
QT Interval Effect
- A study of cardiac conduction showed that ezogabine produced a mean 7.7-msec QT prolongation in healthy volunteers titrated to 400 mg 3 times daily. The QT-prolonging effect occurred within 3 hours. The QT interval should be monitored when ezogabine is prescribed with medicines known to increase QT interval and in patients with known prolonged QT interval, congestive heart failure, ventricular hypertrophy, hypokalemia, or hypomagnesemia.
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including ezogabine, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive-therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted relative risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43% compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately 1 case of suicidal thinking or behavior for every 530 patients treated. There were 4 suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as 1 week after starting treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanism of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3. Risk of Suicidal Thoughts or Behaviors by Indication for Antiepileptic Drugs in the Pooled Analysis

- The relative risk for suicidal thoughts or behavior was higher in clinical trials in patients with epilepsy than in clinical trials in patients with psychiatric or other conditions, but the absolute risk differences were similar for epilepsy and psychiatric indications.
- Anyone considering prescribing ezogabine or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression; any unusual changes in mood or behavior; or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Withdrawal Seizures
- As with all AEDs, when ezogabine is discontinued, it should be withdrawn gradually when possible to minimize the potential of increased seizure frequency. The dosage of ezogabine should be reduced over a period of at least 3 weeks, unless safety concerns require abrupt withdrawal.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions and for varying durations, adverse reaction frequencies observed in the clinical trials of a drug cannot be directly compared with frequencies in the clinical trials of another drug and may not reflect the frequencies observed in practice.
- Ezogabine was administered as adjunctive therapy to 1,365 patients with epilepsy in all controlled and uncontrolled clinical studies during the premarketing development. A total of 801 patients were treated for at least 6 months, 585 patients were treated for 1 year or longer, and 311 patients were treated for at least 2 years.
- Adverse Reactions Leading to Discontinuation in All Controlled Clinical Studies: In the 3 randomized, double-blind, placebo-controlled studies, 199 of 813 patients (25%) receiving ezogabine and 45 of 427 patients (11%) receiving placebo discontinued treatment because of adverse reactions. The most common adverse reactions leading to withdrawal in patients receiving ezogabine were dizziness (6%), confusional state (4%), fatigue (3%), and somnolence (3%).
- Common Adverse Reactions in All Controlled Clinical Studies: Overall, the most frequently reported adverse reactions in patients receiving ezogabine (≥4% and occurring approximately twice the placebo rate) were dizziness (23%), somnolence (22%), fatigue (15%), confusional state (9%), vertigo (8%), tremor (8%), abnormal coordination (7%), diplopia (7%), disturbance in attention (6%), memory impairment (6%), asthenia (5%), blurred vision (5%), gait disturbance (4%), aphasia (4%), dysarthria (4%), and balance disorder (4%). In most cases the reactions were of mild or moderate intensity.
- Table 4. Adverse Reaction Incidence in Placebo-Controlled Adjunctive Trials in Adult Patients With Partial Onset seizures (Adverse reactions in at least 2% of patients treated with ezogabine in any treatment group and numerically more frequent than in the placebo group.)

- Other adverse reactions reported in these 3 studies in <2% of patients treated with ezogabine and numerically greater than placebo were increased appetite, Hallucinations, myoclonus, peripheral edema, hypokinesia, dry mouth, dysphagia, hyperhydrosis, Urinary Retention, malaise, and increased liver enzymes.
- Most of the adverse reactions appear to be dose related (especially those classified as psychiatric and nervous system symptoms), including dizziness, somnolence, confusional state, tremor, abnormal coordination, memory impairment, blurred vision, gait disturbance, aphasia, balance disorder, constipation, dysuria, and chromaturia.
- Ezogabine was associated with dose-related weight gain, with mean weight increasing by 0.2 kg, 1.2 kg, 1.6 kg, and 2.7 kg in the placebo, 600 mg per day, 900 mg per day, and 1,200 mg per day groups, respectively.
- Additional Adverse Reactions Observed During All Phase 2 and 3 Clinical Trials: Following is a list of adverse reactions reported by patients treated with ezogabine during all clinical trials: rash, nystagmus, dyspnea, leukopenia, muscle spasms, alopecia, nephrolithiasis, syncope, neutropenia, thrombocytopenia, euphoric mood, renal colic, coma, encephalopathy.
- Comparison of Gender, Age, and Race: The overall adverse reaction profile of ezogabine was similar for females and males.
- There are insufficient data to support meaningful analyses of adverse reactions by age or race. Approximately 86% of the population studied was Caucasian, and 0.8% of the population was older than 65 years.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience.
Drug Interactions
Antiepileptic Drugs
- The potentially significant interactions between ezogabine and concomitant AEDs are summarized in Table 5.

Digoxin
- Data from an in vitro study showed that the N-acetyl metabolite of ezogabine (NAMR) inhibited P-glycoprotein–mediated transport of digoxin in a concentration-dependent manner, indicating that NAMR may inhibit renal clearance of digoxin. Administration of ezogabine at therapeutic doses may increase digoxin serum concentrations. Serum levels of digoxin should be monitored.
Alcohol
- Alcohol increased systemic exposure to ezogabine. Patients should be advised of possible worsening of ezogabine’s general dose-related adverse reactions if they take ezogabine with alcohol.
Laboratory Tests
- Ezogabine has been shown to interfere with clinical laboratory assays of both serum and urine bilirubin, which can result in falsely elevated readings.
Use in Specific Populations
Pregnancy
- Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. ezogabine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In animal studies, doses associated with maternal plasma exposures (AUC) to ezogabine and its major circulating metabolite, NAMR, similar to or below those expected in humans at the maximum recommended human dose (MRHD) of 1,200 mg per day produced developmental toxicity when administered to pregnant rats and rabbits. The maximum doses evaluated were limited by maternal toxicity (acute neurotoxicity).
- Treatment of pregnant rats with ezogabine (oral doses of up to 46 mg/kg/day) throughout organogenesis increased the incidences of fetal skeletal variations. The no-effect dose for embryo-fetal toxicity in rats (21 mg/kg/day) was associated with maternal plasma exposures (AUC) to ezogabine and NAMR less than those in humans at the MRHD. Treatment of pregnant rabbits with ezogabine (oral doses of up to 60 mg/kg/day) throughout organogenesis resulted in decreased fetal body weights and increased incidences of fetal skeletal variations. The no-effect dose for embryo-fetal toxicity in rabbits (12 mg/kg/day) was associated with maternal plasma exposures to ezogabine and NAMR less than those in humans at the MRHD.
- Administration of ezogabine (oral doses of up to 61.9 mg/kg/day) to rats throughout pregnancy and lactation resulted in increased pre- and postnatal mortality, decreased body weight gain, and delayed reflex development in the offspring. The no-effect dose for pre- and postnatal developmental effects in rats (17.8 mg/kg/day) was associated with maternal plasma exposures to ezogabine and NAMR less than those in humans at the MRHD.
- Pregnancy Registry: To provide information regarding the effects of in utero exposure to ezogabine, physicians are advised to recommend that pregnant patients taking ezogabine enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll-free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website www.aedpregnancyregistry.org.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ezogabine in women who are pregnant.
Labor and Delivery
- The effects of ezogabine on labor and delivery in humans are unknown.
Nursing Mothers
- It is not known whether ezogabine is excreted in human milk. However, ezogabine and/or its metabolites are present in the milk of lactating rats. Because of the potential for serious adverse reactions in nursing infants from ezogabine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of ezogabine in patients under 18 years of age have not been established.
- In juvenile animal studies, increased sensitivity to acute neurotoxicity and urinary bladder toxicity was observed in young rats compared to adults. In studies in which rats were dosed starting on postnatal day 7, ezogabine-related mortality, clinical signs of neurotoxicity, and renal and urinary tract toxicities were observed at doses ≥2 mg/kg/day. The no-effect level was associated with plasma ezogabine exposures (AUC) less than those expected in human adults at the MRHD of 1,200 mg per day. In studies in which dosing began on postnatal day 28, acute central nervous system effects, but no apparent renal or urinary tract effects, were observed at doses of up to 30 mg/kg/day. These doses were associated with plasma ezogabine exposures less than those achieved clinically at the MRHD.
Geriatic Use
- There were insufficient numbers of elderly patients enrolled in partial-onset seizure controlled trials (n = 8 patients on ezogabine) to determine the safety and efficacy of ezogabine in this population. Dosage adjustment is recommended in patients aged 65 years and older.
- Ezogabine may cause Urinary Retention. Elderly men with symptomatic BPH may be at increased risk for urinary retention.
Gender
There is no FDA guidance on the use of Ezogabine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ezogabine with respect to specific racial populations.
Renal Impairment
- Dosage adjustment is recommended for patients with creatinine clearance <50 mL/min or patients with end-stage renal disease (ESRD) receiving dialysis treatments.
Hepatic Impairment
- No dosage adjustment is required for patients with mild hepatic impairment.
- In patients with moderate or severe hepatic impairment, the initial and maintenance dosage of ezogabine should be reduced.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ezogabine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ezogabine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- All patients taking ezogabine should have baseline and periodic (every 6 months) systematic visual monitoring by an ophthalmic professional. Testing should include visual acuity and dilated fundus photography.
- Closer monitoring is recommended for patients who have other risk factors for Urinary Retention (e.g., benign prostatic hyperplasia [BPH]), patients who are unable to communicate clinical symptoms (e.g., cognitively impaired patients), or patients who use concomitant medications that may affect voiding (e.g., anticholinergics).
- Monitoring of cardiac rhythm and vital sign measurement is recommended in management of overdose.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
Signs, Symptoms, and Laboratory Findings
- There is limited experience of overdose with ezogabine. Total daily doses of ezogabine over 2,500 mg were reported during clinical trials. In addition to adverse reactions seen at therapeutic doses, symptoms reported with ezogabine overdose included agitation, aggressive behavior, and irritability. There were no reported sequelae.
- In an abuse potential study, cardiac arrhythmia (asystole or ventricular tachycardia) occurred in 2 volunteers within 3 hours of receiving a single 900-mg dose of ezogabine. The arrhythmias spontaneously resolved and both volunteers recovered without sequelae.
Management of Overdose
- There is no specific antidote for overdose with ezogabine. In the event of overdose, standard medical practice for the management of any overdose should be used. An adequate airway, oxygenation, and ventilation should be ensured; monitoring of cardiac rhythm and vital sign measurement is recommended. A certified poison control center should be contacted for updated information on the management of overdose with ezogabine.
Pharmacology
| |
| |
Ezogabine
| |
| Systematic (IUPAC) name | |
| ethyl N-2-amino-4-(4-fluorophenyl)methylamino-phenyl-carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 303.331 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 60–80% |
| Metabolism | Hepatic glucuronidation and acetylation. CYP not involved |
| Half life | 8 hours (mean), range: 7–11 hours[1] |
| Excretion | Renal (84%) |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- The mechanism by which ezogabine exerts its therapeutic effects has not been fully elucidated. In vitro studies indicate that ezogabine enhances transmembrane potassium currents mediated by the KCNQ (Kv7.2 to 7.5) family of ion channels. By activating KCNQ channels, ezogabine is thought to stabilize the resting membrane potential and reduce brain excitability. In vitro studies suggest that ezogabine may also exert therapeutic effects through augmentation of GABA-mediated currents.
Structure
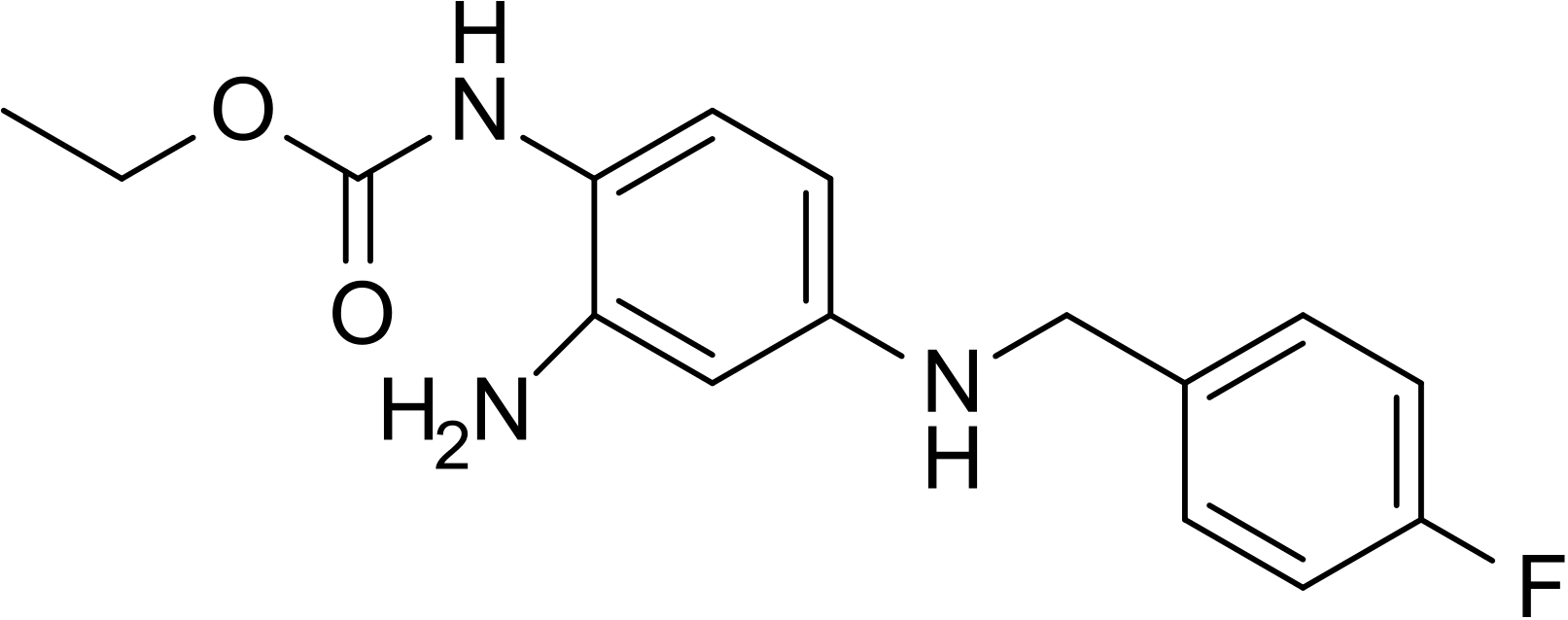
- The chemical name of ezogabine is N-[2-amino-4-(4-fluorobenzylamino)-phenyl] carbamic acid ethyl ester, and it has the following structure:

- The empirical formula is C16H18FN3O2, representing a molecular weight of 303.3. Ezogabine is a white to slightly colored, odorless, tasteless, crystalline powder. At room temperature, ezogabine is practically insoluble in aqueous media at pH values above 4, while the solubility is higher in polar organic solvents. At gastric pH, ezogabine is sparingly soluble in water (about 16 g/L). The pKa is approximately 3.7 (basic).
- Ezogabine is supplied for oral administration as 50-mg, 200-mg, 300-mg, and 400-mg film-coated immediate-release tablets. Each tablet contains the labeled amount of ezogabine and the following inactive ingredients: carmine (50-mg and 400-mg tablets), croscarmellose sodium, FD&C Blue No. 2 (50-mg, 300-mg, and 400-mg tablets), hypromellose, iron oxide yellow (200‑mg and 300-mg tablets), lecithin, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, talc, titanium dioxide, and xanthan gum.
Pharmacodynamics
- The QTc prolongation risk of ezogabine was evaluated in healthy subjects. In a randomized, double-blind, active- and placebo-controlled parallel-group study, 120 healthy subjects (40 in each group) were administered ezogabine titrated up to the final dose of 400 mg 3 times daily, placebo, and placebo and moxifloxacin (on day 22). After 22 days of dosing, the maximum mean (upper 1-sided, 95% CI) increase of baseline- and placebo-adjusted QTc interval based on Fridericia correction method (QTcF) was 7.7 msec (11.9 msec) and was observed at 3 hours after dosing in subjects who achieved 1,200 mg per day. No effects on heart rate, PR, or QRS intervals were noted.
- Patients who are prescribed ezogabine with medicines known to increase QT interval or who have known prolonged QT interval, congestive heart failure, ventricular hypertrophy, hypokalemia, or hypomagnesemia should be observed closely.
Pharmacokinetics
- The pharmacokinetic profile is approximately linear in daily doses between 600 mg and 1,200 mg in patients with epilepsy, with no unexpected accumulation following repeated administration. The pharmacokinetics of ezogabine are similar in healthy volunteers and patients with epilepsy.
- Absorption: After both single and multiple oral doses, ezogabine is rapidly absorbed with median time to maximum plasma concentration (Tmax) values generally between 0.5 and 2 hours. Absolute oral bioavailability of ezogabine relative to an intravenous dose of ezogabine is approximately 60%. High-fat food does not affect the extent to which ezogabine is absorbed based on plasma AUC values, but it increases peak concentration (Cmax) by approximately 38% and delays Tmax by 0.75 hour.
- Ezogabine can be taken with or without food.
- Distribution: Data from in vitro studies indicate that ezogabine and NAMR are approximately 80% and 45% bound to plasma protein, respectively. Clinically significant interactions with other drugs through displacement from proteins are not anticipated. The steady-state volume of distribution of ezogabine is 2 to 3 L/kg following intravenous dosing, suggesting that ezogabine is well distributed in the body.
- Metabolism: Ezogabine is extensively metabolized primarily via glucuronidation and acetylation in humans. A substantial fraction of the ezogabine dose is converted to inactive N-glucuronides, the predominant circulating metabolites in humans. Ezogabine is also metabolized to NAMR that is also subsequently glucuronidated. NAMR has antiepileptic activity, but it is less potent than ezogabine in animal seizure models. Additional minor metabolites of ezogabine are an N-glucoside of ezogabine and a cyclized metabolite believed to be formed from NAMR. In vitro studies using human biomaterials showed that the N-acetylation of ezogabine was primarily carried out by NAT2, while glucuronidation was primarily carried out by UGT1A4, with contributions by UGT1A1, UGT1A3, and UGT1A9.
- In vitro studies showed no evidence of oxidative metabolism of ezogabine or NAMR by cytochrome P450 enzymes. Coadministration of ezogabine with medications that are inhibitors or inducers of cytochrome P450 enzymes is therefore unlikely to affect the pharmacokinetics of ezogabine or NAMR.
- Elimination: Results of a mass balance study suggest that renal excretion is the major route of elimination for ezogabine and NAMR. About 85% of the dose was recovered in the urine, with the unchanged parent drug and NAMR accounting for 36% and 18% of the administered dose, respectively, and the total N-glucuronides of ezogabine and NAMR accounting for 24% of the administered dose. Approximately 14% of the radioactivity was recovered in the feces, with unchanged ezogabine accounting for 3% of the total dose. Average total recovery in both urine and feces within 240 hours after dosing is approximately 98%.
- Ezogabine and its N-acetyl metabolite have similar elimination half-lives (t½) of 7 to 11 hours. The clearance of ezogabine following intravenous dosing was approximately 0.4 to 0.6 L/hr/kg. Ezogabine is actively secreted into the urine.
- Specific Populations: Race: No study has been conducted to investigate the impact of race on pharmacokinetics of ezogabine. A population pharmacokinetic analysis comparing Caucasians and non-Caucasians (predominately African American and Hispanic patients) showed no significant pharmacokinetic difference. No adjustment of the ezogabine dose for race is recommended.
- Gender: The impact of gender on the pharmacokinetics of ezogabine was examined following a single dose of ezogabine to healthy young (aged 21 to 40 years) and elderly (aged 66 to 82 years) subjects. The AUC values were approximately 20% higher in young females compared to young males and approximately 30% higher in elderly females compared to elderly males. The Cmax values were approximately 50% higher in young females compared to young males and approximately 100% higher in elderly females compared to elderly males. There was no gender difference in weight-normalized clearance. Overall, no adjustment of the dosage of ezogabine is recommended based on gender.
- Pediatric Patients: The pharmacokinetics of ezogabine in pediatric patients have not been investigated.
- Geriatric: The impact of age on the pharmacokinetics of ezogabine was examined following a single dose of ezogabine to healthy young (aged 21 to 40 years) and elderly (aged 66 to 82 years) subjects. Systemic exposure (AUC) of ezogabine was approximately 40% to 50% higher and terminal half-life was prolonged by approximately 30% in the elderly compared to the younger subjects. The peak concentration (Cmax) was similar to that observed in younger subjects. A dosage reduction in the elderly is recommended.
- Renal Impairment: The pharmacokinetics of ezogabine were studied following a single 100-mg dose of ezogabine in subjects with normal (CrCL >80 ml/min), mild (CrCL ≥50 to <80 mL/min), moderate (CrCL ≥30 to <50 mL/min), or severe renal impairment (CrCL <30 mL/min) (n = 6 in each cohort) and in subjects with ESRD requiring hemodialysis (n = 6). The ezogabine AUC was increased by approximately 30% in patients with mild renal impairment and doubled in patients with moderate impairment to ESRD (CrCL <50 mL/min) relative to healthy subjects. Similar increases in NAMR exposure were observed in the various degrees of renal impairment. The effect of hemodialysis on ezogabine clearance has not been established. Dosage reduction is recommended for patients with creatinine clearance <50 mL/min and for patients with ESRD receiving dialysis.
- Hepatic Impairment: The pharmacokinetics of ezogabine were studied following a single 100-mg dose of ezogabine in subjects with normal, mild (Child-Pugh score 5 to 6), moderate (Child-Pugh score 7 to 9), or severe hepatic (Child-Pugh score >9) impairment (n = 6 in each cohort). Relative to healthy subjects, ezogabine AUC was not affected by mild hepatic impairment, but was increased by approximately 50% in subjects with moderate hepatic impairment and doubled in subjects with severe hepatic impairment. There was an increase of approximately 30% in exposure to NAMR in patients with moderate to severe impairment. Dosage reduction is recommended for patients with moderate and severe hepatic impairment.
- Drug Interactions: In vitro studies using human liver microsomes indicated that ezogabine does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4/5. Inhibition of CYP2B6 by ezogabine has not been evaluated. In addition, in vitro studies in human primary hepatocytes showed that ezogabine and NAMR did not induce CYP1A2 or CYP3A4/5 activity. Therefore, ezogabine is unlikely to affect the pharmacokinetics of substrates of the major cytochrome P450 isoenzymes through inhibition or induction mechanisms.
- Ezogabine is neither a substrate nor an inhibitor of P-glycoprotein, an efflux transporter. NAMR is a P-glycoprotein inhibitor. Data from an in vitro study showed that NAMR inhibited P-glycoprotein–mediated transport of digoxin in a concentration-dependent manner, indicating that NAMR may inhibit renal clearance of digoxin. Administration of ezogabine at therapeutic doses may increase digoxin serum concentration.
- Interactions with Antiepileptic Drugs: The interactions between ezogabine and concomitant AEDs are summarized in Table 6.

- Oral Contraceptives: In one study examining the potential interaction between ezogabine (150 mg 3 times daily for 3 days) and the combination oral contraceptive norgestrel/ethinyl estradiol (0.3 mg/0.03 mg) tablets in 20 healthy females, no significant alteration in the pharmacokinetics of either drug was observed.
- In a second study examining the potential interaction of repeated ezogabine dosing (250 mg 3 times daily for 14 days) and the combination oral contraceptive norethindrone/ethinyl estradiol (1 mg/0.035 mg) tablets in 25 healthy females, no significant alteration in the pharmacokinetics of either drug was observed.
- Alcohol: In a healthy volunteer study, the coadministration of ethanol 1g/kg (5 standard alcohol drinks) over 20 minutes and ezogabine (200 mg) resulted in an increase in the ezogabine Cmax and AUC by 23% and 37%, respectively.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis: In a one-year neonatal mouse study of ezogabine (2 single-dose oral administrations of up to 96 mg/kg on postnatal days 8 and 15), a dose-related increase in the frequency of lung neoplasms (bronchioalveolar carcinoma and/or adenoma) was observed in treated males. No evidence of carcinogenicity was observed in rats following oral administration of ezogabine (oral gavage doses of up to 50 mg/kg/day) for 2 years. Plasma exposure (AUC) to ezogabine at the highest doses tested was less than that in humans at the maximum recommended human dose (MRHD) of 1,200 mg per day.
- Mutagenesis: Highly purified ezogabine was negative in the in vitro Ames assay, the in vitro Chinese hamster ovary (CHO) Hprt gene mutation assay, and the in vivo mouse micronucleus assay. Ezogabine was positive in the in vitro chromosomal aberration assay in human lymphocytes. The major circulating metabolite of ezogabine, NAMR, was negative in the in vitro Ames assay, but positive in the in vitro chromosomal aberration assay in CHO cells.
- Impairment of Fertility: Ezogabine had no effect on fertility, general reproductive performance, or early embryonic development when administered to male and female rats at doses of up to 46.4 mg/kg/day (associated with a plasma ezogabine exposure [AUC] less than that in humans at the MRHD) prior to and during mating, and continuing in females through gestation day 7.
Clinical Studies
- The efficacy of ezogabine as adjunctive therapy in partial-onset seizures was established in 3 multicenter, randomized, double-blind, placebo-controlled studies in 1,239 adult patients. The primary endpoint consisted of the percent change in seizure frequency from baseline in the double-blind treatment phase.
- Patients enrolled in the studies had partial onset seizures with or without secondary generalization and were not adequately controlled with 1 to 3 concomitant AEDs, with or without concomitant vagus nerve stimulation. More than 75% of patients were taking 2 or more concomitant AEDs. During an 8-week baseline period, patients experienced at least 4 partial onset seizures per 28 days on average with no seizure-free period exceeding 3 to 4 weeks. Patients had a mean duration of epilepsy of 22 years. Across the 3 studies, the median baseline seizure frequency ranged from 8 to 12 seizures per month. The criteria for statistical significance was P<0.05.
- Patients were randomized to the total daily maintenance dosages of 600 mg per day, 900 mg per day, or 1,200 mg per day, each administered in 3 equally divided doses. During the titration phase of all 3 studies, treatment was initiated at 300 mg per day (100 mg 3 times per day) and increased in weekly increments of 150 mg per day to the target maintenance dosage.
- Figure 1 shows the median percent reduction in 28-day seizure frequency (baseline to double-blind phase) as compared with placebo across all 3 studies. A statistically significant effect was observed with ezogabine at doses of 600 mg per day (Study 1), at 900 mg per day (Studies 1 and 3), and at 1,200 mg per day (Studies 2 and 3).
Figure 1. Median Percent Reduction From Baseline in seizure Frequency per 28 Days by Dose

- Figure 2 shows changes from baseline in the 28-day total partial seizure frequency by category for patients treated with ezogabine and placebo in an integrated analysis across the 3 clinical trials. Patients in whom the seizure frequency increased are shown at left as “worse.” Patients in whom the seizure frequency decreased are shown in five categories.
- Figure 2. Proportion of Patients by Category of Seizure Response for ezogabine and Placebo Across All Three Double-blind Trials

How Supplied
- Ezogabine is supplied as film-coated immediate-release tablets for oral administration containing 50 mg, 200 mg, 300 mg, or 400 mg of ezogabine in the following packs:
- 50-mg Tablets: purple, round, film-coated tablets debossed with “RTG 50” on one side in bottles of 90 tablets with desiccant (NDC 0173-0810-59).
- 200-mg Tablets: yellow, oblong, film-coated tablets debossed with “RTG-200” on one side in bottles of 90 tablets with desiccant (NDC 0173-0812-59).
- 300-mg Tablets: green, oblong, film-coated tablets debossed with “RTG-300” on one side in bottles of 90 tablets with desiccant (NDC 0173-0813-59).
- 400-mg Tablets: purple, oblong, film-coated tablets debossed with “RTG-400” on one side in bottles of 90 tablets with desiccant (NDC 0173-0814-59).
Storage
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature.]
Images
Drug Images
{{#ask: Page Name::Ezogabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Ezogabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Retinal Abnormalities and Potential Vision Loss
- Inform patients of the risk of retinal abnormalities and possible risk of vision loss, which may be permanent. All patients taking ezogabine should participate in baseline and periodic ophthalmologic monitoring of vision by an ophthalmic professional. Inform patients that if they suspect any vision changes, they should notify their physician immediately.
Urinary Retention
- Patients should be informed that ezogabine can cause Urinary Retention (including urinary hesitation and dysuria). If patients experience any symptoms of urinary retention, inability to urinate, and/or pain with urination, they should be instructed to seek immediate medical assistance. For patients who cannot reliably report symptoms of urinary retention (for example, patients with cognitive impairment), urologic consultation may be helpful.
Skin Discoloration
- Inform patients that ezogabine can cause discoloration of nails, lips, skin, palate, and parts of the eye and that it is not known if the discoloration is reversible upon drug discontinuation. Most skin discoloration has been reported after at least 2 years of treatment with ezogabine, but may happen earlier. Inform patients that the possibility of more extensive systemic involvement has not been excluded. Instruct patients to notify their physician if they develop skin discoloration.
Psychiatric Symptoms
- Patients should be informed that ezogabine can cause psychiatric symptoms such as confusional state, disorientation, hallucinations, and other symptoms of psychosis. Patients and their caregivers should be instructed to notify their physicians if they experience psychotic symptoms.
Central Nervous System Effects
- Patients should be informed that ezogabine may cause dizziness, somnolence, memory impairment, abnormal coordination/balance, disturbance in attention, and ophthalmological effects such as diplopia or blurred vision. Patients taking ezogabine should be advised not to drive, operate complex machinery, or engage in other hazardous activities until they have become accustomed to any such effects associated with ezogabine.
Suicidal Thinking and Behavior
- Patients, their caregivers, and families should be informed that AEDs, including ezogabine, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Pregnancy
- Patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy. Patients should be advised to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
- Patients should be encouraged to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry collects information about the safety of AEDs during pregnancy. To enroll, patients can call the toll-free number 1-888-233-2334.
Precautions with Alcohol
- Alcohol-Ezogabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- POTIGA®[2]
Look-Alike Drug Names
There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Ferron GM, Paul J, Fruncillo R; et al. (February 2002). "Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers". Journal of Clinical Pharmacology. 42 (2): 175–82. doi:10.1177/00912700222011210. PMID 11831540.
- ↑ "POTIGA- ezogabine tablet, film coated".