Eslicarbazepine acetate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Eslicarbazepine acetate is a anticonvulsant that is FDA approved for the treatment of partial-onset seizures. Common adverse reactions include dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Partial-Onset Seizures
- APTIOM (eslicarbazepine acetate) is indicated as adjunctive treatment of partial-onset seizures.
Dosage
Important Administration Instructions
- Instruct patients to administer APTIOM either as whole or as crushed tablets. Instruct patients to take APTIOM either with or without food.
Dosage for Partial-Onset Seizures
- Start treatment at 400 mg once daily. After one week, increase dosage to 800 mg once daily, which is the recommended maintenance dosage. Some patients may benefit from the maximum recommended maintenance dosage of 1200 mg once daily, although this dosage is associated with an increase in adverse reactions. A maximum dose of 1200 mg daily should only be initiated after the patient has tolerated 800 mg daily for at least a week. For some patients, treatment may be initiated at 800 mg once daily if the need for additional seizure reduction outweighs an increased risk of adverse reactions during initiation.
Dosage Modifications with Other Antiepileptic Drugs
- APTIOM should not be taken as an adjunctive therapy with oxcarbazepine.
- Some adverse reactions occur more frequently when patients take APTIOM with carbamazepine. However, carbamazepine reduces the plasma concentration of eslicarbazepine. When APTIOM and carbamazepine are taken concomitantly, the dose of APTIOM or carbamazepine may need to be adjusted based on efficacy and tolerability. For patients taking other enzyme-inducing antiepileptic drugs (AEDs) (i.e., phenobarbital, phenytoin, and primidone), higher doses of APTIOM may be needed.
Dosage Modifications in Patients with Renal Impairment
- A dose reduction is recommended in patients with moderate and severe renal impairment (i.e., creatinine clearance < 50 mL/min). Start treatment at 200 mg once daily. After two weeks, increase dosage to 400 mg once daily, which is the recommended maintenance dosage. Some patients may benefit from the maximum recommended maintenance dosage of 600 mg once daily.
Patients with Hepatic Impairment
- Dose adjustments are not required in patients with mild to moderate hepatic impairment. Use of APTIOM in patients with severe hepatic impairment has not been studied, and use in these patients is not recommended.
Discontinuation of APTIOM
- When discontinuing APTIOM, reduce the dosage gradually and avoid abrupt discontinuation in order to minimize the risk of increased seizure frequency and status epilepticus
DOSAGE FORMS AND STRENGTHS
- APTIOM tablets are available in the following shapes and color (TABLE 1) with respective one-sided engraving:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eslicarbazepine acetate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eslicarbazepine acetate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Eslicarbazepine acetate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eslicarbazepine acetate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eslicarbazepine acetate in pediatric patients.
Contraindications
- APTIOM is contraindicated in patients with a hypersensitivity to eslicarbazepine acetate or oxcarbazepine
Warnings
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including APTIOM, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number of events is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
- TABLE 2 shows absolute and relative risk by indication for all evaluated AEDs.
- The relative risk for suicidal thoughts or behavior was higher in clinical trials in patients with epilepsy than in clinical trials in patients with psychiatric or other conditions, but the absolute risk differences were similar for epilepsy and psychiatric indications.
- Anyone considering prescribing APTIOM or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression; any unusual changes in mood or behavior; or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Serious Dermatologic Reactions
- Serious dermatologic reactions including Stevens-Johnson Syndrome (SJS) have been reported in association with APTIOM use. Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and SJS, have been reported in patients using oxcarbazepine or carbamazepine which are chemically related to APTIOM. The reporting rate of these reactions associated with oxcarbazepine use exceeds the background incidence rate estimates by a factor of 3- to 10-fold. Risk factors for development of serious dermatologic reactions with APTIOM use have not been identified.
- If a patient develops a dermatologic reaction while taking APTIOM, discontinue APTIOM use, unless the reaction is clearly not drug-related. Patients with a prior dermatologic reaction with either oxcarbazepine or APTIOM should not be treated with APTIOM.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, has been reported in patients taking APTIOM. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. APTIOM should be discontinued and not be resumed if an alternative etiology for the signs or symptoms cannot be established. Patients with a prior DRESS reaction with either oxcarbazepine or APTIOM should not be treated with APTIOM.
Anaphylactic Reactions and Angioedema
- Rare cases of anaphylaxis and angioedema have been reported in patients taking APTIOM. Anaphylaxis and angioedema associated with laryngeal edema can be fatal. If a patient develops any of these reactions after treatment with APTIOM, the drug should be discontinued. Patients with a prior anaphylactic-type reaction with either oxcarbazepine or APTIOM should not be treated with APTIOM.
Hyponatremia
- Clinically significant hyponatremia (sodium <125 mEq/L) can develop in patients taking APTIOM. In the controlled epilepsy trials, 4/415 patients (1.0%) treated with 800 mg and 6/410 (1.5%) patients treated with 1200 mg of APTIOM had at least one serum sodium value less than 125 mEq/L, compared to none of the patients assigned to placebo. A higher percentage of APTIOM-treated patients (5.1%) than placebo-treated patients (0.7%) experienced decreases in sodium values of more than 10 mEq/L. These effects were dose-related and generally appeared within the first 8 weeks of treatment (as early as after 3 days). Serious, life-threatening complications were reported with APTIOM-associated hyponatremia (as low as 112 mEq/L) including seizures, severe nausea/vomiting leading to dehydration, severe gait instability, and injury. Some patients required hospitalization and discontinuation of APTIOM. Concurrent hypochloremia was also present in patients with hyponatremia. Depending on the severity of hyponatremia, the dose of APTIOM may need to be reduced or discontinued.
- Measurement of serum sodium and chloride levels should be considered during maintenance treatment with APTIOM, particularly if the patient is receiving other medications known to decrease serum sodium levels and should be performed if symptoms of hyponatremia develop (e.g., nausea/vomiting, malaise, headache, lethargy, confusion, irritability, muscle weakness/spasms, obtundation, or increase in seizure frequency or severity).
Neurological Adverse Reactions
- Dizziness and Disturbance in Gait and Coordination
- APTIOM causes dose-related increases in adverse reactions related to dizziness and disturbance in gait and coordination (dizziness, ataxia, vertigo, balance disorder, gait disturbance, nystagmus, and abnormal coordination). In controlled epilepsy trials, these events were reported in 26% and 38% of patients randomized to receive APTIOM at doses of 800 mg and 1200 mg/day, respectively, compared to 12% of placebo-treated patients. Events related to dizziness and disturbance in gait and coordination were more often serious in APTIOM-treated patients than in placebo-treated patients (2% vs. 0%), and more often led to study withdrawal in APTIOM-treated patients than in placebo-treated patients (9% vs. 0.7%). There was an increased risk of these adverse reactions during the titration period (compared to the maintenance period) and there also may be an increased risk of these adverse reactions in patients 60 years of age and older compared to younger adults. Nausea and vomiting also occurred with these events.
- The incidence of dizziness was greater with the concomitant use of APTIOM and carbamazepine compared to the use of APTIOM without carbamazepine (up to 37% vs. 19%, respectively). Therefore, consider dosage modifications of both APTIOM and carbamazepine if these drugs are used concomitantly.
Somnolence and Fatigue
- APTIOM causes dose-dependent increases in somnolence and fatigue-related adverse reactions (fatigue, asthenia, malaise, hypersomnia, sedation, and lethargy). In the controlled epilepsy trials, these events were reported in 13% of placebo patients, 16% of patients randomized to receive 800 mg/day APTIOM, and 28% of patients randomized to receive 1200 mg/day APTIOM. Somnolence and fatigue-related events were serious in 0.3% of APTIOM-treated patients (and 0 placebo patients) and led to discontinuation in 3% of APTIOM-treated patients (and 0.7% of placebo-treated patients).
Cognitive Dysfunction
- APTIOM causes dose-dependent increases in cognitive dysfunction-related events (memory impairment, disturbance in attention, amnesia, confusional state, aphasia, speech disorder, slowness of thought, disorientation, and psychomotor retardation). In the controlled epilepsy trials, these events were reported in 1% of placebo patients, 4% of patients randomized to receive 800 mg/day APTIOM, and 7% of patients randomized to receive 1200 mg/day APTIOM. Cognitive dysfunction-related events were serious in 0.2% of APTIOM-treated patients (and 0.2% of placebo patients) and led to discontinuation in 1% of APTIOM-treated patients (and 0.5% of placebo-treated patients).
Visual Changes
- APTIOM causes dose-dependent increases in events related to visual changes including diplopia, blurred vision, and impaired vision. In the controlled epilepsy trials, these events were reported in 16% of patients randomized to receive APTIOM compared to 6% of placebo patients. Eye events were serious in 0.7% of APTIOM-treated patients (and 0 placebo patients) and led to discontinuation in 4% of APTIOM-treated patients (and 0.2% of placebo-treated patients). There was an increased risk of these adverse reactions during the titration period (compared to the maintenance period) and also in patients 60 years of age and older (compared to younger adults). The incidence of diplopia was greater with the concomitant use of APTIOM and carbamazepine compared to the use of APTIOM without carbamazepine (up to 16% vs. 6%, respectively) .
Hazardous Activities
- Prescribers should advise patients against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of APTIOM is known.
Withdrawal of AEDs
- As with all antiepileptic drugs, APTIOM should be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus.
Drug Induced Liver Injury
- Hepatic effects, ranging from mild to moderate elevations in transaminases (>3 times the upper limit of normal) to rare cases with concomitant elevations of total bilirubin (>2 times the upper limit of normal) have been reported with APTIOM use. Baseline evaluations of liver laboratory tests are recommended. The combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury. APTIOM should be discontinued in patients with jaundice or other evidence of significant liver injury (e.g., laboratory evidence).
Abnormal Thyroid Function Tests
- Dose-dependent decreases in serum T3 and T4 (free and total) values have been observed in patients taking APTIOM. These changes were not associated with other abnormal thyroid function tests suggesting hypothyroidism. Abnormal thyroid function tests should be clinically evaluated.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are described in more detail in the Warnings and Precautions section of the label:
- Suicidal Behavior and Ideation
- Serious Dermatologic Reactions
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Anaphylactic Reactions and Angioedema
- Hyponatremia
- Neurological Adverse Reactions
- Drug Induced Liver Injury
- Abnormal Thyroid Function Tests
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In all controlled and uncontrolled trials in patients with partial-onset seizures, 1195 patients received APTIOM of whom 586 were treated for longer than 6 months and 462 for longer than 12 months. In the placebo controlled trials in patients with partial-onset seizures, 1021 patients received APTIOM. Of the patients in those trials, approximately 95% were between 18 and 60 years old, approximately 50% were male, and approximately 80% were Caucasian.
Adverse Reactions Leading to Discontinuation
- In the controlled epilepsy trials (Studies 1, 2, and 3), the rate of discontinuation as a result of any adverse reaction was 14% for the 800 mg dose, 25% for the 1200 mg dose, and 7% in subjects randomized to placebo. The adverse reactions most commonly (≥1% in any APTIOM treatment group, and greater than placebo) leading to discontinuation, in descending order of frequency, were dizziness, nausea, vomiting, ataxia, diplopia, somnolence, headache, blurred vision, vertigo, asthenia, fatigue, rash, dysarthria, and tremor.
Most Common Adverse Reactions
- The most frequently reported adverse reactions in patients receiving APTIOM at doses of 800 mg or 1200 mg (≥4% and ≥2% greater than placebo) were dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor.
- TABLE 3 gives the incidence of adverse reactions that occurred in ≥2% of subjects with partial-onset seizures in any APTIOM treatment group and for which the incidence was greater than placebo during the controlled clinical trials. Adverse reactions during titration were less frequent for patients who began therapy at an initial dose of 400 mg for 1 week and then increased to 800 mg compared to patients who initiated therapy at 800 mg.
Other Adverse Reactions with APTIOM Use
- Compared to placebo, APTIOM use was associated with slightly higher frequencies of decreases in hemoglobin and hematocrit, increases in total cholesterol, triglycerides, and LDL, and increases in creatine phosphokinase.
Adverse Reactions Based on Gender and Race
- No significant gender differences were noted in the incidence of adverse reactions. Although there were few non-Caucasian patients, no differences in the incidences of adverse reactions compared to Caucasian patients were observed.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Eslicarbazepine acetate in the drug label.
Drug Interactions
General Information
- Several AEDs (e.g., carbamazepine, phenobarbital, phenytoin, and primidone) can induce enzymes that metabolize APTIOM and can cause decreased plasma concentrations of eslicarbazepine (see FIGURE 1).
- APTIOM can inhibit CYP2C19, which can cause increased plasma concentrations of drugs that are metabolized by this isoenzyme (e.g., phenytoin, clobazam, and omeprazole). In vivo studies suggest that APTIOM can induce CYP3A4, decreasing plasma concentrations of drugs that are metabolized by this isoenzyme (e.g., simvastatin) (see FIGURE 2).
Potential for Other AEDs to Affect Eslicarbazepine
- The potential impact of other AEDs on the systemic exposure (area under the curve, AUC) of eslicarbazepine, the active metabolite of APTIOM, is shown in FIGURE 1:
Oral Contraceptives
- Because concomitant use of APTIOM and ethinylestradiol and levonorgestrel is associated with lower plasma levels of these hormones, females of reproductive potential should use additional or alternative non-hormonal birth control.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women. In oral studies conducted in pregnant mice, rats, and rabbits, eslicarbazepine acetate demonstrated developmental toxicity, including teratogenicity (mice), embryolethality (rats), and fetal growth retardation, at clinically relevant doses. APTIOM should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- When eslicarbazepine acetate was orally administered (150, 350, 650 mg/kg/day) to pregnant mice throughout organogenesis, increased incidences of fetal malformations was observed at all doses and fetal growth retardation was observed at the mid and high doses. A no-effect dose for adverse developmental effects was not identified. At the lowest dose tested, plasma eslicarbazepine exposure (Cmax, AUC) is less than that in humans at the maximum recommended human dose (MRHD) of 1200 mg/day.
- Oral administration of eslicarbazepine acetate (40, 160, 320 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in fetal growth retardation and increased incidences of skeletal variations at the mid and high doses. The no-effect dose (40 mg/kg/day) is less than the MRHD on a mg/m2 basis.
- Oral administration to pregnant rats (65, 125, 250 mg/kg/day) throughout organogenesis resulted in embryolethality at all doses, increased incidences of skeletal variations at the mid and high doses, and fetal growth retardation at the high dose. The lowest dose tested (65 mg/kg/day) is less than the MRHD on a mg/m2 basis.
- When eslicarbazepine acetate was orally administered to female mice during pregnancy and lactation (150, 350, 650 mg/kg/day), the gestation period was prolonged at the highest dose tested. In offspring, a persistent reduction in offspring body weight and delayed physical development and sexual maturation were observed at the mid and high doses. The lowest dose tested (150 mg/kg/day) is less than the MRHD on a mg/m2 basis.
- When eslicarbazepine acetate was orally administered (65, 125, 250 mg/kg/day) to rats during pregnancy and lactation, reduced offspring body weight was seen at the mid and high doses. Delayed sexual maturation and a neurological deficit (decreased motor coordination) were observed at the highest dose tested. The no-effect dose for adverse developmental effects (65 mg/kg/day) is less than the MRHD on a mg/m2 basis.
- The rat data are of uncertain relevance to humans because of differences in metabolic profile between species.
Pregnancy Registry
- Physicians are advised to recommend that pregnant patients taking APTIOM enroll in the North American Antiepileptic Drug Pregnancy Registry. This can be done by calling 1-888-233-2334 (toll-free), and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eslicarbazepine acetate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eslicarbazepine acetate during labor and delivery.
Nursing Mothers
- Eslicarbazepine is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from APTIOM, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in patients below 18 years of age have not been established.
- In a juvenile animal study in which eslicarbazepine acetate (40, 60, 160 mg/kg/day) was orally administered to young dogs for 10 months starting on postnatal day 21, mortality and evidence of immunotoxicity (bone marrow hypocellularity and lymphoid tissue depletion) were observed at all doses. Convulsions were seen at the highest dose tested. Adverse effects on bone growth (decreased bone mineral content and density) were seen in females at all doses at the end of the dosing period, but not at the end of a 2-month recovery period. None of these findings were reported in adult dogs dosed with eslicarbazepine acetate for up to 12 months in duration. A no-effect dose for adverse effects on juvenile dogs was not identified.
Geriatic Use
- There were insufficient numbers of patients ≥65 years old enrolled in the controlled epilepsy trials (N=15) to determine the efficacy of APTIOM in this patient population. The pharmacokinetics of APTIOM were evaluated in elderly healthy subjects (N=12) (FIGURE 3). Although the pharmacokinetics of eslicarbazepine are not affected by age independently, dose selection should take in consideration the greater frequency of renal impairment and other concomitant medical conditions and drug therapies in the elderly patient. Dose adjustment is necessary if CrCl is <50 mL/min
Gender
- No dosage adjustment is recommended on the basis of gender (FIGURE 3)
Race
There is no FDA guidance on the use of Eslicarbazepine acetate with respect to specific racial populations.
Renal Impairment
- Clearance of eslicarbazepine is decreased in patients with impaired renal function and is correlated with creatinine clearance. Dosage adjustment is necessary in patients with CrCl<50 mL/min (FIGURE 3)
Hepatic Impairment
- Dose adjustments are not required in patients with mild to moderate hepatic impairment (FIGURE 3). Use of APTIOM in patients with severe hepatic impairment has not been evaluated, and use in these patients is not recommended
Females of Reproductive Potential and Males
- Because concomitant use of APTIOM and ethinylestradiol and levonorgestrel is associated with lower plasma levels of these hormones, females of reproductive potential should use additional or alternative non-hormonal birth control
Immunocompromised Patients
There is no FDA guidance one the use of Eslicarbazepine acetate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Monitor for suicidal thoughts or behavior
- Monitor for dermatologic reactions and discontinue in the case of serious dermatologic reaction
- Monitor sodium levels in patients at risk or patients experiencing hyponatremia symptoms
IV Compatibility
There is limited information regarding IV Compatibility of Eslicarbazepine acetate in the drug label.
Overdosage
Signs, Symptoms, and Laboratory Findings of Acute Overdose in Humans
- Symptoms of overdose are consistent with the known adverse reactions of APTIOM and include hyponatremia (sometimes severe), dizziness, nausea, vomiting, somnolence, euphoria, oral paraesthesia, ataxia, walking difficulties, and diplopia.
Treatment or Management of Overdose
- There is no specific antidote for overdose with APTIOM. Symptomatic and supportive treatment should be administered as appropriate. Removal of the drug by gastric lavage and/or inactivation by administering activated charcoal should be considered.
- Standard hemodialysis procedures result in partial clearance of APTIOM. Hemodialysis may be considered based on the patient's clinical state or in patients with significant renal impairment.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
- APTIOM is not a controlled substance.
Abuse
- Prescription drug abuse is the intentional non-therapeutic use of a drug, even once, for its rewarding psychological or physiological effects. Drug addiction, which develops after repeated drug abuse, is characterized by a strong desire to take a drug despite harmful consequences, difficulty in controlling its use, giving a higher priority to drug use than to obligations, increased tolerance, and sometimes physical withdrawal. Drug abuse and drug addiction are separate and distinct from physical dependence (for example, abuse may not be accompanied by physical dependence).
- In a human abuse study in recreational sedative abusers APTIOM showed no evidence of abuse. In Phase 1, 1.5% of the healthy volunteers taking APTIOM reported euphoria compared to 0.4% taking placebo.
Dependence
- Physical dependence is characterized by withdrawal symptoms after abrupt discontinuation or a significant dose reduction of a drug.
- The potential for APTIOM to produce withdrawal symptoms has not been adequately evaluated. In general, antiepileptic drugs should not be abruptly discontinued in patients with epilepsy because of the risk of increased seizure frequency and status epilepticus.
Pharmacology
| |
Eslicarbazepine acetate
| |
| Systematic (IUPAC) name | |
| (S)-10-Acetoxy- 10,11-dihydro- 5H-dibenz[b,f]azepine- 5-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 296.320 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ~90% renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) Rx only |
| Routes | Oral |
Mechanism of Action
- APTIOM is extensively converted to eslicarbazepine, which is considered to be responsible for therapeutic effects in humans. The precise mechanism(s) by which eslicarbazepine exerts anticonvulsant activity is unknown but is thought to involve inhibition of voltage-gated sodium channels.
Structure
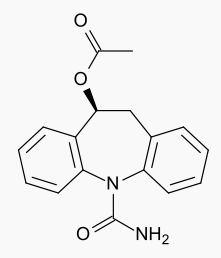
- The chemical name of APTIOM (eslicarbazepine acetate) is (S)-10-Acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide. APTIOM is a dibenz[b,f]azepine-5-carboxamide derivative. Its molecular formula is C17H16N2O3 and its molecular weight is 296.32. The chemical structure is:
- APTIOM is a white to off-white, odorless crystalline solid. It is insoluble in hexane, very slightly soluble in aqueous solvents and soluble in organic solvents such as acetone, acetonitrile, and methanol.
- Each APTIOM tablet contains 200 mg, 400 mg, 600 mg or 800 mg of eslicarbazepine acetate and the following inactive ingredients: povidone, croscarmellose sodium, and magnesium stearate.
Pharmacodynamics
- The effect of APTIOM on cardiac repolarization was evaluated in a randomized, double-blind, placebo- and active-controlled 4-period crossover trial in healthy adult men and women. Subjects received APTIOM 1200 mg once daily × 5 days, APTIOM 2400 mg once daily × 5 days, an active-control, moxifloxacin 400 mg × 1 dose on Day 5, and placebo once daily × 5 days. At both doses of APTIOM, no significant effect on the QTc interval was detected.
Pharmacokinetics
- The pharmacokinetics of eslicarbazepine is linear and dose-proportional in the dose range of 400 mg to 1200 mg once daily, both in healthy subjects and patients. The apparent half-life of eslicarbazepine in plasma was 13 -20 hours in epilepsy patients. Steady-state plasma concentrations are attained after 4 to 5 days of once daily dosing.
Absorption, Distribution, Metabolism, and Excretion
Absorption
- APTIOM is mostly undetectable (0.01% of the systemic exposure) after oral administration. Eslicarbazepine, the major metabolite, is primarily responsible for the pharmacological effect of APTIOM. Peak plasma concentrations (Cmax) of eslicarbazepine are attained at 1-4 hours post-dose. Eslicarbazepine is highly bioavailable, because the amount of eslicarbazepine and glucuronide metabolites recovered in urine corresponded to more than 90% of an APTIOM dose. Food has no effect on the pharmacokinetics of eslicarbazepine after oral administration of APTIOM.
Distribution
- The binding of eslicarbazepine to plasma proteins is relatively low (<40%) and independent of concentration. In vitro studies have shown that plasma protein binding was not relevantly affected by the presence of warfarin, diazepam, digoxin, phenytoin, or tolbutamide. Similarly, the binding of warfarin, diazepam, digoxin, phenytoin or tolbutamide was not significantly affected by the presence of eslicarbazepine. The apparent volume of distribution of eslicarbazepine is 61 L for body weight of 70 kg based on population PK analysis.
Metabolism
- APTIOM is rapidly and extensively metabolized to its major active metabolite eslicarbazepine by hydrolytic first-pass metabolism. Eslicarbazepine corresponds to 91% of systemic exposure. The systemic exposure to minor active metabolites of (R)-licarbazepine is 5% and oxcarbazepine is 1%. The inactive glucuronides of these active metabolites correspond to approximately 3% of systemic exposure.
- In in vitro studies in human liver microsomes, eslicarbazepine had no clinically relevant inhibitory effect on the activity of CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4, and only a moderate inhibitory effect on CYP2C19. Studies with eslicarbazepine in fresh human hepatocytes showed no induction of enzymes involved in glucuronidation and sulfation of 7-hydroxy-coumarin. A mild activation of UGT1A1- mediated glucuronidation was observed in human hepatic microsomes.
- No apparent autoinduction of metabolism has been observed with APTIOM in humans.
Excretion
- APTIOM metabolites are eliminated from the systemic circulation primarily by renal excretion, in the unchanged and glucuronide conjugate forms. In total, eslicarbazepine and its glucuronide account for more than 90% of total metabolites excreted in urine, approximately two thirds in the unchanged form and one third as glucuronide conjugate. Other minor metabolites account for the remaining 10% excreted in the urine. In healthy subjects with normal renal function, the renal clearance of eslicarbazepine (approximately 20 mL/min) is substantially lower than glomerular filtration rate (80-120 mL/min), suggesting that renal tubular reabsorption occurs. The apparent plasma half-life of eslicarbazepine was 13-20 hours in epilepsy patients.
Specific Populations
- Elderly (≥65 years of age)
- The pharmacokinetic profile of eslicarbazepine was unaffected in elderly subjects with creatinine clearance >60 mL/min compared to healthy subjects (18-40 years) after single and repeated doses of 600 mg APTIOM during 8 days of dosing. No dose adjustment is necessary in adults based on age, if CrCl is ≥50 mL/min.
- Gender
- Studies in healthy subjects and patients showed that pharmacokinetics of eslicarbazepine was not affected by gender.
- Race
- No clinically significant effect of race (Caucasian N=849, Black N=53, Asian N=65, and Other N=51) on the pharmacokinetics of eslicarbazepine was noted in a population pharmacokinetic analysis of pooled data from the clinical studies.
- Renal Impairment
- APTIOM metabolites are eliminated from the systemic circulation primarily by renal excretion. The extent of systemic exposure of eslicarbazepine following an 800 mg single dose was increased by 62% in patients with mild renal impairment (CrCl 50-80 mL/min), by 2-fold in patients with moderate renal impairment (CrCl 30-49 mL/min) and by 2.5-fold in patients with severe renal impairment (CrCl <30 mL/min) in comparison to the healthy subjects (CrCl >80 mL/min). Dosage adjustment is recommended in patients with creatinine clearance below 50 mL/min.
- In patients with end stage renal disease, repeated hemodialysis removed APTIOM metabolites from systemic circulation.
- Hepatic Impairment
- The pharmacokinetics and metabolism of APTIOM was evaluated in healthy subjects and patients with moderate liver impairment (7-9 points on the Child-Pugh assessment) after multiple oral doses. Moderate hepatic impairment did not affect the pharmacokinetics of APTIOM. No dose adjustment is recommended in patients with mild to moderate liver impairment.
- The pharmacokinetics of APTIOM has not been studied in patients with severe hepatic impairment.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
=====Carcinogenesis
- In a two-year carcinogenicity study in mice, eslicarbazepine acetate was administered orally at doses of 100, 250, and 600 mg/kg/day. An increase in the incidence of hepatocellular adenomas and carcinomas was observed at 250 and 600 mg/kg/day in males and at 600 mg/kg/day in females. The dose not associated with an increase in tumors (100 mg/kg/day) is less than the maximum recommended human dose of 1200 mg/day on a mg/m2 basis.
Mutagenesis
- Eslicarbazepine acetate and eslicarbazepine were not mutagenic in the in vitro Ames assay. In in vitro assays in mammalian cells, eslicarbazepine acetate and eslicarbazepine were not clastogenic in human peripheral blood lymphocytes; however, eslicarbazepine acetate was clastogenic in Chinese hamster ovary (CHO) cells, with and without metabolic activation. Eslicarbazepine acetate was positive in the in vitro mouse lymphoma tk assay in the absence of metabolic activation. Eslicarbazepine acetate was not clastogenic in the in vivo mouse micronucleus assay.
Impairment of Fertility
- When eslicarbazepine acetate (150, 350, and 650 mg/kg/day) was orally administered to male and female mice prior to and throughout the mating period, and continuing in females to gestation day 6, there was an increase in embryolethality at all doses. The lowest dose tested is less than the maximum recommended human dose (1200 mg/day) on a mg/m2 basis.
- When eslicarbazepine acetate (65, 125, 250 mg/kg/day) was orally administered to male and female rats prior to and throughout the mating period, and continuing in females to implantation, lengthening of the estrus cycle was observed at the highest dose tested. The data in rats are of uncertain relevance to humans because of differences in metabolic profile between species.
Clinical Studies
Clinical Studies in Adults with Partial-Onset Seizures
- The efficacy of APTIOM as adjunctive therapy in partial-onset seizures was established in three randomized, double-blind, placebo-controlled, multicenter trials in adult patients with epilepsy (Studies 1, 2, and 3). Patients enrolled had partial-onset seizures with or without secondary generalization and were not adequately controlled with 1 to 3 concomitant AEDs. During an 8-week baseline period, patients were required to have an average of ≥4 partial-onset seizures per 28 days with no seizure-free period exceeding 21 days. In these three trials, patients had a median duration of epilepsy of 19 years and a median baseline seizure frequency of 8 seizures per 28 days. Two-thirds (69%) of subjects used 2 concomitant AEDs and 28% used 1 concomitant AED. The most commonly used AEDs were carbamazepine (50%), lamotrigine (24%), valproic acid (21%), and levetiracetam (18%). Oxcarbazepine was not allowed as a concomitant AED.
- Studies 1 and 2 compared dosages of APTIOM 400, 800, and 1200 mg once daily with placebo. Study 3 compared dosages of APTIOM 800 and 1200 mg once daily with placebo. In all three trials, following an 8-week Baseline Phase, which established a baseline seizure frequency, subjects were randomized to a treatment arm. Patients entered a treatment period consisting of an initial titration phase (2 weeks), and a subsequent maintenance phase (12 weeks). The specific titration schedule differed amongst the three studies. Thus, patients were started on a daily dose of 400 mg or 800 mg and subsequently increased by 400 mg/day following one or two weeks, until the final daily target dose was achieved.
- The standardized seizure frequency during the Maintenance Phase over 28 days was the primary efficacy endpoint in all three trials. TABLE 4 presents the results for the primary endpoint, as well as the secondary endpoint of percent reduction from baseline in seizure frequency. The APTIOM treatment at 400 mg/day was studied in Studies 1 and 2 and did not show significant treatment effect. A statistically significant effect was observed with APTIOM treatment at doses of 800 mg/day in Studies 1 and 2 but not in Study 3, and at doses of 1200 mg/day in all 3 studies.
How Supplied
- APTIOM tablets are white, oblong and with functional scoring on one side (200 mg, 600 mg, and 800 mg) or white, circular bi-convex and plain on one side (400 mg) and identified with strength-specific one-sided engraving on the other side, “ESL 200” (200 mg), “ESL 400” (400 mg), “ESL 600” (600 mg), or “ESL 800” (800 mg). Tablets are supplied in the following strengths and package configurations
Storage
- Store APTIOM tablets at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)
Images
Drug Images
{{#ask: Page Name::Eslicarbazepine acetate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Eslicarbazepine acetate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking APTIOM. Instruct patients to take APTIOM only as prescribed.
Suicidal Behavior and Ideation
- Counsel patients, their caregivers, and families that AEDs, including APTIOM, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to healthcare providers.
Serious Dermatologic Reactions
- Advise patients and caregivers about the risk of potentially fatal serious skin reactions. Educate patients about the signs and symptoms that may signal a serious skin reaction. Instruct patients to consult with their healthcare provider immediately if a skin reaction occurs during treatment with APTIOM.
DRESS/Multiorgan Hypersensitivity
- Instruct patients that a fever associated with signs of other organ system involvement (e.g., rash, lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately .
Anaphylactic Reactions and Angioedema
- Advise patients of life threatening symptoms suggesting anaphylaxis or angioedema (swelling of the face, eyes, lips, tongue, or difficulty in swallowing or breathing) that can occur with APTIOM. Instruct them to immediately report these symptoms to their healthcare provider.
Hyponatremia
- Advise patients that APTIOM may reduce serum sodium concentrations, especially if they are taking other medications that can lower sodium. Advise patients to report symptoms of low sodium such as nausea, tiredness, lack of energy, irritability, confusion, muscle weakness/spasms, or more frequent or more severe seizures.
Neurological Adverse Reactions
- Counsel patients that APTIOM may cause dizziness, gait disturbance, somnolence/fatigue, cognitive dysfunction, and visual changes. These adverse reactions, if observed, are more likely to occur during the titration period compared to the maintenance period. Advise patients not to drive or operate machinery until they have gained sufficient experience on APTIOM to gauge whether it adversely affects their ability to drive or operate machinery.
Withdrawal of APTIOM
- Advise patients not to discontinue use of APTIOM without consulting with their healthcare provider. APTIOM should be gradually withdrawn to minimize the potential of increased seizure frequency and status epilepticus.
Interaction with Oral Contraceptives
- Inform patients that APTIOM can significantly decrease the effectiveness of hormonal contraceptives. Recommend that female patients of childbearing potential use additional or alternative non-hormonal forms of contraception during treatment with APTIOM and after treatment has been discontinued for at least one menstrual cycle or until otherwise instructed by their healthcare provider.
Pregnancy Registry
- Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This Registry is collecting information about the safety of AEDs during pregnancy. To enroll, patients can call 1-888-233-2334 (toll-free)
MEDICATION GUIDE
Precautions with Alcohol
- Alcohol-Eslicarbazepine acetate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Aptiom®[1]
Look-Alike Drug Names
There is limited information regarding Eslicarbazepine acetate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.











