Metamizole
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
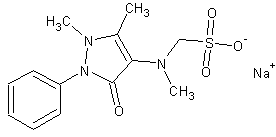
| Formula | C13H16N3NaO4S |
| Molar mass | 311.358 g/mol |
|
WikiDoc Resources for Metamizole |
|
Articles |
|---|
|
Most recent articles on Metamizole |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Metamizole at Clinical Trials.gov Clinical Trials on Metamizole at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Metamizole
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Metamizole Discussion groups on Metamizole Patient Handouts on Metamizole Directions to Hospitals Treating Metamizole Risk calculators and risk factors for Metamizole
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Metamizole |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Metamizole sodium is a non-steroidal anti-inflammatory drug (NSAID), commonly used in the past as a powerful painkiller and fever reducer. It is better known under the names Dipyrone, Analgin and Novalgin.
Metamizole was first synthesized by the German company Hoechst AG in 1920, and its mass production started in 1922. It remained freely available worldwide until the 1970s, when it was discovered that the drug carries a small risk of causing agranulocytosis - a very dangerous and potentially fatal condition. Controversy remains regarding the level of risk. Several national medical authorities have banned metamizole either totally or have restricted it to be available only on prescription.
Risks of agranulocytosis
According to comments by Dr Anthony Wong of the University of São Paulo, Brazil in a WHO newsletter,[1] recent studies estimate that the incidence rate of metamizole-induced agranulocytosis is between 0.2 and 2 cases per million person days of use, with approximately 7% of all cases fatal (provided that all patients have access to urgent medical care). In other words, one should expect 50 to 500 deaths annually due to metamizole in a country of 300 million, assuming that every citizen takes the drug once a month. This is not a very high rate compared to other drugs - for example, the prescription drug clozapine is known to be at least 50 times more likely to trigger agranulocytosis. However, at the time the risk was assumed to be much greater and, as such, excessive for an over-the-counter analgesic, especially considering the existence of safer alternatives (aspirin and ibuprofen).
A study in Northern Sweden published in 2002 estimated the total risk during metamizole therapy for patients in hospitals (inpatients) and outside of hospital (outpatients) about 3 to 100 times greater than that estimated by Dr Wong: "Given certain assumptions including the actual amounts prescribed the calculated risks of agranulocytosis would be approximately one out of every 31,000 metamizole-treated inpatients and one of every 1400 metamizole-treated outpatients."[2]
Availability around the world
Metamizole was banned in Sweden in 1974, in the United States in 1977; more than 30 countries, including Japan, Australia, Iran, and part of the European Union, have followed suit. In these countries metamizole is still occasionally used as a veterinary drug. In Germany it became a prescription drug. Some European pharmaceutical companies, notably Hoechst and Merck, continue to develop metamizole-containing drugs and market them in some countries. In Sweden, the ban was lifted in 1995 and re-introduced in 1999 only to be taken off the market again just a few years later.
In other parts of the world (notably in Spain, Mexico, India, Brazil, Russia, Bulgaria, Romania, Israel and Third World countries) metamizole is still freely available over-the-counter, remains one of the most popular analgesics, and plays an important role in self-medication. For example, metamizole and metamizole-containing drugs account for 80% of OTC analgesic market in Russia, whereas ibuprofen accounts for 2.5%. In Brazil, metamizole (Novalgina) products, although over-the-counter, carry warnings to avoid usage by those under 19 years old, and have several informations about early detection and treatment of agranulocytosis. Although the Brazilian government did not push for a ban on the drug, its use has seen a decline on the past years as pharmaceutical companies and doctors pushed aspirin, paracetamol and ibuprofen based products as replacement, specialy regarding child care. Amongst adults it is still widely used. Some of the most widely available metamizole-containing product still in use in Brazil are: Buscopan Plus (under the name of Buscopan Composto), Novalgina and Neosaldina. Generic Dipyrone is also available.
Media attention
Metamizole received brief period of attention by American media in 2001[3], when a Latino immigrant boy was admitted into a Salt Lake City clinic with symptoms of agranulocytosis. It was discovered that the drug remained freely available in Latino shops and highly popular among Mexican immigrants, despite the ban. The ongoing "LATIN" Study, a multicenter international case-control study, is examining the incidence of agranulocytosis in Latin America and the role of metamizole.
Brand names
- Brazil: Novalgina, Neosaldina, Sedalgina, Doridina, Migranette, Benegrip, Anador, Magnopyrol, Conmel, Difebril, Termopirona, Dipifarma, Termosil, Dorona, Hynalgin, Lisador, among others.
- Bulgaria: Proalgin, Analgin
- Croatia: Analgin
- Finland: Litalgin
- Germany: Novalgin, Analgin, Berlosin, Metalgin, Metamizol-Puren, Novaminsulfon.
- Hungary: Algopyrin
- India: Novalgin
- Israel: Optalgin
- Italy: Novalgina
- Macedonia: Analgin
- Mexico: Neo-Melubrina
- Poland: Pyralgina
- Romania: Algocalmin, Novocalmin, Algozone, Nevralgin
- Russia/Bulgaria: Tempalgin (combination drug; metamizole is one of its components)
- Slovenia: Analgin
- Serbia: Analgin
- Spain: Nolotil
- Switzerland: Novalgin
- Turkey: Novalgin
- Venezuela: Novalcina
References
- ↑ Dr Anthony Wong in WHO Pharmaceuticals Newsletter No. 1, 2002, p.15
- ↑ Bäckström, M. (April–May 2002). "Utilization pattern of metamizole in northern Sweden and risk estimates of agranulocytosis". Retrieved 2007-08-21. Unknown parameter
|coauthors=ignored (help) - ↑ Metamizole Use by Latino Immigrants: A Common and Potentially Harmful Home Remedy
bg:Норамидопирин de:Metamizol he:דיפירון hu:Metamizol-nátrium
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Date format
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Analgesics
- Antipyretics
- Withdrawn drugs
- Drugs