Daratumumab Test2
{{DrugProjectFormSinglePage
|authorTag=AKT
|genericName=Daratumumab
|aOrAn=an
|drugClass=antineoplastic agent
|indicationType=treatment
|indication=multiple myeloma
|adverseReactions=fatigue, headache, nausea, diarrhea, constipation, decreased appetite, vomiting, lymphocytopenia, neutropenia, thrombocytopenia, anemia, back pain, arthralgia, leg pain, musculoskeletal chest pain, cough, nasal congestion, dyspnea, nasopharyngitis, pneumonia, and infusion-related reaction
|blackBoxWarningTitle=TITLE
|blackBoxWarningBody=Condition Name: (Content)
|fdaLIADAdult=Daratumumab is indicated for, in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone, treatment of patients with multiple myeloma who have received at least one prior therapy; for, in combination with pomalidomide and dexamethasone, treatment of patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor; and as monotherapy, for the treatment of patients with multiple myeloma who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double refractory to a PI and an immunomodulatory agent.
Multiple Myeloma
- Administer post-infusion medication to reduce the risk of delayed infusion reactions to all patients as follows:
- Administer corticosteroids (monotherapy: methylprednisolone 100 mg, or equivalent, administered intravenously. Following the second infusion, the dose of corticosteroid may be reduced (oral or intravenous methylprednisolone 60 mg) or combination therapy: administer 20 mg dexamethasone prior to every daratumumab infusion. Dexamethasone is given intravenously prior to the first daratumumab infusion and oral administration may be considered prior to subsequent infusions), antipyretics (oral acetaminophen 650 to 1000 mg), and antihistamine (oral or intravenous diphenhydramine 25 to 50 mg or equivalent) to reduce the risk of infusion reactions to all patients 1–3 hours prior to every infusion of daratumumab.
- Administer post-infusion medication to reduce the risk of delayed infusion reactions to all patients as follows:
- Monotherapy: Administer oral corticosteroid (20 mg methylprednisolone or equivalent dose of an intermediate-acting or long-acting corticosteroid in accordance with local standards) on each of the 2 days following all daratumumab infusions (beginning the day after the infusion).
- Combination therapy: Consider administering low-dose oral methylprednisolone (≤ 20 mg) or equivalent, the day after the daratumumab infusion. However, if a background regimen-specific corticosteroid (e.g. dexamethasone) is administered the day after the daratumumab infusion, additional post-infusion medications may not be needed.
- In addition, for any patients with a history of chronic obstructive pulmonary disease, consider prescribing post-infusion medications such as short and long-acting bronchodilators, and inhaled corticosteroids. Following the first four infusions, if the patient experiences no major infusion reactions, these additional inhaled post-infusion medications may be discontinued.
- Initiate antiviral prophylaxis to prevent herpes zoster reactivation within 1 week after starting daratumumab and continue for 3 months following treatment.
- Dosing Information
- Administer only as an intravenous infusion after dilution in 0.9% Sodium Chloride Injection, USP. Daratumumab should be administered by a healthcare professional, with immediate access to emergency equipment and appropriate medical support to manage infusion reactions if they occur.
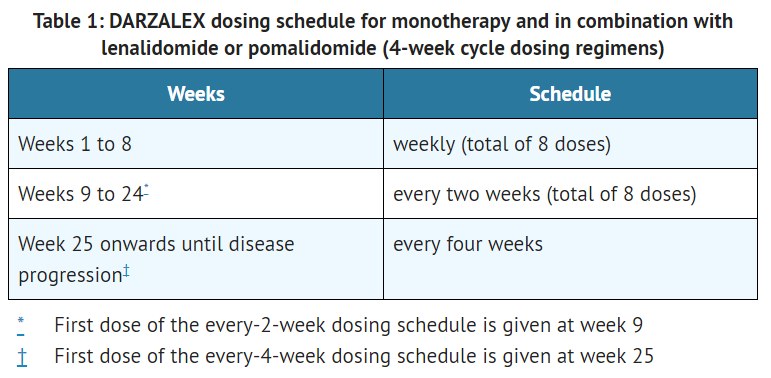
- The recommended dose of daratumumab for monotherapy and combination therapy with lenalidomide or pomalidomide and low-dose dexamethasone (4-week cycle regimens) is 16 mg/kg actual body weight administered as an intravenous infusion according to the following dosing schedule
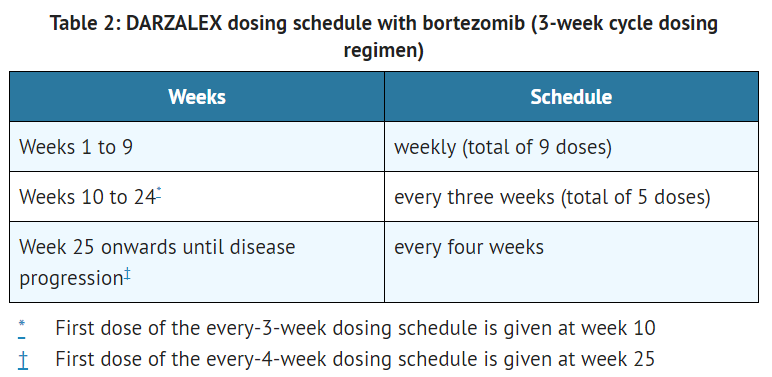
This image is provided by the National Library of Medicine. - The recommended dose of daratumumab for combination therapy with bortezomib and dexamethasone (3-week cycle regimen) is 16 mg/kg actual body weight administered as an intravenous infusion according to the following dosing schedule
This image is provided by the National Library of Medicine. - If a planned dose of daratumumab is missed, administer the dose as soon as possible and adjust the dosing schedule accordingly, maintaining the treatment interval.
- Administer daratumumab infusion intravenously at the infusion rate described below. Consider incremental escalation of the infusion rate only in the absence of infusion reactions.
This image is provided by the National Library of Medicine.
- For infusion reactions of any grade/severity, immediately interrupt the daratumumab infusion and manage symptoms. Management of infusion reactions may further require reduction in the rate of infusion, or treatment discontinuation of daratumumab as outlined below:
- Grade 1–2 (mild to moderate): Once reaction symptoms resolve, resume the infusion at no more than half the rate at which the reaction occurred. If the patient does not experience any further reaction symptoms, infusion rate escalation may resume at increments and intervals as clinically appropriate up to the maximum rate of 200 mL/hour (Table 3).
- Grade 3 (severe): Once reaction symptoms resolve, consider restarting the infusion at no more than half the rate at which the reaction occurred. If the patient does not experience additional symptoms, resume infusion rate escalation at increments and intervals as outlined in Table 3. Repeat the procedure above in the event of recurrence of Grade 3 symptoms. Permanently discontinue daratumumab upon the third occurrence of a Grade 3 or greater infusion reaction.
- Grade 4 (life threatening): Permanently discontinue daratumumab treatment.
- No dose reductions of daratumumab are recommended. Dose delay may be required to allow recovery of blood cell counts in the event of hematological toxicity.
- Daratumumab is for single use only. Prepare the solution for infusion using aseptic technique as follows:
- Calculate the dose (mg), total volume (mL) of daratumumab solution required and the number of daratumumab vials needed based on patient actual body weight.
- Check that the daratumumab solution is colorless to pale yellow. Do not use if opaque particles, discoloration or other foreign particles are present.
- Remove a volume of 0.9% Sodium Chloride Injection, USP from the infusion bag/container that is equal to the required volume of daratumumab solution.
- Withdraw the necessary amount of daratumumab solution and dilute to the appropriate volume by adding to the infusion bag/container containing 0.9% Sodium Chloride Injection, USP as specified in Table 3. Infusion bags/containers must be made of either polyvinyl chloride (PVC), polypropylene (PP), polyethylene (PE) or polyolefin blend (PP+PE). Dilute under appropriate aseptic conditions. Discard any unused portion left in the vial.
- Gently invert the bag/container to mix the solution. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The diluted solution may develop very small, translucent to white proteinaceous particles, as daratumumab is a protein. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
- Since daratumumab does not contain a preservative, administer the diluted solution immediately at room temperature 15°C–25°C (59°F–77°F) and in room light. Diluted solution may be kept at room temperature for a maximum of 15 hours (including infusion time).
- If not used immediately, the diluted solution can be stored prior to administration for up to 24 hours at refrigerated conditions 2°C – 8°C (36°F–46°F) and protected from light. Do not freeze.
- Administer daratumumab as follows:
- If stored in the refrigerator, allow the solution to come to room temperature. Administer the diluted solution by intravenous infusion using an infusion set fitted with a flow regulator and with an in-line, sterile, non-pyrogenic, low protein-binding polyethersulfone (PES) filter (pore size 0.22 or 0.2 micrometer). Administration sets must be made of either polyurethane (PU), polybutadiene (PBD), PVC, PP or PE.
- Do not store any unused portion of the infusion solution for reuse. Any unused product or waste material should be disposed of in accordance with local requirements.
- Do not infuse daratumumab concomitantly in the same intravenous line with other agents.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Daratumumab Test2 in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Daratumumab Test2 in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Daratumumab Test2 in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Daratumumab Test2 in pediatric patients. |warnings=Infusion Reactions
- Daratumumab can cause severe infusion reactions. Approximately half of all patients experienced a reaction, most during the first infusion. Infusion reactions can also occur with subsequent infusions. Nearly all reactions occurred during infusion or within 4 hours of completing daratumumab. Prior to the introduction of post-infusion medication in clinical trials, infusion reactions occurred up to 48 hours after infusion.
- Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, laryngeal edema and pulmonary edema. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting and nausea. Less common symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, and hypotension.
- Pre-medicate patients with antihistamines, antipyretics and corticosteroids. Frequently monitor patients during the entire infusion. Interrupt daratumumab infusion for reactions of any severity and institute medical management as needed. Permanently discontinue daratumumab therapy for life-threatening (Grade 4) reactions. For patients with Grade 1, 2, or 3 reactions, reduce the infusion rate when re-starting the infusion.
- To reduce the risk of delayed infusion reactions, administer oral corticosteroids to all patients following daratumumab infusions. Patients with a history of chronic obstructive pulmonary disease may require additional post-infusion medications to manage respiratory complications. Consider prescribing short- and long-acting [[bronchodilators] and inhaled corticosteroids for patients with chronic obstructive pulmonary disease.
- Interference with serological testing
- Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive Indirect Antiglobulin Test (Indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab infusion. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient's serum. The determination of a patient's ABO and Rh blood type are not impacted.
- Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received daratumumab. Type and screen patients prior to starting daratumumab.
- Neutropenia
- Daratumumab may increase neutropenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Daratumumab dose delay may be required to allow recovery of neutrophils. No dose reduction of daratumumab is recommended. Consider supportive care with growth factors.
- Thrombocytopenia
- Daratumumab may increase thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer's prescribing information for background therapies. Daratumumab dose delay may be required to allow recovery of platelets. No dose reduction of daratumumab is recommended. Consider supportive care with transfusions.
- Interference with determination of complete response
- Daratumumab is a human IgG kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response of disease progression in some patients with IgG kappa myeloma protein.
|alcohol=Alcohol-Daratumumab Test2 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }}