Chronic obstructive pulmonary disease pathophysiology
|
Chronic obstructive pulmonary disease Microchapters |
|
Differentiating Chronic obstructive pulmonary disease from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Chronic obstructive pulmonary disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chronic obstructive pulmonary disease pathophysiology |
|
FDA on Chronic obstructive pulmonary disease pathophysiology |
|
CDC on Chronic obstructive pulmonary disease pathophysiology |
|
Chronic obstructive pulmonary disease pathophysiology in the news |
|
Blogs on Chronic obstructive pulmonary disease pathophysiology |
|
Directions to Hospitals Treating Chronic obstructive pulmonary disease |
|
Risk calculators and risk factors for Chronic obstructive pulmonary disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editors-In-Chief: Seyedmahdi Pahlavani, M.D. [2], Mehrian Jafarizade, M.D [3], Cafer Zorkun, M.D., Ph.D. [4]
Overview
Pathologic changes in chronic obstructive pulmonary disease (COPD) occur in the large (central) airways, the bronchioles, and the lung parenchyma. Increased numbers of activated polymorphonuclear leukocytes and macrophages release elastases, proteinase-3 and macrophage-derived matrix metalloproteinases (MMPs), cysteine proteinases, and a plasminogen activator resulting in lung destruction. The antiprotease in the body cannot counteract effectively these elastases. Additionally, increased oxidative stress caused by free radicals in cigarette smoke, phagocytes, and polymorphonuclear leukocytes all may lead to apoptosis. In addition to macrophages, T lymphocytes, particularly CD8+, play an important role in the pathogenesis of smoking-induced airflow limitation.
Pathophysiology
- Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs.
- In COPD, the greatest reduction in air flow occurs when breathing out (during expiration) because the pressure in the chest tends to compress rather than expand the airways.
- In theory, air flow could be increased by breathing more forcefully, increasing the pressure in the chest during expiration. In COPD, there is often a limit to how much this can actually increase air flow, a situation known as expiratory flow limitation.[1]
- If the rate of airflow is too low, a person with COPD may not be able to completely finish breathing out (expiration) before he or she needs to take another breath. This is particularly common during exercise, when breathing has to be faster. A little of the air of the previous breath remains within the lungs when the next breath is started, resulting in an increase in the volume of air in the lungs, a process called dynamic hyperinflation.[1]
- Dynamic hyperinflation is closely linked to dyspnea in COPD.[2]
- It is less comfortable to breathe with hyperinflation because it takes more effort to move the lungs and chest wall when they are already stretched by hyperinflation.
- Another factor contributing to shortness of breath in COPD is the loss of the surface area available for the exchange of oxygen and carbon dioxide with emphysema. This reduces the rate of transfer of these gases between the body and the atmosphere and can lead to low oxygen and high carbon dioxide levels in the body.
- A person with emphysema may have to breathe faster or more deeply to compensate, which can be difficult to do if there is also flow limitation or hyperinflation.
- Some people with advanced COPD do manage to breathe fast to compensate, but usually have dyspnea as a result. Others, who may be less short of breath, tolerate low oxygen and high carbon dioxide levels in their bodies, but this can eventually lead to headaches, drowsiness and heart failure.
- It is not fully understood how tobacco smoke and other inhaled particles damage the lungs to cause COPD. The most important processes causing lung damage are:
- Oxidative stress produced by the high concentrations of free radicals in tobacco smoke,
- Cytokine release due to inflammation as the body responds to irritant particles such as tobacco smoke in the airway,
- Tobacco smoke and free radicals impair the activity of antiprotease enzymes such as alpha 1-antitrypsin, allowing protease enzymes to damage the lung.
- Several molecular signatures associated to lung function decline and corollaries of disease severity have been proposed, a majority of which are characterized in easily accessible surrogate tissue, including blood derivatives such as serum and plasma. A recent 2010 clinical study proposes alpha 1B-glycoprotein precursor/A1BG, alpha 2-antiplasmin, apolipoprotein A-IV precursor/APOA4, and complement component 3 precursor, among other coagulation and complement system proteins as corollaries of lung function decline, although ambiguity between cause and effect is unresolved.[3]
 |
Chronic Bronchitis
Pathogenesis
- Hallmark features include: hyperplasia (increased number) and hypertrophy (increased size) of the goblet cells (mucous gland) of the airway, resulting in an increase in secretion of mucus, which contributes to the airway obstruction.[5]
- Narrowing of the airways reduces the rate at which air can flow to and from the air sacs (alveoli) and limits the effectiveness of the lungs.
Microscopy
- On microscopic histopathological analysis, there is infiltration of the airway walls with inflammatory cells, particularly CD8+ T-lymphocytes and neutrophils.[6] Inflammation is followed by scarring and remodeling that thickens the walls resulting in narrowing of the small airways.
Emphysema
- Emphysema is the destruction of pulmonary acinus. Acinus is the structures distal to the terminal bronchiole, consisting of the respiratory bronchiole, alveolar ducts, alveolar sacs, and alveoli.
Gross Pathology
Microscopic Pathology
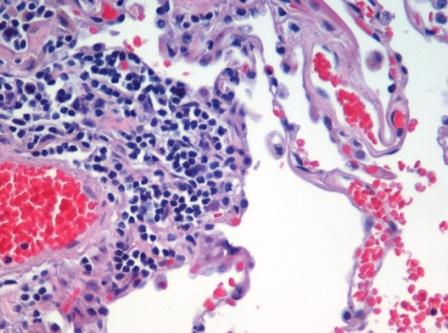 |
Acute Exacerbations of COPD
An acute exacerbation of COPD is a sudden worsening of COPD symptoms (shortness of breath, quantity and color of phlegm) that typically lasts for several days. Airway inflammation is increased during the exacerbation, resulting in increased hyperinflation, reduced expiratory air flow and worsening of gas transfer. This can also lead to hypoventilation and eventually hypoxia, insufficient tissue perfusion, and then cell necrosis.
References
- ↑ 1.0 1.1 Calverley PM, Koulouris NG (2005). "Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology". Eur Respir J. 25 (1): 186–199. doi:10.1183/09031936.04.00113204. PMID 15640341.
- ↑ O'Donnell DE (2006). "Hyperinflation, Dyspnea, and Exercise Intolerance in Chronic Obstructive Pulmonary Disease". The Proceedings of the American Thoracic Society. 3 (2): 180–4. doi:10.1513/pats.200508-093DO. PMID 16565429.
- ↑ Rana GS, York TP, Edmiston JS, Zedler BK; et al. (2010). "Proteomic biomarkers in plasma that differentiate rapid and slow decline in lung function in adult cigarette smokers with chronic obstructive pulmonary disease (COPD)". Anal Bioanal Chem. 397 (5): 1809–19. doi:10.1007/s00216-010-3742-4. PMID 20442989.
- ↑ "File:Copd 2010.jpg - Wikimedia Commons".
- ↑ Hogg JC (2004). "Pathophysiology of airflow limitation in chronic obstructive pulmonary disease". Lancet. 364 (9435): 709–21. doi:10.1016/S0140-6736(04)16900-6. PMID 15325838.
- ↑ Baraldo S, Turato G, Badin C, Bazzan E, Beghé B, Zuin R, Calabrese F, Casoni G, Maestrelli P, Papi A, Fabbri LM, Saetta M (2004). "Neutrophilic infiltration within the airway smooth muscle in patients with COPD". Thorax. 59 (4): 308–12. PMC 1763819. PMID 15047950.
