Choline Magnesium Trisalicylate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Choline Magnesium Trisalicylate is an analgesic that is FDA approved for the treatment of osteoarthritis, rheumatoid arthritis and acute painful shoulder, analgesic and antipyretic action. Common adverse reactions include pruritis, rash, constipation, diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indicaions
Osteoarthritis, Rheumatoid Arthritis and Acute Painful Shoulder
- Salicylates are considered the base therapy of choice in the arthritides; and choline magnesium trisalicylate preparation is indicated for the relief of the signs and symptoms of rheumatoid arthritis, osteoarthritis and other arthritides. Choline Magnesium Trisalicylate Liquid is indicated in the long-term management of these diseases and especially in the acute flare of rheumatoid arthritis. Choline Magnesium Trisalicylate Liquid is also indicated for the treatment of acute painful shoulder.
- Choline Magnesium Trisalicylate Liquid is effective and generally well tolerated, and is logical choice whenever salicylate treatment is indicated. It is particularly suitable when a once-a-day or b.i.d. dosage regimen is important to patient compliance; when gastrointestinal intolerance to aspirin is encountered; when gastrointestinal microbleeding or hematologic effects of aspirin are considered a patient hazard; and when interference (or the risk of interference) with normal platelet function by aspirin or by propionic acid derivatives is considered to be clinically undesirable. Use of Choline Magnesium Trisalicylate Liquid is appropriate when a liquid dosage form is preferred, as in the elderly patient.
- The efficacy of Choline Magnesium Trisalicylate Liquid has not been studied in those patients who are designated by the American Rheumatism Association as belonging in
- Functional Class IV (incapacitated, largely or wholly bedridden or confined to a wheelchair, with little or no self-care).
Analgesic and Antipyretic Action
- Choline Magnesium Trisalicylate Liquid is also indicated for the relief of mild to moderate pain and for antipyresis.
Dosage
Adults
- In rheumatoid arthritis, osteoarthritis, the more severe arthritides, and acute painful shoulder, the recommended starting dosage is 1500 mg given b.i.d. Some patients may be treated with 3000 mg given once per day (h.s.). Dosage should be adjusted in accordance with the patient's response. In patients with renal dysfunction, monitor salicylate levels and adjust dose accordingly.
Elderly
- In the elderly patient, a daily dosage of 2250 mg given as 750 mg t.i.d. may be efficacious and well tolerated. Dosage should be adjusted in accordance with the patient's response. In patients with renal dysfunction, monitor salicylate levels and adjust dose accordingly.
- For mild to moderate pain or for antipyresis, the usual dosage is 2000 mg to 3000 mg daily in divided doses (b.i.d.). Based on patient response or salicylate blood levels, dosage may be adjusted to achieve optimum therapeutic effect. Salicylate blood levels should be in the range of 15 to 30 mg/100 mL for anti-inflammatory effect and 5 to 15 mg/100 mL for analgesia and antipyresis.
- If the physician prefers, the recommended daily dosage may be administered on a t.i.d. schedule.
- As with other therapeutic agents, individual dosage adjustment is advisable, and a number of patients may require higher or lower dosages than those recommended. Certain patients require 2 to 3 weeks of therapy for optimal effect.
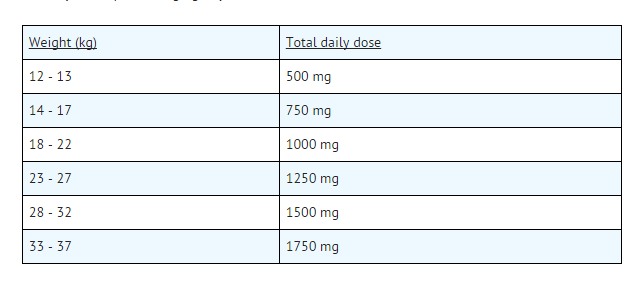
- Total daily doses should be administered in divided doses (b.i.d.). The dose of Choline Magnesium Trisalicylate Liquid is calculated as the total daily dose of 50 mg/kg/day for children of 37 kg body weight or less and 2250 mg/day for heavier children.
- Choline Magnesium Trisalicylate Liquid is available for greater convenience in treating younger patients and those adult patients unable to swallow a solid dosage form. Choline Magnesium Trisalicylate Liquid may be mixed with fruit juices just before ingestion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Choline Magnesium Trisalicylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Choline Magnesium Trisalicylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Pediatric Use
- In children, Choline Magnesium Trisalicylate Liquid is indicated for conditions requiring anti-inflammatory or analgesic action-such as juvenile rheumatoid arthritis and other appropriate conditions.
Children
- Usual daily dose for children for anti-inflammatory or analgesic action: Choline Magnesium Trisalicylate Liquid, 50 mg/kg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Choline Magnesium Trisalicylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Choline Magnesium Trisalicylate in pediatric patients.
Contraindications
- Patients who are hypersensitive to non-acetylated salicylates should not take Choline Magnesium Trisalicylate Liquid.
Warnings
- Reye Syndrome is a rare but serious disease which may develop in children and teenagers who have chicken pox, influenza, or flu symptoms. While the cause of Reye Syndrome is unknown, some studies suggest a possible association between the development of Reye Syndrome and the use of medicines containing acetylated salicylates or aspirin. Choline Magnesium Trisalicylate Liquid is a combination of choline salicylate and magnesium salicylate which are nonacetylated salicylates, and there have been no reported cases associating Choline Magnesium Trisalicylate Liquid with Reye Syndrome. Nevertheless, Choline Magnesium Trisalicylate Liquid, as a salicylate-containing product, is not recommended for use in children and teenagers with chicken pox, influenza or flu symptoms.
- The FDA has determined that routine heavy alcohol use (three or more alcoholic drinks every day), in combination with analgesic/antipyretic drug products containing NSAID ingredients (including choline and magnesium salicylates), increases the risk of adverse GI events, including stomach bleeding.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Choline Magnesium Trisalicylate in the drug label.
Postmarketing Experience
- The most frequent adverse reactions observed with Choline Magnesium Trisalicylate Liquid in clinical trials7-12 are tinnitus and gastrointestinal complaints (including nausea, vomiting, gastric upset, indigestion, heartburn, diarrhea, constipation and epigastric pain). These occur in less than twenty percent (20%) of patients. Should tinnitus develop, reduction of daily dosage is recommended until the tinnitus is resolved. Less frequent adverse reactions, occurring in less than two percent (2%) of patients, are: hearing impairment, headache, lightheadedness, dizziness, drowsiness, and lethargy. Adverse reactions occurring in less than one percent (1%) of patients, are: gastric ulceration, positive fecal occult blood, elevation in serum BUN and creatinine, rash, pruritus, anorexia, weight gain, edema, epistaxis and dysgeusia.
- Spontaneous reporting has yielded isolated or rare reports of the following adverse experiences: duodenal ulceration, elevated hepatic transaminases, hepatitis, esophagitis,
- Asthma, erythema multiforme, urticaria, ecchymoses, irreversible hearing loss and/or tinnitus, mental confusion and hallucinations.
Drug Interactions
There is limited information regarding Choline Magnesium Trisalicylate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Choline Magnesium Trisalicylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Choline Magnesium Trisalicylate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Choline Magnesium Trisalicylate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Choline Magnesium Trisalicylate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Choline Magnesium Trisalicylate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Choline Magnesium Trisalicylate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Choline Magnesium Trisalicylate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Choline Magnesium Trisalicylate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Choline Magnesium Trisalicylate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Choline Magnesium Trisalicylate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Choline Magnesium Trisalicylate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Choline Magnesium Trisalicylate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Choline Magnesium Trisalicylate in the drug label.
Overdosage
- Death in adults has been reported following ingestion of doses from 10 to 30 grams of salicylate; however, larger doses have been taken without resulting fatality.
- Symptoms: Salicylate intoxication, known as salicylism, may occur with large doses or extended therapy. Common symptoms of salicylism include headache, dizziness, tinnitus, hearing impairment, confusion, drowsiness, sweating, vomiting, diarrhea, and hyperventilation. A more severe degree of salicylate intoxication can lead to CNS disturbances, alteration in electrolyte balance, respiratory and metabolic acidosis, hyperthermia, and dehydration.
- Treatment: Reduction of further absorption of salicylate from the gastrointestinal tract can be achieved via emesis, gastric lavage, use of activated charcoal, or a combination of the above. Appropriate I.V. fluids should be administered to correct dehydration, electrolyte imbalance, and acidosis and to maintain adequate renal function. To accelerate salicylate excretion, forced diuresis with alkalinizing solution is recommended. In extreme cases, peritoneal dialysis or hemodialysis should be considered for effective salicylate removal.
Pharmacology
There is limited information regarding Choline Magnesium Trisalicylate Pharmacology in the drug label.
Mechanism of Action
- Choline Magnesium Trisalicylate Liquid contains salicylate with anti-inflammatory, analgesic and antipyretic action. On ingestion of Choline Magnesium Trisalicylate Liquid, the salicylate moiety is absorbed rapidly and reaches peak blood levels within an average of one to two hours after single dose of the liquid.
- The primary route of excretion is renal: the excretion products are chiefly the glycine and glucuronide conjugates. At higher serum salicylate concentrations, the glycine conjugation pathway becomes rapidly saturated. Thus, the slower glucuronide conjugation pathway becomes the rate limiting step for salicylate excretion. In addition, salicylate excreted in the bile as glucuronide conjugate may be reabsorbed. * These factors account for the prolongation of salicylate half-life and the nonlinear increase in plasma salicylate level as the salicylate dose is increased.
- The serum concentration of salicylate is increased by conditions that decrease glomerular filtration rate or proximal tubular secretion.
- Unlike aspirin and certain other non-steroidal anti-inflammatory agents, such as arylpropionic acid derivatives and arylacetic acid derivatives, choline magnesium trisalicylate at therapeutic dosage levels does not affect platelet aggregation, as shown by in-vitro and in-vivo studies.
Structure
- Choline Magnesium Trisalicylate Liquid is a nonsteroidal, anti-inflammatory preparation containing choline magnesium trisalicylate which is freely soluble in water. The absolute structure of choline magnesium trisalicylate is not known at this time. Choline magnesium trisalicylate has a molecular formula of C26H29O10NMg, a molecular weight of 539.8, and it may be represented in the solid form as:
- Choline Magnesium Trisalicylate Liquid is a cherry-flavored liquid providing 500 mg salicylate content per teaspoonful (5 mL) for oral administration.
- Inactive Ingredients: Each teaspoonful (5 mL) of Choline Magnesium Trisalicylate Liquid contains: caramel, carboxymethylcellulose sodium, edetate disodium, FD&C yellow no. 6, flavor, glycerin, methylparaben, potassium sorbate, sodium citrate, sorbic acid, sorbitol, and water.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Choline Magnesium Trisalicylate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Choline Magnesium Trisalicylate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Choline Magnesium Trisalicylate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Choline Magnesium Trisalicylate in the drug label.
How Supplied
- Choline Magnesium Trisalicylate Liquid is supplied in bottles of 240 mL.
Storage
- Store at controlled room temperature 15° to 30°C (59° to 86°F).
Images
Drug Images
{{#ask: Page Name::Choline Magnesium Trisalicylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Choline Magnesium Trisalicylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Choline Magnesium Trisalicylate in the drug label.
Precautions with Alcohol
- Alcohol-Choline Magnesium Trisalicylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CHOLINE MAGNESIUM TRISALICYLATE ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "CHOLINE MAGNESIUM TRISALICYLATE - choline magnesium trisalicylate liquid".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Choline Magnesium Trisalicylate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Choline Magnesium Trisalicylate |Label Name=Choline Magnesium Trisalicylate11.png
}}
{{#subobject:
|Label Page=Choline Magnesium Trisalicylate |Label Name=Choline Magnesium Trisalicylate11.png
}}