Tetralogy of Fallot pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| (37 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Tetralogy of fallot}} | {{Tetralogy of fallot}} | ||
{{CMG}}; '''Associate Editors-In-Chief:''' [[Priyamvada Singh| Priyamvada Singh, M.B.B.S.]] [mailto:psingh13579@gmail.com], [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu]; [[User:Omar Toubat|Omar Toubat]]; '''Assistant Editor-In-Chief:''' [[Kristin Feeney|Kristin Feeney, B.S.]] [mailto:kfeeney@elon.edu] | {{CMG}}; '''Associate Editors-In-Chief:''' {{Fs}}, [[Priyamvada Singh| Priyamvada Singh, M.B.B.S.]] [mailto:psingh13579@gmail.com], [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu]; [[User:Omar Toubat|Omar Toubat]]; '''Assistant Editor-In-Chief:''' [[Kristin Feeney|Kristin Feeney, B.S.]] [mailto:kfeeney@elon.edu] | ||
==Overview== | ==Overview== | ||
Tetralogy of Fallot is a congenital heart lesion characterized by a constellation of four morphologic abnormalities present in the newborn heart. These features include a ventricular septal defect, overriding aorta, pulmonary stenosis, and right ventricular hypertrophy. The obstruction of right ventricular outflow in tetralogy of Fallot causes [[blood]] to [[shunt]] or flow from the right to left side of heart through the [[ventricular septal defect]]. This causes [[right ventricular hypertrophy]] and eventual [[right sided heart failure]]. There is flow of deoxygenated venous blood from the right side of the [[heart]] to the [[systemic circulation]] resulting in [[cyanosis]]. | Tetralogy of Fallot is a [[Congenital heart defect|congenital heart lesion]] characterized by a constellation of four [[Morphology|morphologic]] [[abnormalities]] present in the [[newborn]] [[heart]]. It is understood that tetralogy of fallot is the result of improper positioning of the outlet [[septum]]. In the normal [[heart]], the outlet septum is an indistinguishable component of the crista supraventricularis that communicates with the [[Septomarginal trabecula|septomarginal trabeculae]] to divide the [[Right ventricle|right]] and [[Left ventricle|left]] [[Ventricle|ventricular cavities]]. In Tetralogy of Fallot, proper [[Ventricle (heart)|ventricular]] septation is perturbed by anterocephalad displacement of the outlet [[Septum (disambiguation)|septum]] relative to the [[septomarginal trabecula]]. These features include a ventricular septal defect, overriding aorta, pulmonary stenosis, and right ventricular hypertrophy. The obstruction of right ventricular outflow in tetralogy of Fallot causes [[blood]] to [[shunt]] or flow from the [[Right-to-left shunt|right to left]] side of heart through the [[ventricular septal defect]]. This causes [[right ventricular hypertrophy]] and eventual [[right sided heart failure]]. There is [[Blood flow|flow]] of deoxygenated [[venous blood]] from the right side of the [[heart]] to the [[systemic circulation]] resulting in [[cyanosis]]. | ||
==Pathophysiology== | ==Pathophysiology== | ||
=== | === Physiology === | ||
[[ | The normal [[physiology]] of [[heart]] [[development]] can be understood as follows: | ||
* Subsequent to the lateral folding and looping events that give rise to the [[primitive heart tube]] are a series of complex septation processes that will ultimately complete [[morphogenesis]] of the four [[Heart chamber|chambered]] [[heart]]. | |||
[[ | * The goals of cardiac septation are two-fold: | ||
** To create four distinct [[cardiac chambers]] | |||
** To correctly position the [[great vessels]] relative to these [[Heart chamber|chambers]] | |||
[[File:2037 Embryonic Development of Heart.jpg|500px|none|thumb|https://en.wikipedia.org/wiki/File:2037_Embryonic_Development_of_Heart.jpg]]<br /> | |||
=== | === Pathogenesis === | ||
{{ | * It is understood that tetralogy of fallot is the result of improper positioning of the outlet [[septum]].<ref name="pmid19094375">{{cite journal| author=Anderson RH, Jacobs ML| title=The anatomy of tetralogy of Fallot with pulmonary stenosis. | journal=Cardiol Young | year= 2008 | volume= 18 Suppl 3 | issue= | pages= 12-21 | pmid=19094375 | doi=10.1017/S1047951108003259 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19094375 }} </ref><ref name="pmid17420363">{{cite journal| author=Bashore TM| title=Adult congenital heart disease: right ventricular outflow tract lesions. | journal=Circulation | year= 2007 | volume= 115 | issue= 14 | pages= 1933-47 | pmid=17420363 | doi=10.1161/CIRCULATIONAHA.105.592345 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17420363 }} </ref><ref name="pmid19144126">{{cite journal| author=Bailliard F, Anderson RH| title=Tetralogy of Fallot. | journal=Orphanet J Rare Dis | year= 2009 | volume= 4 | issue= | pages= 2 | pmid=19144126 | doi=10.1186/1750-1172-4-2 | pmc=PMC2651859 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19144126 }} </ref> | ||
* In the normal [[heart]], the outlet septum is an indistinguishable component of the crista supraventricularis that communicates with the [[Septomarginal trabecula|septomarginal trabeculae]] to divide the [[Right ventricle|right]] and [[Left ventricle|left]] [[Ventricle|ventricular cavities]]. | |||
* In Tetralogy of Fallot, proper [[Ventricle (heart)|ventricular]] septation is perturbed by anterocephalad displacement of the outlet [[Septum (disambiguation)|septum]] relative to the [[septomarginal trabecula]]. | |||
* The direct consequence of this misalignment is an [[Overriding aorta|overriding aortic orifice]] and a [[ventricular septal defect]], resulting in an intracardiac [[Right-to-left shunt|right to left shunt]] of blood. | |||
* In addition, anterocephalad displacement of the outlet septum indirectly predisposes the [[pulmonary trunk]] to [[Pulmonary stenosis|stenosis]] in the setting of septoparietal trabecular [[hypertrophy]]. | |||
* Together, the displacement of the outlet [[septum]] coupled with the [[Hypertrophy (medical)|hypertrophic]] arrangement of the septoparietal trabeculae account for the three [[Anatomy|anatomical]] cardinal [[Defect|defects]] in Tetralogy of Fallot - [[Aorta|aortic]] dextroposition, [[Ventricular septal defect|interventricular communication]] ([[VSD]]), and [[pulmonary stenosis]]. | |||
* The fourth [[defect]] - [[right ventricular hypertrophy]] - is a [[hemodynamic]] consequence of these three [[Morphology|morphologic]] changes, as the [[right ventricle]] physiologically adapts to the increased [[resistance]] of a [[Pulmonary stenosis|stenotic pulmonary trunk]]. | |||
* | |||
[[File:1024px-Tetralogy of Fallot.svg.png|500px|none|thumb|Image by Mariana Ruiz LadyofHats| https://en.wikipedia.org/wiki/File:Tetralogy_of_Fallot.svg]]<br /> | |||
==== Anatomy ==== | |||
=== | {| | ||
| | |||
[[Image:Heart tetralogy fallot.svg.png|300px|thumb|right|Image by Wapcaplet| https://en.wikipedia.org/wiki/File:Heart_tetralogy_fallot.svg]] | |||
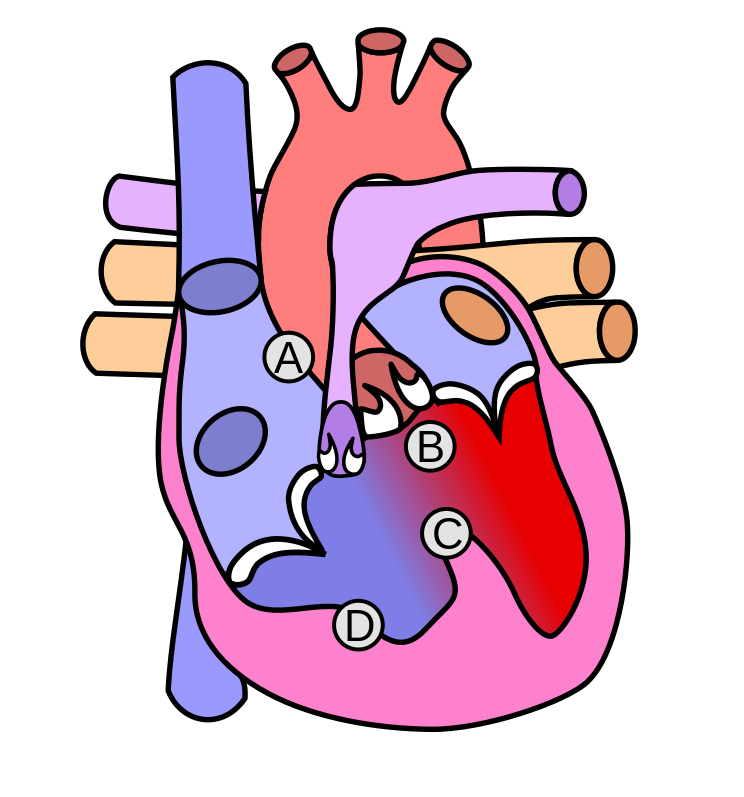
* This defect in outlet septum in turn leads to the four characteristic features: | |||
* A: Pulmonic Stenosis | |||
**[[Pulmonic stenosis]] is a right ventricular outflow tract obstruction | |||
*** A narrowing at ([[valvular stenosis]], seen in approximately 20-25% case) or just below (infundibular stenosis, seen in around 50% of cases) the [[heart|pulmonary valve]]. | |||
*** The stenosis is mostly the result of [[hypertrophy]] of the septoparietal trabeculae,<ref name="gatzoulis" /> however the deviated outlet septum is believed to play a role. | |||
*** The stenosis is the major cause of the malformations, with the other associated malformations acting as compensatory mechanisms to the [[pulmonic stenosis]].<ref>{{cite journal |author=Bartelings M, Gittenberger-de Groot A |title=Morphogenetic considerations on congenital malformations of the outflow tract. Part 1: Common arterial trunk and tetralogy of Fallot |journal=Int. J. Cardiol. |volume=32 |issue=2 |pages=213-30 |year=1991 |pmid=1917172}}</ref> | |||
*** The degree of stenosis varies between individuals with TOF, and is the primary determinant of symptoms and severity. This malformation is infrequently described as sub-pulmonary stenosis or subpulmonary obstruction.<ref>Anderson RH, Weinberg. The clinical anatomy of tetralogy of Fallot. Cardiol Young. 2005 15;38-47. PMID 15934690.</ref> | |||
***Tetralogy of Fallot with [[pulmonary atresia]] or pseudotruncus arteriosus is a severe variant in which there is complete obstruction of the right ventricular outflow tract and absence of the [[pulmonary trunk]]. In these individuals, there is complete [[right-to-left shunt]]ing of [[blood]]. The [[lung]]s are perfused via extensive collaterals from the systemic arteries. | |||
*B: Overriding Aorta | |||
**[[Overriding aorta]] is defined as when the [[aortic valve]] is not restricted to the [[left ventricle]], thus having biventricular connections. The aortic root can be moved anteriorly or override the septal defect, but it is still to the right of the root of the [[pulmonary artery]]. The degree of override is quite variable, with 5-95% of the valve being connected to the [[right ventricle]].<ref name="gatzoulis" /> | |||
*C: Ventricular Septal Defect | |||
**[[Ventricular septal defect]] is a hole between the two bottom chambers ([[ventricle]]s) of the [[heart]]. The defect is centered around the outlet septum, the most superior aspect of the [[septum]], and in the majority of cases is single and large. In some cases septal [[hypertrophy]] can narrow the margins of the defect.<ref name="gatzoulis">Gatzoulis MA, Webb GD, Daubeney PE. (2005) ''Diagnosis and Management of Adult Congenital Heart Disease''. Churchill Livingstone, Philadelphia. ISBN 0443071039.</ref> | |||
*D: Right Ventricular Hypertrophy | |||
**The [[right ventricle]] is more muscular than normal, causing a characteristic coeur-en-sabot (boot-shaped) appearance as seen by [[chest x-ray]]. Due to the misarrangement of the external ventricular septum, the right ventricular wall increase in size to deal with the increased obstruction to the right outflow tract. This feature is now generally agreed to be a secondary anomaly, as the level of hypertrophy generally increases with age.<ref>Anderson RH, Tynan M. Tetralogy of Fallot – a centennial review. Int J Cardiol. 1988 21; 219-232. PMID 3068155.</ref> | |||
# | * There is anatomic variation between the hearts of individuals with tetralogy of Fallot. | ||
*The degree of right ventricular outflow tract obstruction varies between patients and is generally determines clinical symptoms and disease progression. | |||
{{#ev:youtube|ACRfFkxow7w}} | |||
| | |||
|} | |||
====Embrylogy==== | |||
*[[Embryology|Embryologic]] studies show that tetralogy of Fallot is a result of anterior misalignment of the conal septum, resulting in the clinical combination of a [[ventricular septal defect]] ([[VSD]]), [[pulmonary stenosis]], and an [[overriding aorta]]. | |||
* [[Right ventricular hypertrophy]] is secondary to this combination of [[abnormalities]], which causes resistance to [[blood flow]] from the [[right ventricle]]. | |||
* Tetralogy of Fallot results in [[cyanosis]], ([[hypoxia]] or [[low oxygenation]]) of the blood due to mixing of [[Venous blood|deoxygenated venous blood]] from the [[right ventricle]] with [[oxygenated blood]] in the [[left ventricle]] through the [[ventricular septal defect]] and preferential flow of both [[Oxygenated blood|oxygenated]] and deoxygenated [[blood]] from the [[Ventricle (heart)|ventricles]] through the [[aorta]] because of obstruction to flow through the [[pulmonary valve]]. | |||
*This is known as a [[right-to-left shunt]]. | |||
*If [[Pulmonary valve|pulmonary]] [[blood flow]] is dramatically reduced, [[Pulmonary valve|pulmonary]] [[blood flow]] may depend on a [[patent ductus arteriosus]] ([[PDA]]) or [[bronchial]] collaterals. | |||
* If obstruction of the [[right ventricular outflow tract]] is minimal, the intracardiac [[Shunt (medical)|shunt]] may be mostly from left to right, and this may result in what is termed a pink tet or pink tetralogy. | |||
*Although [[cyanosis]] is absent on [[Clinical examination|clinical exam]], [[laboratory]] testing will often show [[systemic]] [[oxygen]] [[desaturation]]. | |||
*[[Children]] with tetralogy of Fallot may develop [[Acute (medicine)|acute]] severe [[cyanosis]] or [[hypoxic]] [[tet spells]]. | |||
*The mechanism underlying these episodes is not entirely clear, but may be due to [[spasm]] of the infundibular septum and the [[right ventricular outflow tract]]. | |||
*Whatever the mechanism, there is an increase in [[resistance]] to [[blood flow]] to the [[lung]]s with increased preferential flow of desaturated [[blood]] to the [[systemic circulation]]. | |||
*The [[child]] will often squat during a [[tet spell]] to improve [[venous return]] to the right side of the [[heart]]. | |||
*Squatting increases the [[systemic vascular resistance]] and thereby [[shunt]]s flow to [[Pulmonary circulation|pulmonary circuit]]. | |||
*These spells can be [[fatal]], and can occur in [[patients]] who are not [[cyanotic]]. | |||
==Genetics== | |||
=== | *[[Genes]] involved in the [[pathogenesis]] of tetralogy of fallot include:<ref name="pmid17008524">{{cite journal| author=Olson EN| title=Gene regulatory networks in the evolution and development of the heart. | journal=Science | year= 2006 | volume= 313 | issue= 5795 | pages= 1922-7 | pmid=17008524 | doi=10.1126/science.1132292 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17008524 }} </ref><ref name="pmid24000169">{{cite journal| author=Yang YQ, Gharibeh L, Li RG, Xin YF, Wang J, Liu ZM et al.| title=GATA4 loss-of-function mutations underlie familial tetralogy of fallot. | journal=Hum Mutat | year= 2013 | volume= 34 | issue= 12 | pages= 1662-71 | pmid=24000169 | doi=10.1002/humu.22434 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24000169 }} </ref><ref name="pmid18288184">{{cite journal| author=Bruneau BG| title=The developmental genetics of congenital heart disease. | journal=Nature | year= 2008 | volume= 451 | issue= 7181 | pages= 943-8 | pmid=18288184 | doi=10.1038/nature06801 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18288184 }} </ref><ref name="pmid24526674">{{cite journal| author=Bruneau BG, Srivastava D| title=Congenital heart disease: entering a new era of human genetics. | journal=Circ Res | year= 2014 | volume= 114 | issue= 4 | pages= 598-9 | pmid=24526674 | doi=10.1161/CIRCRESAHA.113.303060 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24526674 }} </ref><ref name="pmid11431700">{{cite journal| author=Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R et al.| title=Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. | journal=Nat Genet | year= 2001 | volume= 28 | issue= 3 | pages= 276-80 | pmid=11431700 | doi=10.1038/90123 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11431700 }} </ref><ref name="pmid12845333">{{cite journal| author=Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA et al.| title=GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. | journal=Nature | year= 2003 | volume= 424 | issue= 6947 | pages= 443-7 | pmid=12845333 | doi=10.1038/nature01827 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12845333 }} </ref><ref name="pmid24182332">{{cite journal| author=Sheng W, Qian Y, Wang H, Ma X, Zhang P, Diao L et al.| title=DNA methylation status of NKX2-5, GATA4 and HAND1 in patients with tetralogy of fallot. | journal=BMC Med Genomics | year= 2013 | volume= 6 | issue= | pages= 46 | pmid=24182332 | doi=10.1186/1755-8794-6-46 | pmc=PMC3819647 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24182332 }} </ref> | ||
** The [[cellular]] processes that underlie cardiogenesis are extensively regulated in the developing [[heart]]. | |||
** Proper [[Heart|cardiac]] development requires the complex orchestration of [[cardiac]] [[Transcription factor|transcription factors]] and [[Signaling pathway|signaling pathways]] in a spatiotemporal specific manner. | |||
** Previous [[Genetics|genetic]] studies demonstrated that [[mutations]] in numerous [[genes]] encoding [[Heart|cardiac]] [[transcription factors]] and [[cell]] [[Signaling protein|signaling proteins]] have a role in the [[development]] of Tetralogy of Fallot. | |||
** Specifically, [[Heterozygous|heterozygous mutations]] in [[NKX2-5]], [[HAND1]], [[TBX5 (gene)|TBX5]], and [[GATA4]] have been reported in familial forms of [[disease]]. | |||
** Many of these single [[Gene mutation|gene mutations]] result in [[haploinsufficiency]] and suggest a dose dependent relationship between [[genetic]] expression and [[disease]]. | |||
** While the mechanistic basis of this relationship is currently poorly understood, it is hypothesized that disruption of the direct [[protein]]-[[protein]] interactions that allow these [[transcription factors]] to work synergistically impedes the activation of downstream targets and [[signaling pathway]]<nowiki/>s central to [[cardiac]] morphogenesis. | |||
** In addition, recent whole-exome sequencing investigations have introduced a novel role for [[Epigenetics|epigenetic]] dysregulation in the [[pathogenesis]] of Tetralogy of Fallot. | |||
** Aberrant [[epigenetic]] modifications are thought to provide an alternative mechanism to perturb normal spatiotemporal expression of these essential [[developmental]] [[genes]]. | |||
==Associated Conditions== | |||
* Conditions associated with tetralogy of fallot include:<ref name="pmid7349946">{{cite journal |vauthors=Dabizzi RP, Caprioli G, Aiazzi L, Castelli C, Baldrighi G, Parenzan L, Baldrighi V |title=Distribution and anomalies of coronary arteries in tetralogy of fallot |journal=Circulation |volume=61 |issue=1 |pages=95–102 |date=January 1980 |pmid=7349946 |doi=10.1161/01.cir.61.1.95 |url=}}</ref><ref name="Satyanarayana RaoAnderson1971">{{cite journal|last1=Satyanarayana Rao|first1=B.N.|last2=Anderson|first2=Ray C.|last3=Edwards|first3=Jesse E.|title=Anatomic variations in the tetralogy of Fallot|journal=American Heart Journal|volume=81|issue=3|year=1971|pages=361–371|issn=00028703|doi=10.1016/0002-8703(71)90106-2}}</ref><ref name="MusterPaul1973">{{cite journal|last1=Muster|first1=Alexander J.|last2=Paul|first2=Milton H.|last3=Nikaidoh|first3=Hisashi|title=Tetralogy of Fallot Associated with Total Anomalous Pulmonary Venous Drainage|journal=Chest|volume=64|issue=3|year=1973|pages=323–326|issn=00123692|doi=10.1378/chest.64.3.323}}</ref><ref name="SaifiMatsumoto2012">{{cite journal|last1=Saifi|first1=Comron|last2=Matsumoto|first2=Hiroko|last3=Vitale|first3=Michael G.|last4=Roye|first4=David P.|last5=Hyman|first5=Joshua E.|title=The incidence of congenital scoliosis in infants with tetralogy of Fallot based on chest radiographs|journal=Journal of Pediatric Orthopaedics B|volume=21|issue=4|year=2012|pages=313–316|issn=1060-152X|doi=10.1097/BPB.0b013e3283536872}}</ref> | |||
**Left [[superior vena cava]] | |||
**[[Anomaly|Anomalies]] of the [[mitral valve]] | |||
**[[Anomaly|Anomalies]] of the [[tricuspid valve]] | |||
**[[pulmonic stenosis|Stenosis of the left pulmonary artery]], in 40% of [[Patient|patients]] | |||
** A bicuspid [[Pulmonic regurgitation|pulmonary valve]], in 40% of [[patients]] | |||
** Right sided [[aortic arch]], in 25% of [[patients]] | |||
**[[Coronary artery anomalies]], in 10% of [[patients]] | |||
** An [[atrial septal defect]], in which case the [[DRESS syndrome|syndrome]] is sometimes called a [[pentalogy of Fallot]]. | |||
** An [[atrioventricular septal defect]] | |||
** Partially or totally [[anomalous pulmonary venous return]] | |||
** Forked ribs and [[scoliosis]] | |||
**Associated [[abnormalities]] include [[cleft lip]], [[cleft palate]], [[hypospadias]], [[Skeleton|skeletal]] and [[craniofacial]] [[abnormalities]]. | |||
=== | ==Gross Pathology== | ||
* On [[gross pathology]] characteristic features findings of tetralogy of fallot include: | |||
**[[Right ventricular hypertrophy]] | |||
**[[VSD]] | |||
**[[Overriding aorta]] | |||
**Subpulmonic stenosis | |||
** | |||
== | [[File:PLC Fallots tetralogy.jpg|300px|none|thumb|https:// by Jurgennizer| https://en.wikipedia.org/wiki/File:PLC_Fallots_tetralogy.jpg]]<br /> | ||
== Microscopic Pathology == | |||
There is no characteristic findings of tetralogy of fallot on microscopic [[histopathological]] analysis. | |||
==References== | ==References== | ||
Latest revision as of 01:40, 23 February 2020
|
Tetralogy of fallot Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
|
|
Tetralogy of Fallot pathophysiology On the Web |
|
American Roentgen Ray Society Images of Tetralogy of Fallot pathophysiology |
|
Risk calculators and risk factors for Tetralogy of Fallot pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editors-In-Chief: Fahimeh Shojaei, M.D., Priyamvada Singh, M.B.B.S. [2], Keri Shafer, M.D. [3]; Omar Toubat; Assistant Editor-In-Chief: Kristin Feeney, B.S. [4]
Overview
Tetralogy of Fallot is a congenital heart lesion characterized by a constellation of four morphologic abnormalities present in the newborn heart. It is understood that tetralogy of fallot is the result of improper positioning of the outlet septum. In the normal heart, the outlet septum is an indistinguishable component of the crista supraventricularis that communicates with the septomarginal trabeculae to divide the right and left ventricular cavities. In Tetralogy of Fallot, proper ventricular septation is perturbed by anterocephalad displacement of the outlet septum relative to the septomarginal trabecula. These features include a ventricular septal defect, overriding aorta, pulmonary stenosis, and right ventricular hypertrophy. The obstruction of right ventricular outflow in tetralogy of Fallot causes blood to shunt or flow from the right to left side of heart through the ventricular septal defect. This causes right ventricular hypertrophy and eventual right sided heart failure. There is flow of deoxygenated venous blood from the right side of the heart to the systemic circulation resulting in cyanosis.
Pathophysiology
Physiology
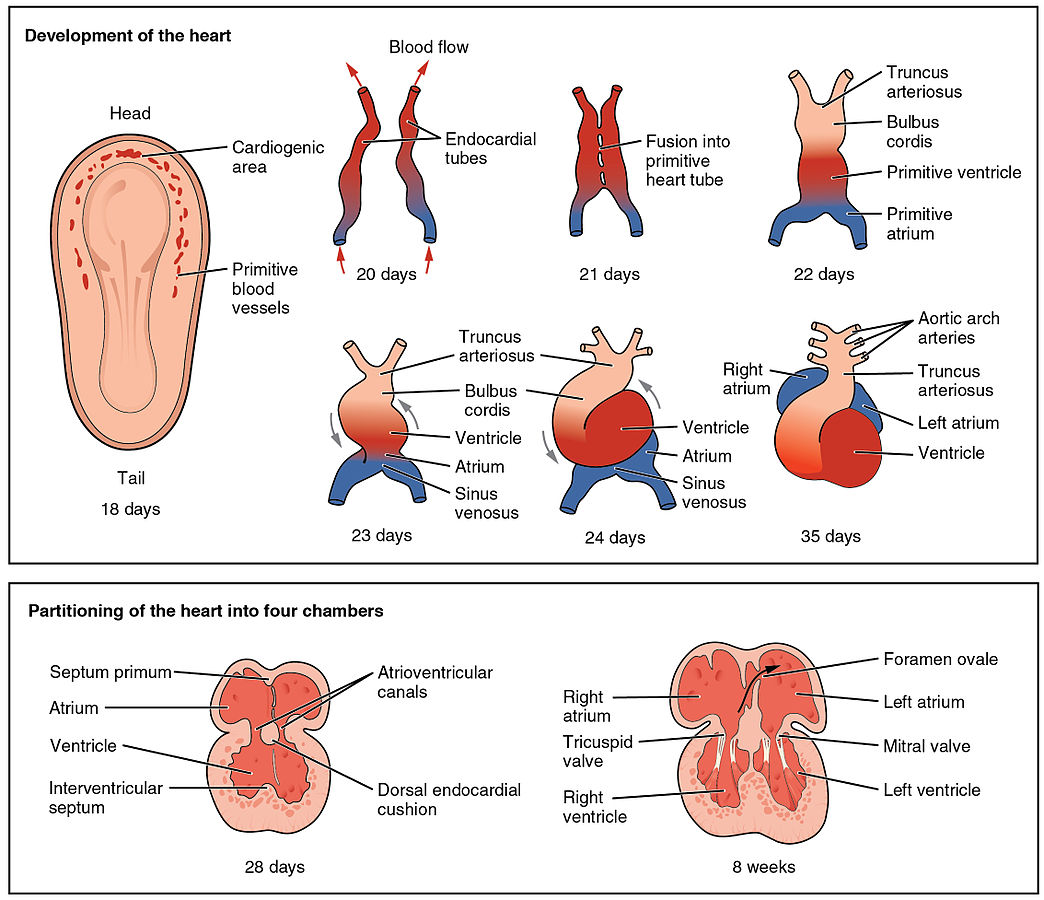
The normal physiology of heart development can be understood as follows:
- Subsequent to the lateral folding and looping events that give rise to the primitive heart tube are a series of complex septation processes that will ultimately complete morphogenesis of the four chambered heart.
- The goals of cardiac septation are two-fold:
- To create four distinct cardiac chambers
- To correctly position the great vessels relative to these chambers
Pathogenesis
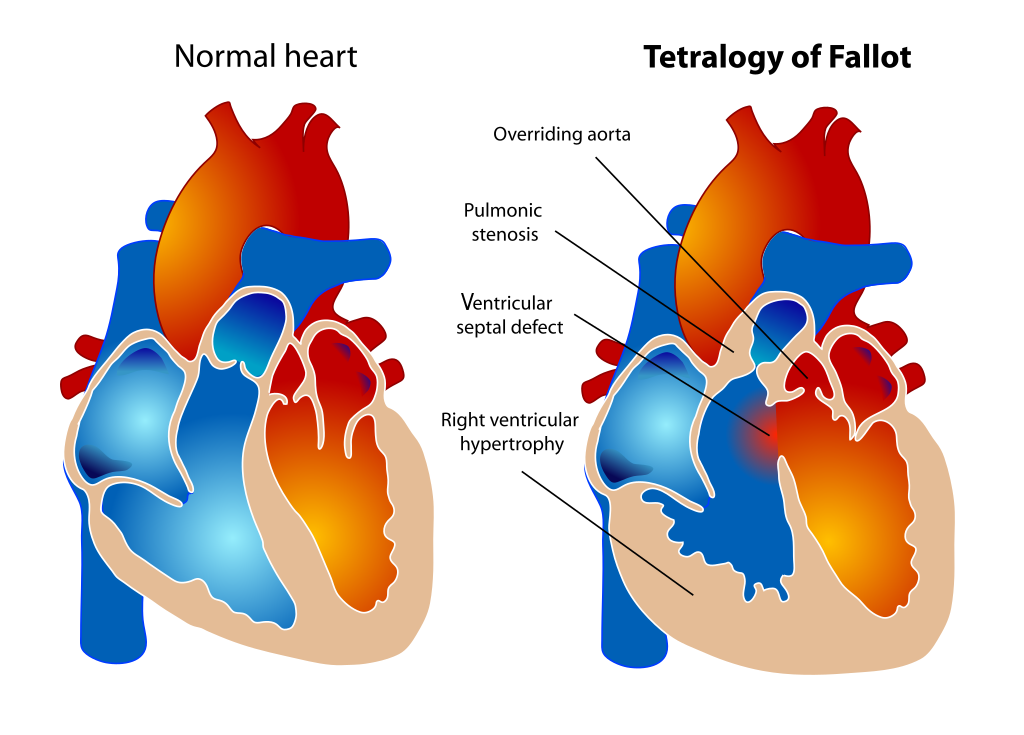
- It is understood that tetralogy of fallot is the result of improper positioning of the outlet septum.[1][2][3]
- In the normal heart, the outlet septum is an indistinguishable component of the crista supraventricularis that communicates with the septomarginal trabeculae to divide the right and left ventricular cavities.
- In Tetralogy of Fallot, proper ventricular septation is perturbed by anterocephalad displacement of the outlet septum relative to the septomarginal trabecula.
- The direct consequence of this misalignment is an overriding aortic orifice and a ventricular septal defect, resulting in an intracardiac right to left shunt of blood.
- In addition, anterocephalad displacement of the outlet septum indirectly predisposes the pulmonary trunk to stenosis in the setting of septoparietal trabecular hypertrophy.
- Together, the displacement of the outlet septum coupled with the hypertrophic arrangement of the septoparietal trabeculae account for the three anatomical cardinal defects in Tetralogy of Fallot - aortic dextroposition, interventricular communication (VSD), and pulmonary stenosis.
- The fourth defect - right ventricular hypertrophy - is a hemodynamic consequence of these three morphologic changes, as the right ventricle physiologically adapts to the increased resistance of a stenotic pulmonary trunk.
Anatomy
{{#ev:youtube|ACRfFkxow7w}} |
Embrylogy
- Embryologic studies show that tetralogy of Fallot is a result of anterior misalignment of the conal septum, resulting in the clinical combination of a ventricular septal defect (VSD), pulmonary stenosis, and an overriding aorta.
- Right ventricular hypertrophy is secondary to this combination of abnormalities, which causes resistance to blood flow from the right ventricle.
- Tetralogy of Fallot results in cyanosis, (hypoxia or low oxygenation) of the blood due to mixing of deoxygenated venous blood from the right ventricle with oxygenated blood in the left ventricle through the ventricular septal defect and preferential flow of both oxygenated and deoxygenated blood from the ventricles through the aorta because of obstruction to flow through the pulmonary valve.
- This is known as a right-to-left shunt.
- If pulmonary blood flow is dramatically reduced, pulmonary blood flow may depend on a patent ductus arteriosus (PDA) or bronchial collaterals.
- If obstruction of the right ventricular outflow tract is minimal, the intracardiac shunt may be mostly from left to right, and this may result in what is termed a pink tet or pink tetralogy.
- Although cyanosis is absent on clinical exam, laboratory testing will often show systemic oxygen desaturation.
- Children with tetralogy of Fallot may develop acute severe cyanosis or hypoxic tet spells.
- The mechanism underlying these episodes is not entirely clear, but may be due to spasm of the infundibular septum and the right ventricular outflow tract.
- Whatever the mechanism, there is an increase in resistance to blood flow to the lungs with increased preferential flow of desaturated blood to the systemic circulation.
- The child will often squat during a tet spell to improve venous return to the right side of the heart.
- Squatting increases the systemic vascular resistance and thereby shunts flow to pulmonary circuit.
- These spells can be fatal, and can occur in patients who are not cyanotic.
Genetics
- Genes involved in the pathogenesis of tetralogy of fallot include:[8][9][10][11][12][13][14]
- The cellular processes that underlie cardiogenesis are extensively regulated in the developing heart.
- Proper cardiac development requires the complex orchestration of cardiac transcription factors and signaling pathways in a spatiotemporal specific manner.
- Previous genetic studies demonstrated that mutations in numerous genes encoding cardiac transcription factors and cell signaling proteins have a role in the development of Tetralogy of Fallot.
- Specifically, heterozygous mutations in NKX2-5, HAND1, TBX5, and GATA4 have been reported in familial forms of disease.
- Many of these single gene mutations result in haploinsufficiency and suggest a dose dependent relationship between genetic expression and disease.
- While the mechanistic basis of this relationship is currently poorly understood, it is hypothesized that disruption of the direct protein-protein interactions that allow these transcription factors to work synergistically impedes the activation of downstream targets and signaling pathways central to cardiac morphogenesis.
- In addition, recent whole-exome sequencing investigations have introduced a novel role for epigenetic dysregulation in the pathogenesis of Tetralogy of Fallot.
- Aberrant epigenetic modifications are thought to provide an alternative mechanism to perturb normal spatiotemporal expression of these essential developmental genes.
Associated Conditions
- Conditions associated with tetralogy of fallot include:[15][16][17][18]
- Left superior vena cava
- Anomalies of the mitral valve
- Anomalies of the tricuspid valve
- Stenosis of the left pulmonary artery, in 40% of patients
- A bicuspid pulmonary valve, in 40% of patients
- Right sided aortic arch, in 25% of patients
- Coronary artery anomalies, in 10% of patients
- An atrial septal defect, in which case the syndrome is sometimes called a pentalogy of Fallot.
- An atrioventricular septal defect
- Partially or totally anomalous pulmonary venous return
- Forked ribs and scoliosis
- Associated abnormalities include cleft lip, cleft palate, hypospadias, skeletal and craniofacial abnormalities.
Gross Pathology
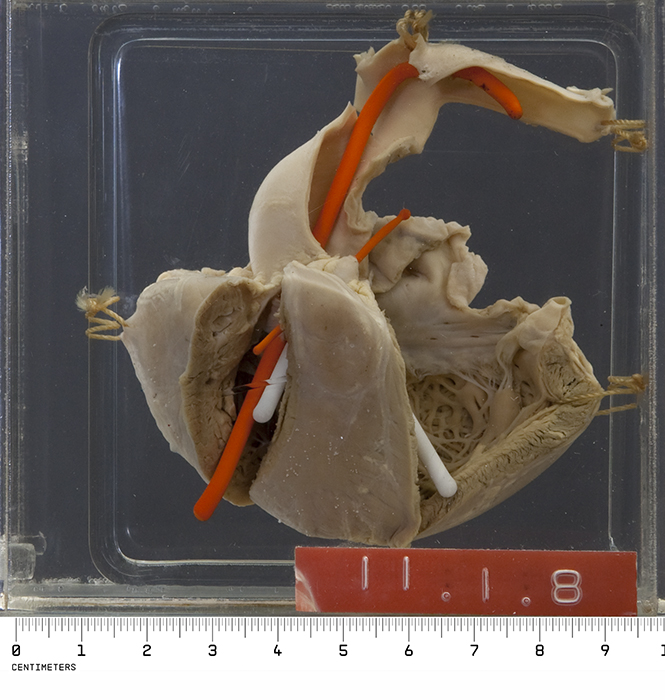
- On gross pathology characteristic features findings of tetralogy of fallot include:
- Right ventricular hypertrophy
- VSD
- Overriding aorta
- Subpulmonic stenosis
Microscopic Pathology
There is no characteristic findings of tetralogy of fallot on microscopic histopathological analysis.
References
- ↑ Anderson RH, Jacobs ML (2008). "The anatomy of tetralogy of Fallot with pulmonary stenosis". Cardiol Young. 18 Suppl 3: 12–21. doi:10.1017/S1047951108003259. PMID 19094375.
- ↑ Bashore TM (2007). "Adult congenital heart disease: right ventricular outflow tract lesions". Circulation. 115 (14): 1933–47. doi:10.1161/CIRCULATIONAHA.105.592345. PMID 17420363.
- ↑ Bailliard F, Anderson RH (2009). "Tetralogy of Fallot". Orphanet J Rare Dis. 4: 2. doi:10.1186/1750-1172-4-2. PMC 2651859. PMID 19144126.
- ↑ 4.0 4.1 4.2 Gatzoulis MA, Webb GD, Daubeney PE. (2005) Diagnosis and Management of Adult Congenital Heart Disease. Churchill Livingstone, Philadelphia. ISBN 0443071039.
- ↑ Bartelings M, Gittenberger-de Groot A (1991). "Morphogenetic considerations on congenital malformations of the outflow tract. Part 1: Common arterial trunk and tetralogy of Fallot". Int. J. Cardiol. 32 (2): 213–30. PMID 1917172.
- ↑ Anderson RH, Weinberg. The clinical anatomy of tetralogy of Fallot. Cardiol Young. 2005 15;38-47. PMID 15934690.
- ↑ Anderson RH, Tynan M. Tetralogy of Fallot – a centennial review. Int J Cardiol. 1988 21; 219-232. PMID 3068155.
- ↑ Olson EN (2006). "Gene regulatory networks in the evolution and development of the heart". Science. 313 (5795): 1922–7. doi:10.1126/science.1132292. PMID 17008524.
- ↑ Yang YQ, Gharibeh L, Li RG, Xin YF, Wang J, Liu ZM; et al. (2013). "GATA4 loss-of-function mutations underlie familial tetralogy of fallot". Hum Mutat. 34 (12): 1662–71. doi:10.1002/humu.22434. PMID 24000169.
- ↑ Bruneau BG (2008). "The developmental genetics of congenital heart disease". Nature. 451 (7181): 943–8. doi:10.1038/nature06801. PMID 18288184.
- ↑ Bruneau BG, Srivastava D (2014). "Congenital heart disease: entering a new era of human genetics". Circ Res. 114 (4): 598–9. doi:10.1161/CIRCRESAHA.113.303060. PMID 24526674.
- ↑ Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R; et al. (2001). "Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation". Nat Genet. 28 (3): 276–80. doi:10.1038/90123. PMID 11431700.
- ↑ Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA; et al. (2003). "GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5". Nature. 424 (6947): 443–7. doi:10.1038/nature01827. PMID 12845333.
- ↑ Sheng W, Qian Y, Wang H, Ma X, Zhang P, Diao L; et al. (2013). "DNA methylation status of NKX2-5, GATA4 and HAND1 in patients with tetralogy of fallot". BMC Med Genomics. 6: 46. doi:10.1186/1755-8794-6-46. PMC 3819647. PMID 24182332.
- ↑ Dabizzi RP, Caprioli G, Aiazzi L, Castelli C, Baldrighi G, Parenzan L, Baldrighi V (January 1980). "Distribution and anomalies of coronary arteries in tetralogy of fallot". Circulation. 61 (1): 95–102. doi:10.1161/01.cir.61.1.95. PMID 7349946.
- ↑ Satyanarayana Rao, B.N.; Anderson, Ray C.; Edwards, Jesse E. (1971). "Anatomic variations in the tetralogy of Fallot". American Heart Journal. 81 (3): 361–371. doi:10.1016/0002-8703(71)90106-2. ISSN 0002-8703.
- ↑ Muster, Alexander J.; Paul, Milton H.; Nikaidoh, Hisashi (1973). "Tetralogy of Fallot Associated with Total Anomalous Pulmonary Venous Drainage". Chest. 64 (3): 323–326. doi:10.1378/chest.64.3.323. ISSN 0012-3692.
- ↑ Saifi, Comron; Matsumoto, Hiroko; Vitale, Michael G.; Roye, David P.; Hyman, Joshua E. (2012). "The incidence of congenital scoliosis in infants with tetralogy of Fallot based on chest radiographs". Journal of Pediatric Orthopaedics B. 21 (4): 313–316. doi:10.1097/BPB.0b013e3283536872. ISSN 1060-152X.