Somatostatin: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Protein | ||
| | |Name=Somatostatin | ||
| | |image=somatostatin.png | ||
| | |caption= | ||
| | |Symbol=SST | ||
| | |AltSymbols= | ||
| | |HGNCid=11329 | ||
|Chromosome=3 | |||
|Arm=q | |||
|Band=28 | |||
|LocusSupplementaryData= | |||
|ECnumber= | |||
|OMIM=182450 | |||
| | |EntrezGene=6750 | ||
|RefSeq=NM_001048 | |||
|UniProt=P61278 | |||
|PDB= | |||
}} | |||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
| | |||
= | |||
| | |||
= | |||
{{SK}} GHIH; Growth hormone inhibiting hormone; somatotropin release-inhibiting factor; SRIF | |||
==Overview== | |||
'''Somatostatin''' (also known as '''growth hormone inhibiting hormone''' ('''GHIH''') or '''somatotropin release-inhibiting hormone''' ('''SRIF''')) is a [[peptide hormone]] that regulates the [[endocrine system]] and affects [[neurotransmission]] and [[cell proliferation]] via interaction with [[G-protein-coupled]] [[somatostatin receptors]] and inhibition of the release of numerous secondary hormones. | |||
Somatostatin has two active forms produced by alternative cleavage of a single preproprotein: one of 14 [[amino acid]]s, the other of 28 amino acids.<ref name="GP5416">{{GeorgiaPhysiology|5/5ch4/s5ch4_16}}</ref> | |||
==Production== | |||
===Digestive system=== | |||
Somatostatin is secreted in several locations in the digestive system: | |||
* [[Stomach]] | |||
* [[Intestine]] | |||
* [[Delta cells]] of the [[pancreas]]<ref>Costanzo, LS. ''Board Review Series: Physiology'' 3rd Ed. Lippincott, Williams & Wilkins. 2003. p. 280.</ref> | |||
===Brain=== | |||
Somatostatin is produced by [[neuroendocrine]] neurons of the [[periventricular nucleus]] of the [[hypothalamus]]. These neurons project to the [[median eminence]], where somatostatin is released from neurosecretory nerve endings into the hypothalamo-hypophysial portal circulation. These blood vessels carry somatostatin to the [[anterior pituitary gland]], where somatostatin inhibits the secretion of growth hormone from [[somatotrope]] cells. The somatostatin neurons in the periventricular nucleus mediate negative feedback effects of growth hormone on its own release; the somatostatin neurons respond to high circulating concentrations of growth hormone and [[somatomedin]]s by increasing the release of somatostatin, so reducing the rate of secretion of growth hormone. | |||
Somatostatin is also produced by several other populations that project centrally - i.e. to other areas of the brain, and somatostatin receptors are expressed at many different sites in the brain. In particular, there are populations of somatostatin neurons in the [[arcuate nucleus]], the [[hippocampus]] and the brainstem [[nucleus of the solitary tract]]. | |||
== | ==Actions== | ||
[[Image:Control-of-stomach-acid-sec.png|thumb|left|250px|[[D cell (biology)|D cell]] is visible at upper right, and somatostatinis represented by middle arrow pointing left]] | |||
Somatostatin is classified as an [[inhibitory]] hormone,<ref name="GP5416"> </ref> whose actions are spread to different parts of the body: | |||
===Anterior pituitary=== | |||
In the [[anterior pituitary gland]], the effects of somatostatin are: | |||
* Inhibit the release of [[growth hormone]] (GH) <ref name="Colorado"> http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/otherendo/somatostatin.html Colorado State University - Biomedical Hypertextbooks - Somatostatin </ref> (thus opposing the effects of [[Growth Hormone-Releasing Hormone]] (GHRH)) | |||
* Inhibit the release of [[thyroid-stimulating hormone]] (TSH) | |||
===Gastrointestinal system=== | |||
< | * Suppress the release of [[gastrointestinal hormone]]s | ||
** [[Gastrin]] | |||
** [[Cholecystokinin]] (CCK) | |||
** [[Secretin]] | |||
** [[Motilin]] | |||
** [[Vasoactive intestinal peptide]] (VIP) | |||
** [[Gastric inhibitory polypeptide]] (GIP) | |||
** [[Enteroglucagon]] | |||
* Lowers the rate of gastric emptying, and reduces smooth muscle contractions and blood flow within the intestine<ref name="Colorado"> </ref> | |||
* Suppress the release of pancreatic hormones | |||
** Inhibit the release of [[insulin]]<ref name="GP5417">{{GeorgiaPhysiology|5/5ch4/s5ch4_17}}</ref> | |||
** Inhibit the release of [[glucagon]]<ref name="GP5417"> </ref> | |||
* Suppress the exocrine secretory action of [[pancreas]]. | |||
==Synthetic substitutes== | |||
'''[[Octreotide]]''' (brand name ''Sandostatin'', [[Novartis|Novartis Pharmaceuticals]]) is an [[peptide|octopeptide]] that mimics natural somatostatin pharmacologically, though is a more potent inhibitor of [[growth hormone]], [[glucagon]], and [[insulin]] than the natural hormone. | |||
==References== | |||
{{reflist|2}} | |||
{{Hormones}} | |||
{{Gastrointestinal physiology}} | |||
{{Neuropeptides}} | |||
{{Pituitary and hypothalamic hormones and analogues}} | |||
[[Category: | [[Category:Antidiarrhoeals]] | ||
[[Category:Hormonal agents]] | |||
[[Category:Endocrine system]] | |||
[[Category:Pancreatic hormones]] | |||
[[Category:Hormones of the hypothalamus]] | |||
[[Category:Somatotropic axis]] | |||
[[Category:Neuropeptides]] | |||
[[Category:Neuroendocrinology]] | |||
[[Category:Endocrinology]] | |||
Revision as of 19:26, 19 December 2014
| Somatostatin | |
|---|---|
 | |
| Identifiers | |
| Symbol | SST |
| Entrez | 6750 |
| HUGO | 11329 |
| OMIM | 182450 |
| RefSeq | NM_001048 |
| UniProt | P61278 |
| Other data | |
| Locus | Chr. 3 q28 |
|
WikiDoc Resources for Somatostatin |
|
Articles |
|---|
|
Most recent articles on Somatostatin Most cited articles on Somatostatin |
|
Media |
|
Powerpoint slides on Somatostatin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Somatostatin at Clinical Trials.gov Clinical Trials on Somatostatin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Somatostatin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Somatostatin Discussion groups on Somatostatin Patient Handouts on Somatostatin Directions to Hospitals Treating Somatostatin Risk calculators and risk factors for Somatostatin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Somatostatin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: GHIH; Growth hormone inhibiting hormone; somatotropin release-inhibiting factor; SRIF
Overview
Somatostatin (also known as growth hormone inhibiting hormone (GHIH) or somatotropin release-inhibiting hormone (SRIF)) is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G-protein-coupled somatostatin receptors and inhibition of the release of numerous secondary hormones.
Somatostatin has two active forms produced by alternative cleavage of a single preproprotein: one of 14 amino acids, the other of 28 amino acids.[1]
Production
Digestive system
Somatostatin is secreted in several locations in the digestive system:
- Stomach
- Intestine
- Delta cells of the pancreas[2]
Brain
Somatostatin is produced by neuroendocrine neurons of the periventricular nucleus of the hypothalamus. These neurons project to the median eminence, where somatostatin is released from neurosecretory nerve endings into the hypothalamo-hypophysial portal circulation. These blood vessels carry somatostatin to the anterior pituitary gland, where somatostatin inhibits the secretion of growth hormone from somatotrope cells. The somatostatin neurons in the periventricular nucleus mediate negative feedback effects of growth hormone on its own release; the somatostatin neurons respond to high circulating concentrations of growth hormone and somatomedins by increasing the release of somatostatin, so reducing the rate of secretion of growth hormone.
Somatostatin is also produced by several other populations that project centrally - i.e. to other areas of the brain, and somatostatin receptors are expressed at many different sites in the brain. In particular, there are populations of somatostatin neurons in the arcuate nucleus, the hippocampus and the brainstem nucleus of the solitary tract.
Actions
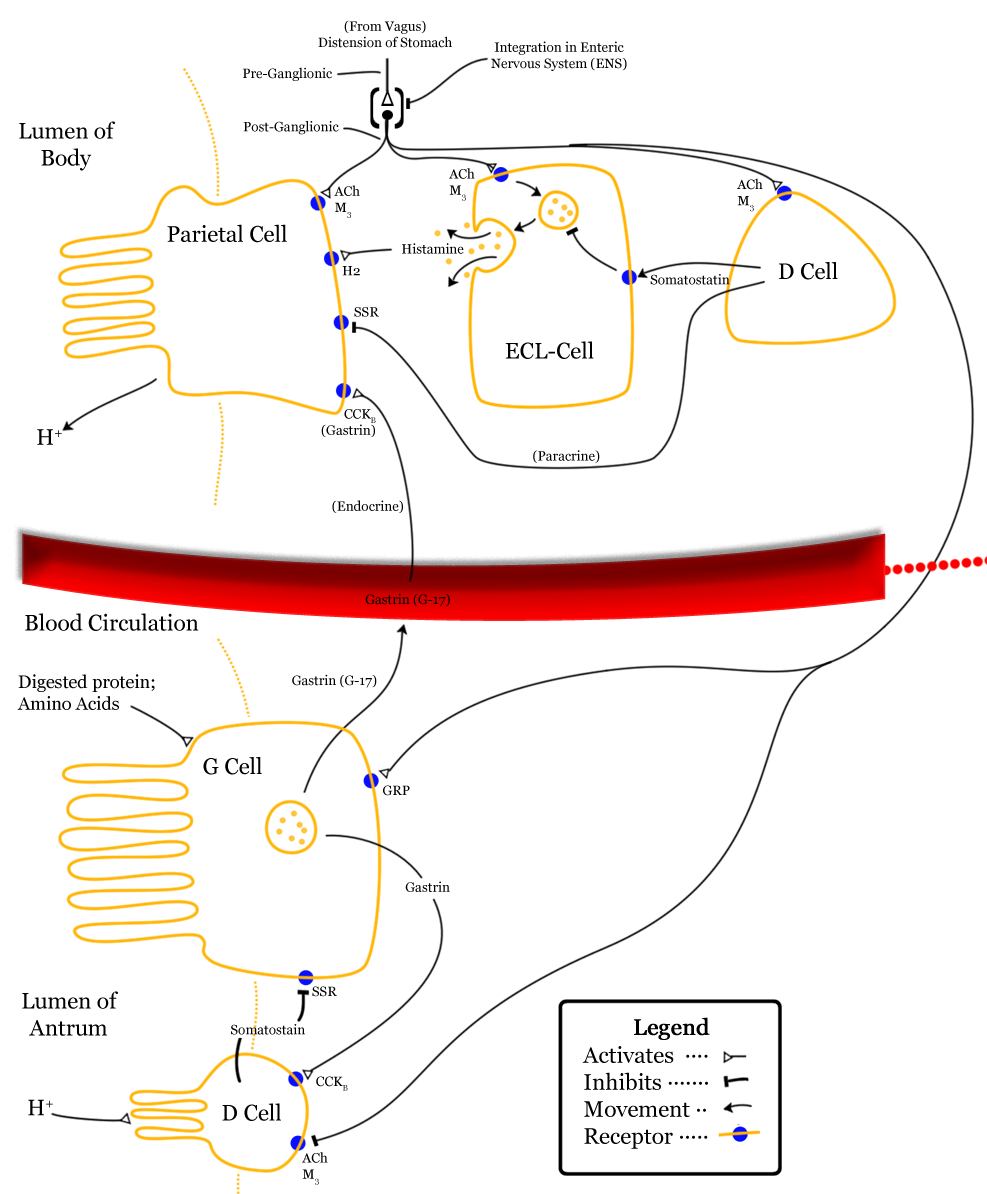
Somatostatin is classified as an inhibitory hormone,[1] whose actions are spread to different parts of the body:
Anterior pituitary
In the anterior pituitary gland, the effects of somatostatin are:
- Inhibit the release of growth hormone (GH) [3] (thus opposing the effects of Growth Hormone-Releasing Hormone (GHRH))
- Inhibit the release of thyroid-stimulating hormone (TSH)
Gastrointestinal system
- Suppress the release of gastrointestinal hormones
- Lowers the rate of gastric emptying, and reduces smooth muscle contractions and blood flow within the intestine[3]
- Suppress the release of pancreatic hormones
- Suppress the exocrine secretory action of pancreas.
Synthetic substitutes
Octreotide (brand name Sandostatin, Novartis Pharmaceuticals) is an octopeptide that mimics natural somatostatin pharmacologically, though is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone.
References
- ↑ 1.0 1.1 Essentials of Human Physiology by Thomas M. Nosek. Section 5/5ch4/s5ch4_16.
- ↑ Costanzo, LS. Board Review Series: Physiology 3rd Ed. Lippincott, Williams & Wilkins. 2003. p. 280.
- ↑ 3.0 3.1 http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/otherendo/somatostatin.html Colorado State University - Biomedical Hypertextbooks - Somatostatin
- ↑ 4.0 4.1 Essentials of Human Physiology by Thomas M. Nosek. Section 5/5ch4/s5ch4_17.