Multiple sclerosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
'''For patient information click [[{{PAGENAME}} (patient information)|here]]''' | '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' | ||
{{CMG}}; '''Associate Editor(s)-In-Chief:''' {{CZ}} | {{CMG}}; '''Associate Editor(s)-In-Chief:''' {{CZ}} | ||
Revision as of 19:15, 27 August 2012
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [15]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [16]
Synonyms and keywords: Disseminated sclerosis, encephalomyelitis disseminata
| Multiple sclerosis | |
 | |
|---|---|
| MRI FLAIR sequence showing four bright spots (plaques) where multiple sclerosis has damaged myelin in the brain | |
| ICD-10 | G35 |
| ICD-9 | 340 |
| OMIM | 126200 |
| DiseasesDB | 8412 |
| MedlinePlus | 000737 |
| MeSH | D009103 |
|
Multiple sclerosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Multiple sclerosis On the Web |
|
American Roentgen Ray Society Images of Multiple sclerosis |
Overview
Classification
Pathophysiology
Causes
Differential Diagnosis
Risk Factors
Natural History, Complications and Prognosis
Diagnosis
History and Symptoms | Physical Examination |Laboratory Findings | ECG | EEG | Chest X Ray |CT | MRI |Echocardiography or Ultrasound |Other Imaging Findings | Other Diagnostic Studies
Treatment
Medical therapy | Surgery | Prevention |Cost-effectiveness of Therapy| Future or Investigational Therapies
See also
Overview
Multiple sclerosis (abbreviated MS, formerly known as disseminated sclerosis or encephalomyelitis disseminata) is a chronic, inflammatory, demyelinating disease that affects the central nervous system (CNS). Disease onset usually occurs in young adults, is more common in women and the disease has a prevalence that ranges between 2 and 150 per 100,000 depending on the country or specific population.[1] MS was first described in 1868 by Jean-Martin Charcot.
Description
MS affects the neurons in the areas of the brain and spinal cord known as the white matter. These cells carry signals in between the grey matter areas, where the processing is done, and between these and the rest of the body. More specifically, MS destroys oligodendrocytes which are the cells responsible for creating and maintaining a fatty layer, known as the myelin sheath, which helps the neurons carry electrical signals. MS results in a thinning or complete loss of myelin and, less frequently, the cutting (transection) of the neuron's extensions or axons. When the myelin is lost, the neurons can no longer effectively conduct their electrical signals. The name multiple sclerosis refers to the scars (scleroses - better known as plaques or lesions) in the white matter. Loss of myelin in these lesions causes some of the symptoms that may vary widely depending upon which signals are interrupted. However, more advanced forms of imaging are now showing that much of the damage happens outside these regions. A consequence of this course of action is that almost any neurological symptom can accompany the disease.
Multiple sclerosis may take several forms, with new symptoms occurring either in discrete attacks (relapsing forms) or slowly accumulating over time (progressive forms). Most people are first diagnosed with relapsing-remitting MS but develop secondary-progressive MS (SPMS) after a number of years. Between attacks, symptoms may resolve completely, but permanent neurological problems often persist, especially as the disease advances.
Although much is known about the mechanisms involved in the disease process, the cause remains elusive. The theory with the most adherents is that it results from attacks to the nervous system by the body's own immune system. Some believe it is a metabolically dependent disease while others think that it might be caused by a virus such as Epstein-Barr. Still other people believe that its virtual absence from the tropics points to a deficiency of vitamin D during childhood.
The disease currently does not have a cure, but several therapies have proven helpful. The aims of treatment are returning function after an attack, preventing new attacks, and preventing disability. As with any treatment, medications have several adverse effects, and many therapies are still under investigation. At the same time different alternative treatments are pursued by many patients, despite the paucity of supporting scientific study.
The prognosis, or expected course of the disease, for a person depends on the subtype of the disease; the characteristics of the individual, the initial symptoms; and the degree of disability the person experiences as time advances. However life expectancy of patients is nearly the same as that of the unaffected population and in many cases a normal life is possible.
Epidemiology
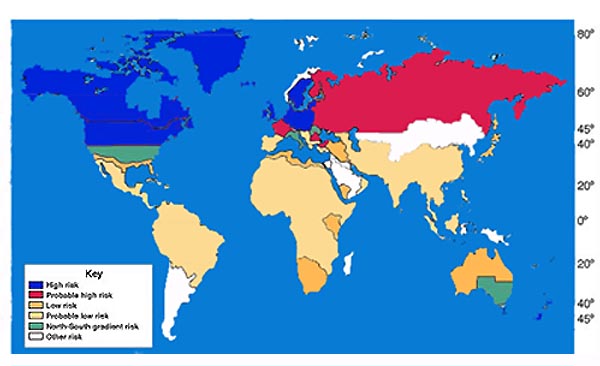
In northern Europe, continental North America, and Australasia, about one of every 1000 citizens suffers from multiple sclerosis, whereas in the [rabian peninsula, Asia, and continental South America, the frequency is much lower. In sub-Saharan Africa, MS is extremely rare. With important exceptions, there is a north-to-south gradient in the northern hemisphere and a south-to-north gradient in the southern hemisphere, with MS being much less common in people living near the equator.[2] Climate, diet, geomagnetism, toxins, sunlight exposure, genetic factors, and infectious diseases have all been discussed as possible reasons for these regional differences. Environmental factors during childhood may play an important role in the development of MS later in life. This idea is based on several studies of migrants showing that if migration occurs before the age of fifteen, the migrant acquires the new region's susceptibility to MS. If migration takes place after age fifteen, the migrant keeps the susceptibility of his home country.[3] However other works suggest that the age/geographical risk for developing multiple sclerosis spans a larger timescale than just the first 15 years of life.[4]
MS occurs mainly in Caucasians. It is twentyfold lower in the Inuit people of Canada than in other Canadians living in the same region. It is also rare in the Native American tribes of North America, Australian Aborigines and the Māori of New Zealand. Scotland appears to have the highest rate of MS in the world.[5] The reasons for this are unknown. These few examples point out that either genetic background or lifestyle and cultural factors play an important role in the development of MS.
As observed in many autoimmune disorders, MS is more common in females than males; the mean sex ratio is about two females for every male. In children (who rarely develop MS) the sex ratio may reach three females for each male. In people over age fifty, MS affects males and females equally. Onset of symptoms usually occurs between fifteen to forty years of age, rarely before age fifteen or after age sixty.
As previously discussed, there is a genetic component to MS. On average one of every 25 siblings of individuals with MS will also develop MS. Almost half of the identical twins of MS-affected individuals will develop MS, but only one of twenty fraternal twins. If one parent is affected by MS, each child has a risk of only about one in forty of developing MS later in life.[6]
Finally, it is important to remark that advances in the study of related diseases have shown that some cases formerly considered MS are not MS at all. In fact, all the studies before 2004 can be affected by the impossibility to distinguish MS and Devic's disease (NMO) reliably before this date. The error can be important in some areas, and is considered to be 30% in Japan.[7]
History
The French neurologist Jean-Martin Charcot (1825–93) was the first person to recognize multiple sclerosis as a distinct, separate disease in 1868. Summarizing previous reports and adding his own important clinical and pathological observations, Charcot called the disease sclerose en plaques. The three signs of MS now known as Charcot's triad are dysarthria (problems with speech), ataxia (problems with coordination), and tremor. Charcot also observed cognition changes in MS since he described his patients as having a "marked enfeeblement of the memory" and "with conceptions that formed slowly".[8]
Prior to Charcot, Robert Hooper (1773–1835), a British pathologist and practicing physician, Robert Carswell (1793–1857), a British professor of pathology, and Jean Cruveilhier (1791–1873), a French professor of pathologic anatomy, had described and illustrated many of the disease's clinical details.
After this, several people, such as Eugène Devic (1858–1930), Jozsef Balo (1895–1979), Paul Ferdinand Schilder (1886–1940), and Otto Marburg (1874–1948) found special cases of the disease that some authors consider different diseases and now are called the borderline forms of multiple sclerosis.
There are several historical accounts of people who probably had MS. Saint Lidwina of Schiedam (1380–1433), a Dutch nun, may be one of the first identifiable MS patients. From the age of sixteen until her death at age 53, she suffered intermittent pain, weakness of the legs, and vision loss—symptoms typical of MS. Almost a hundred years before there is a story from Iceland of a young woman called Halla. This girl suddenly lost her vision and capacity to talk; but after praying to the saints recovered them seven days after.[9] Augustus Frederick d'Este (1794–1848), an illegitimate grandson of King George III of Great Britain, almost certainly suffered from MS. D'Este left a detailed diary describing his 22 years living with the disease. He began his diary in 1822 and it had its last entry in 1846 (only to remain unknown until 1948). His symptoms began at age 28 with a sudden transient visual loss after the funeral of a friend. During the course of his disease he developed weakness of the legs, clumsiness of the hands, numbness, dizziness, bladder disturbances, and erectile dysfunction. In 1844, he began to use a wheelchair. Despite his illness, he kept an optimistic view of life.[10] Another early account of MS was kept by the British diarist W. N. P. Barbellion, who maintained a detailed log of his diagnosis and struggle with MS. His diary was published in 1919 as The Journal of a Disappointed Man.
Pathophysiology
Although much is known about how multiple sclerosis causes damage, the reasons why multiple sclerosis occurs are not known.
Multiple sclerosis is a disease in which the myelin (a fatty substance which covers the axons of nerve cells) degenerates. According to the view of most researchers, a special subset of lymphocytes, called T cells, plays a key role in the development of MS.
According to a strictly immunological explanation of MS, the inflammatory process is triggered by the T cells. T cells gain entry into the brain via the blood-brain barrier (a capillary system that should prevent entrance of T-cells into the nervous system). The blood brain barrier is normally not permeable to these types of cells, unless triggered by either infection or a virus, where the integrity of the tight junctions forming the blood-brain barrier is decreased. When the blood brain barrier regains its integrity (usually after infection or virus has cleared) the T cells are trapped inside the brain. These lymphocytes recognize myelin as foreign and attack it as if it were an invading virus. That triggers inflammatory processes, stimulating other immune cells and soluble factors like cytokines and antibodies. Leaks form in the blood-brain barrier. These leaks, in turn, cause a number of other damaging effects such as swelling, activation of macrophages, and more activation of cytokines and other destructive proteins such as matrix metalloproteinases. A deficiency of uric acid has been implicated in this process.[11]
It is known that a repair process, called remyelination, takes place in early phases of the disease, but the oligodendrocytes that originally formed a myelin sheath cannot completely rebuild a destroyed myelin sheath. The newly-formed myelin sheaths are thinner and often not as effective as the original ones. Repeated attacks lead to successively fewer effective remyelinations, until a scar-like plaque is built up around the damaged axons, according to four different damage patterns.[12] The central nervous system should be able to recruit oligodendrocyte stem cells capable of turning into mature myelinating oligodendrocytes, but it is suspected that something inhibits stem cells in affected areas.
The axons themselves can also be damaged by the attacks.[13] Often, the brain is able to compensate for some of this damage, due to an ability called neuroplasticity. MS symptoms develop as the cumulative result of multiple lesions in the brain and spinal cord. This is why symptoms can vary greatly between different individuals, depending on where their lesions occur.
Causes
Although many risk factors for multiple sclerosis have been identified, no definitive cause has been found. MS likely occurs as a result of some combination of both environmental and genetic factors. Various theories try to combine the known data into plausible explanations. Although most accept an autoimmune explanation, several theories suggest that MS is an appropriate immune response to one or several underlying conditions (the etiology could be heterogeneous[14]). The need for alternative theories is supported by the poor results of present therapies, since autoimmune theory predicted greater success.[15][16][17]
Environmental
The most popular hypothesis is that a viral infection or retroviral reactivation primes a susceptible immune system for an abnormal reaction later in life. On a molecular level, this might occur if there is a structural similarity between the infectious virus and some component of the central nervous system, leading to eventual confusion in the immune system.
Since MS seems to be more common in people who live farther from the equator, another theory proposes that decreased sunlight exposure[18] and possibly decreased vitamin D production may help cause MS. This theory is bolstered by recent research into the biochemistry of vitamin D, which has shown that it is an important immune system regulator. A large, 2006 study by the Harvard School of Public Health, reported evidence of a link between Vitamin D deficiency and the onset of multiple sclerosis.[19] Other data comes from a 2007 study which concluded that sun exposure during childhood reduces the risk of suffering MS, while controlling for genetic factors.[20][21]
Other theories, noting that MS is less common in children with siblings, suggest that less exposure to illness in childhood leads to an immune system which is not primed to fight infection and is thus more likely to attack the body. One explanation for this would be an imbalance between the Th1 type of helper T-cells, which fight infection, and the Th2 type, which are more active in allergy and more likely to attack the body.
Other theories describe MS as an immune response to a chronic infection. The association of MS with the Epstein-Barr virus suggests a potential viral contribution in at least some individuals.[22] Still others believe that MS may sometimes result from a chronic infection with spirochetal bacteria, a hypothesis supported by research in which cystic forms were isolated from the cerebrospinal fluid of all MS patients in a small study.[23] When the cysts were cultured, propagating spirochetes emerged. Another bacterium that has been implicated in MS is Chlamydophila pneumoniae; it or its DNA has been found in the cerebrospinal fluid of MS patients by several research laboratories, with one study finding that the oligoclonal bands of 14 of the 17 MS patients studied consisted largely of antibodies to Chlamydophila antigens.[24]
Severe stress may also be a factor—a large study in Denmark found that parents who had lost a child unexpectedly were 50% more likely to develop MS than parents who had not.[25] Smoking has also been shown to be an independent risk factor for developing MS.[26]
Genetic
MS is not considered a hereditary disease. However, increasing scientific evidence suggests that genetics may play a role in determining a person's susceptibility to MS:
Some populations, such as the Roma, Inuit, and Bantus, rarely if ever get MS. The indigenous peoples of the Americas and Asians have very low incidence rates.
In the population at large, the chance of developing MS is less than a tenth of one percent. However, if one person in a family has MS, that person's first-degree relatives—parents, children, and siblings—have a one to three percent chance of getting the disease.
For identical twins, the likelihood that the second twin may develop MS if the first twin does is about 30%. For fraternal twins (who do not inherit an identical set of genes), the likelihood is closer to that for non-twin siblings, or about 4%. This pattern suggests that, while genetic factors clearly help determine the risk of MS, other factors such as environmental effects or random chance are also involved. The actual correlation may be somewhat higher than reported by these numbers as people with MS lesions remain essentially asymptomatic throughout their lives.
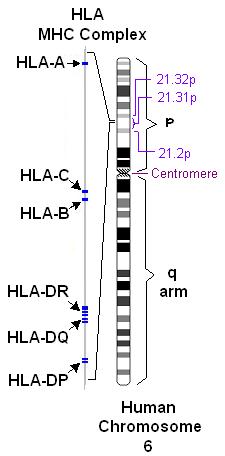
Further indications that more than one gene is involved in MS susceptibility comes from studies of families in which more than one member has MS. Several research teams found that people with MS inherit certain regions on individual genes more frequently than people without MS. Of particular interest is the human leukocyte antigen (HLA) or major histocompatibility complex region on chromosome 6. HLAs are genetically determined proteins that influence the immune system. However, there are other genes in this region which are not related to the immune system.
The HLA patterns of MS patients tend to be different from those of people without the disease. Investigations in northern Europe and America have detected three HLAs that are more prevalent in people with MS than in the general population. Studies of American MS patients have shown that people with MS also tend to exhibit these HLAs in combination—that is, they have more than one of the three HLAs—more frequently than the rest of the population. Furthermore, there is evidence that different combinations of the HLAs may correspond to variations in disease severity and progression.
A large study examining 334,923 single nucleotide polymorphisms (small variations in genes) in 931 families showed that apart from HLA-DRA there were two genes in which polymorphisms strongly predicted MS; these were the IL2RA (a subunit of the receptor for interleukin 2) and the IL7RA (idem for interleukin 7) genes. Mutations in these genes were already known to be associated with diabetes mellitus type 1 and other autoimmune conditions; the findings circumstantially support the notion that MS is an autoimmune disease.[27]
Studies of families with multiple cases of MS and research comparing proteins expressed in humans with MS to those of mice with EAE suggest that another area related to MS susceptibility may be located on chromosome 5. Other regions on chromosomes 2, 3, 7, 11, 17, 19, and X have also been identified as possibly containing genes involved in the development of MS.
These studies strengthen the theory that MS is the result of a number of factors rather than a single gene or other agent. Development of MS is likely to be influenced by the interactions of a number of genes, each of which (individually) has only a modest effect. Additional studies are needed to specifically pinpoint which genes are involved, determine their function, and learn how each gene's interactions with other genes and with the environment make an individual susceptible to MS.
Signs and symptoms
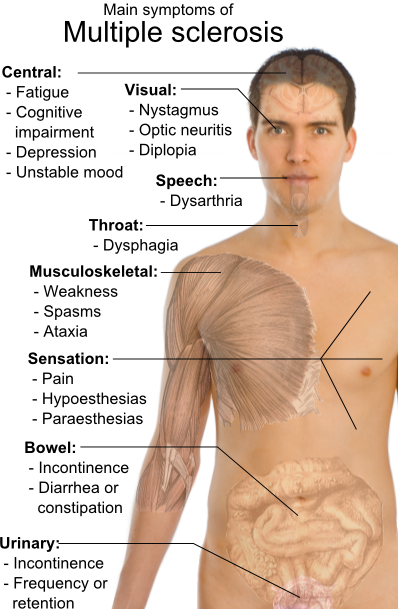
MS can cause a variety of symptoms, including changes in sensation (hypoesthesia), muscle weakness, abnormal muscle spasms, or difficulty in moving; difficulties with coordination and balance (ataxia); problems in speech (dysarthria) or swallowing (dysphagia), visual problems (nystagmus, optic neuritis, or diplopia), fatigue and acute or chronic pain syndromes, bladder and bowel difficulties, cognitive impairment, or emotional symptomatology (mainly depression). Lhermitte's sign is considered a classic MS finding, but it can be seen in several other conditions as well. The main clinical measure of disability progression and severity of the symptoms is the Expanded Disability Status Scale or EDSS.[28]
The initial attacks are often transient, mild (or asymptomatic), and self-limited. They often do not prompt a health care visit and sometimes are only identified in retrospect once the diagnosis has been made based on further attacks. The most common initial symptoms reported are: changes in sensation in the arms, legs or face (33%), complete or partial vision loss (optic neuritis) (16%), weakness (13%), double vision (7%), unsteadiness when walking (5%), and balance problems (3%); but many rare initial symptoms have been reported such as aphasia or psychosis.[29][30] Fifteen percent of individuals have multiple symptoms when they first seek medical attention.[31] For some people the initial MS attack is preceded by infection, trauma, or strenuous physical effort.
Bladder
Bladder problems (See also urinary system and urination) appear in 70-80% of MS patients and they have an important effect both in hygiene habits and social activity.[32][33]
However bladder problems are usually related with high levels of disability and pyramidal signs in lower limbs[34]
The most common problems are an increase of frequency and urgency (incontinence) but difficulties to begin urination, hesitation, leaking, retention and sensation of incomplete urination also appear. When there is retention secondary urinary infections are common.
There are many cortical and subcortical structures implicated in micturition.[35] Accordingly; MS lesions in different central nervous system structures can cause these kind of symptoms.
Treatment objectives are aliviation of symptoms of urinary dysfunction, treatment of urinary infections, reduction of complicating factors and preservation of renal function.Treatments can be classified in two main subtypes: pharmacological and non pharmacological.
Pharmacological treatments vary greatly depending on the origin or type of dysfunction; however some examples of the medications used are:[36] alfuzosin for retention,[37] trospium and flavoxate for urgency and incontinency,[38][39] or desmopressin for nocturia.[40][41]
Non pharmacological treatments involve the use of pelvic floor muscle training, stimulation, biofeedback, pessaries, bladder retraining, and sometimes intermittent catheterization.[42]
Cognitive
Cognitive impairments are common. Neuropsychological studies suggest that 40 to 60 percent of patients have cognitive deficits;[43] with the lowest percentages usually from community-based studies and the highest ones from hospital-based.
Cognitive impairment, sometimes referred to as brain fog, is already present in the beginnings of the disease.[44] Even in probable MS (after the first attack but before a second confirmatory one) up to 50% of patients have mild impairment.[45]
Some of the most common declines are in recent memory, attention, processing speed, visual-spatial abilities and executive functions.[46] Other cognitive-related symptoms are emotional instability, and fatigue, including purely neurological fatigue. The cognitive impairments in MS are usually mild; and only in 5% of patients can we speak of dementia. Nevertheless they are related with unemployment and reduced social interactions.[47] They are also related with driving difficulties.[48]
Neurocognitive testing is important for determining the extent of cognitive deficits. Neuropsychological stimulation may help to reverse or decrease the cognitive defects although its management relies on lifestyle strategies.Interferons have demonstrated that can help to reduce cognitive limitations in multiple sclerosis.[49] Anticholinesterase drugs such as donepezil commonly used in alzheimer disease; although not approved yet for multiple sclerosis; have also shown efficacy in different clinical trials.[50][51][52]
Emotional
Emotional symptoms are also common and are thought to be both the normal response to having a debilitating disease and the result of damage to specific areas of the cental nervous system that generate and control emotions.
Clinical depression is the most common neuropsychiatric condition: lifetime depression prevalence rates of 40-50% and 12 month prevalence rates around 20% have been typically reported for samples of people with MS; these figures are considerably higher than those for the general population or for people with other chronic illnesses.[53][54] Many brain-imaging studies have tried to relate depression to lesions in different brain regions with variable success. On balance the evidence seems to favour an association with neuropathology in the left anterior temporal/parietal regions.[55]
Other feelings such as anger, anxiety, frustration, and hopelessness also appear frequently, and suicide is a very real threat since 15% of deaths in MS sufferers are due to this cause.[56]
Fatigue
Fatigue is very common and disabling in MS. At the same time it has a close relationship with depressive symptomatology.[57] When depression is reduced fatigue also tends to improve, so patients should be evaluated for depression before other therapeutic approaches are used.[58]. In a similar way other factors like disturbed sleep, chronic pain, poor nutrition, or even some medications can contribute to fatigue; and therefore medical professionals are encouraged to identify and modify them.[59] There are also different medications used to treat fatigue; such as amantadine,[60][61] or pemoline;[62][63] as well as psichological interventions of energy conservation;[64][65] but the effects of all of them are small. For this reason fatigue is a very difficult symptom to manage.
Internuclear ophthalmoplegia

Internuclear ophthalmoplegia is a disorder of conjugate lateral gaze. The affected eye shows impairment of adduction. The partner eye diverges from the affected eye during abduction, producing diplopia; during extreme abduction, compensatory nystagmus can be seen in the partner eye. Diplopia means double vision while nystagmus is involuntary eye movement characterized by alternating smooth pursuit in one direction and a saccadic movement in the other direction.
Internuclear ophthalmoplegia occurs when MS affects a part of the brain stem called the medial longitudinal fasciculus, which is responsible for communication between the two eyes by connecting the abducens nucleus of one side to the oculomotor nucleus of the opposite side. This results in the failure of the medial rectus muscle to contract appropriately, so that the eyes do not move equally (called disconjugate gaze).
Different drugs as well as optic compensatory systems and prisms can be used to improve this symptoms.[66][67][68][69] Surgery can also be used in some cases for this problem.[70]
Mobility restrictions
Restrictions in mobility (walking, transfers, bed mobility) are common in individuals suffering from multiple sclerosis. Within 10 years after the onset of MS one-third of patients reach a score of 6 on the Expanded Disability Status Scale (requiring the use of a unilateral walking aid),and by 30 years the proportion increases to 83%. Within 5 years the Expanded Disability Status Score is 6 in 50% of those with the progressive form of MS.[71]
In MS a wide range of impairments may exist which can act either alone or in combination to impact directly on a person's balance, function and mobility. Such impairments include fatigue, weakness, hypertonicity, low exercise tolerance, impaired balance, ataxia and tremor.[72]
Interventions may be aimed at the level of the impairments that reduce mobility; or at the level of disability. At this second level interventions include provision, education and instruction in use of equipment such as walking aids, wheelchairs, motorized scooters and car adaptations; and instruction about compensatory strategies to accomplish an activity, (for example,undertaking safe transfers by pivoting in a flexed posture rather than standing up and stepping around)
Optic neuritis
Up to 50% of patients with MS will develop an episode of optic neuritis, and 20% of the time optic neuritis is the presenting sign of MS. The presence of demyelinating white matter lesions on brain MRI at the time of presentation of optic neuritis is the strongest predictor for developing clinically definite MS. Almost half of the patients with optic neuritis have white matter lesions consistent with multiple sclerosis. At five years follow-up, the overall risk of developing MS is 30%, with or without MRI lesions. Patients with a normal MRI still develop MS (16%), but at a lower rate compared to those patients with three or more MRI lesions (51%). From the other perspective, however, almost half (44%) of patients with any demyelinating lesions on MRI at presentation will not have developed MS ten years later. [73][74]
Individuals experience rapid onset of pain in one eye, followed by blurry vision in part or all of the visual field of that eye. Inflammation of the optic nerve causes loss of vision usually due to the swelling and destruction of the myelin sheath covering the optic nerve.
The blurred vision usually resolves within ten weeks, but individuals are often left with less vivid color vision (especially red) in the affected eye.
Systemic intravenous treatment with corticosteroids, which may quicken the healing of the optic nerve, prevent complete loss of vision, and delay the onset of other symptoms, is often recommended.
Pain
Pain is a common symptom in MS; appearing in 55% of patients at some point of their disease process; specially as time passes.[75] It is strong and debilitating and has a profound effect in the quality of life and mental health of the sufferer.[76] It usually appears after a lesion to the ascending or descending tracts that control the transmission of painful stimulus. such as the anterolateral system, but many other causes are also possible.[77] Most frequent pains reported are headaches (40%), dysesthetic limb pain (19%), back pain (17%), and painful spasms (11%).[78]
Acute pain is mainly due to optic neuritis, being corticosteroids the best treatment available; to trigeminal neuralgia, to Lhermitte's sign or to dysesthesias.[79] Subacute pain is usually secondary to the disease and can be consequence of being too much time in the same position, urinary retention, infected skin ulcers and many others. Treatment will depend on cause. Chronic pain is very common and the harder to treat being its most common cause dysesthesias.
Trigeminal neuralgia
Trigeminal neuralgia or "tic douloureux", is a disorder of the trigeminal nerve that causes episodes of intense pain in the eyes, lips, nose, scalp, forehead, and jaw. It affects 1 to 2% of MS patients during their disease.[80][81] The episodes of pain occur paroxysmally, or suddenly; and the patients describe it as trigger area on the face, so sensitive that touching or even air currents can bring an episode of pain. Usually it's successfully treated with anticonvulsants such as carbamazepine[82] or phenytoin[83] but others such as gabapentin[84] can be used. [85] When drugs are not effective enough surgery may be recommended. Further damage to the nerve to prevent the transmission of pain (Rhyzotomy) has proven its efficacy;[86] however the beneficial effects and risks in multiple sclerosis patients of those procedures that consist in relieving the pressure on the nerve are still under discussion.[87][88]
Lhermitte's sign
Lhermitte's sign is an electrical sensation that runs down the back and into the limbs, and is produced by bending the neck forward. The sign suggests a lesion of the dorsal columns of the cervical cord or of the caudal medulla; correlating significantly with cervical MRI abnormalities.[89] Between 25 and 40% of MS patients report having Lhermitte's sign during the course of their illness. [90][91][92]
Dysesthesias
Dysesthesias are disagreeable sensations produced by ordinary stimuli. The abnormal sensations are often described as painful feelings such as burning, wetness, itching, electric shock or pins and needles; and are caused by lesions of the peripheral or central sensory pathways. Both Lhermitte's sign and painful dysesthesias usually respond well to treatment with carbamazepine, clonazepam or amitriptyline. [93][94][95]
Sexual
Sexual dysfunction (SD) is one of many symptoms affecting persons with a diagnosis of multiple sclerosis (MS) and other neurological disease. SD in men encompasses both erectile and ejaculatory disorder. The prevalence of SD in men with MS ranges from 75 to 91% (O'Leary et al., 2007). Erectile dysfunction appears to be the most common form of SD documented in MS. SD may be due to alteration of the ejaculatory reflexe which may be affected by neurological conditions such as MS [96]
Spasticity
Spasticity is characterised by increased stiffness and slowness in limb movement, the development of certain postures, an association with weakness of voluntary muscle power, and with involuntary and sometimes painful spasms of limbs.[59] A physiotherapist can help to reduce spasticity and avoid the development of contractures with techniques such as passive stretching.[97] There is evidence, albeit limited, of the clinical effectiveness of baclofen,[98] dantrolene,[99] diazepam,[100] and tizanidine.[101][102][103] In the most complicated cases intrathecal injections of baclofen can be used.[104] There are also palliative measures like castings, splints or customised seatings.[59]
Transverse myelitis
Some MS patients develop rapid onset of numbness, weakness, bowel or bladder dysfunction, and/or loss of muscle function, typically in the lower half of the body. This is the result of MS attacking the spinal cord. The symptoms and signs depend upon the level of the spinal cord involved and the extent of the involvement.
Prognosis for complete recovery is generally poor. Recovery from transverse myelitis usually begins between weeks 2 and 12 following onset and may continue for up to 2 years in some patients and as many as 80% of individuals with transverse myelitis are left with lasting disabilities.
Treatment is usually symptomatic only, corticosteroids being used with limited success.
Tremor and ataxia
Tremor is an unintentional, somewhat rhythmic, muscle movement involving to-and-fro movements (oscillations) of one or more parts of the body. It is the most common of all involuntary movements and can affect the hands, arms, head, face, vocal cords, trunk, and legs.
Ataxia is an unsteady and clumsy motion of the limbs or torso due to a failure of the gross coordination of muscle movements. People with ataxia experience a failure of muscle control in their arms and legs, resulting in a lack of balance and coordination or a disturbance of gait.
Tremor and ataxia are frequent in MS. They present in 25 to 60% of patients. They can be very disabling and embarrassing, and are difficult to manage.[105] The origin of tremor in MS is difficult to precise but it can be due to a mixture of different factors such as damage to the cerebellar connections, weakness, spasticity, etc.
In the treatment of tremor many medications have been proposed; however their efficacy is very limited. Medications that have been reported to provide some relief are isoniazid,[106][107][108][109] carbamazepine,[110] propranolol;[111][112][113] and gluthetimide,[114] but published evidence of effectiveness is very limited.[115]
Physical therapy is not indicated as a treatment for tremor or ataxia; however, the use of different orthese devices can help. An example is the use of wrist bandages with weights, which can be useful to increase the inertia of movement and therefore reduce tremor.[116] Daily use objects have also to be adapted so they are easier to grab and use.
If all these measures fail some patients are candidates for thalamus surgery. This kind of surgery can be both a thalamotomy or the implantation of a thalamic stimulator. Complications are frequent (30% in thalamotomy and 10% in deep brain stimulation) and include a worsening of ataxia, dysarthria and hemiparesis.
Thalamotomy is a more efficacious surgical treatment for intractable MS tremor, however the higher incidence of persistent neurological deficits in patients receiving lesional surgery supports the use of deep brain stimulation as the preferred surgical strategy.[117]
Factors triggering a relapse
Multiple sclerosis relapses are often unpredictable and can occur without warning with no obvious inciting factors. Some attacks, however, are preceded by common triggers. In general, relapses occur more frequently during spring and summer than during autumn and winter. Infections, such as the common cold, influenza, and gastroenteritis, increase the risk for a relapse.[118] Emotional and physical stress may also trigger an attack,[119][120][121] as can severe illness of any kind. Statistically, there is no good evidence that either trauma or surgery trigger relapses.[122] People with MS can participate in sports, but they should probably avoid extremely strenuous exertion, such as marathon running. Heat can transiently increase symptoms, which is known as Uhthoff's phenomenon. This is why some people with MS avoid saunas or even hot showers. However, heat is not an established trigger of relapses.[123]
Pregnancy can directly affect the susceptibility for relapse. The last three months of pregnancy offer a natural protection against relapses. However, during the first few months after delivery, the risk for a relapse is increased 20%–40%. Pregnancy does not seem to influence long-term disability. Children born to mothers with MS are not at increased risk for birth defects or other problems.[124]
Many potential triggers have been examined and found not to influence relapse rates in MS. Influenza vaccination is safe, does not trigger relapses, and can therefore be recommended for people with MS. There is also no evidence that vaccines for hepatitis B, varicella, tetanus, or Bacille Calmette-Guerin (BCG—immunization for tuberculosis) increases the risk for relapse.[125]
Prognosis
The prognosis (the expected future course of the disease) for a person with multiple sclerosis depends on the subtype of the disease; the individual's sex, race, age, and initial symptoms; and the degree of disability the person experiences. The life expectancy of people with MS is now nearly the same as that of unaffected people. This is due mainly to improved methods of limiting disability, such as physical therapy, occupational therapy and speech therapy, along with more successful treatment of common complications of disability, such as pneumonia and urinary tract infections.[126] Nevertheless half of the deaths in people with MS are directly related to the consequences of the disease, while 15% more are due to suicide.[127]
- Individuals with progressive subtypes of MS, particularly the primary progressive subtype, have a more rapid decline in function. In the primary progressive subtype, supportive equipment (such as a wheelchair or standing frame) is often needed after six to seven years. However, when the initial disease course is the relapsing-remitting subtype, the average time until such equipment is needed is twenty years. This means that many individuals with MS will never need a wheelchair. There is also more cognitive impairment in the progressive forms than in the relapsing-remitting course.
- The earlier in life MS occurs, the slower disability progresses. Individuals who are older than fifty when diagnosed are more likely to experience a chronic progressive course, with more rapid progression of disability. Those diagnosed before age 35 have the best prognosis. Females generally have a better prognosis than males. Although individuals of African descent tend to develop MS less frequently, they are often older at the time of onset and may have a worse prognosis.
- Initial MS symptoms of visual loss or sensory problems, such as numbness or tingling, are markers for a relatively good prognosis, whereas difficulty walking and weakness are markers for a relatively poor prognosis. Better outcomes are also associated with the presence of only a single symptom at onset, the rapid development of initial symptoms, and the rapid regression of initial symptoms.
- The degree of disability varies among individuals with MS. In general, one of three individuals will still be able to work after 15–20 years. Fifteen percent of people diagnosed with MS never have a second relapse, and these people have minimal or no disability after ten years.[128] The degree of disability after five years correlates well with the degree of disability after fifteen years. This means that two-thirds of people with MS with low disability after five years will not get much worse during the next ten years. It should be noted that most of these outcomes were observed before the use of medications such as interferon, which can delay disease progression for several years.
Currently there are no clinically established laboratory investigations available that can predict prognosis or response to treatment. However, several promising approaches have been proposed. These include measurement of the two antibodies anti-myelin oligodendrocyte glycoprotein and anti-myelin basic protein, and measurement of TRAIL (TNF-related apoptosis-inducing ligand).[129]
Additional Images
-
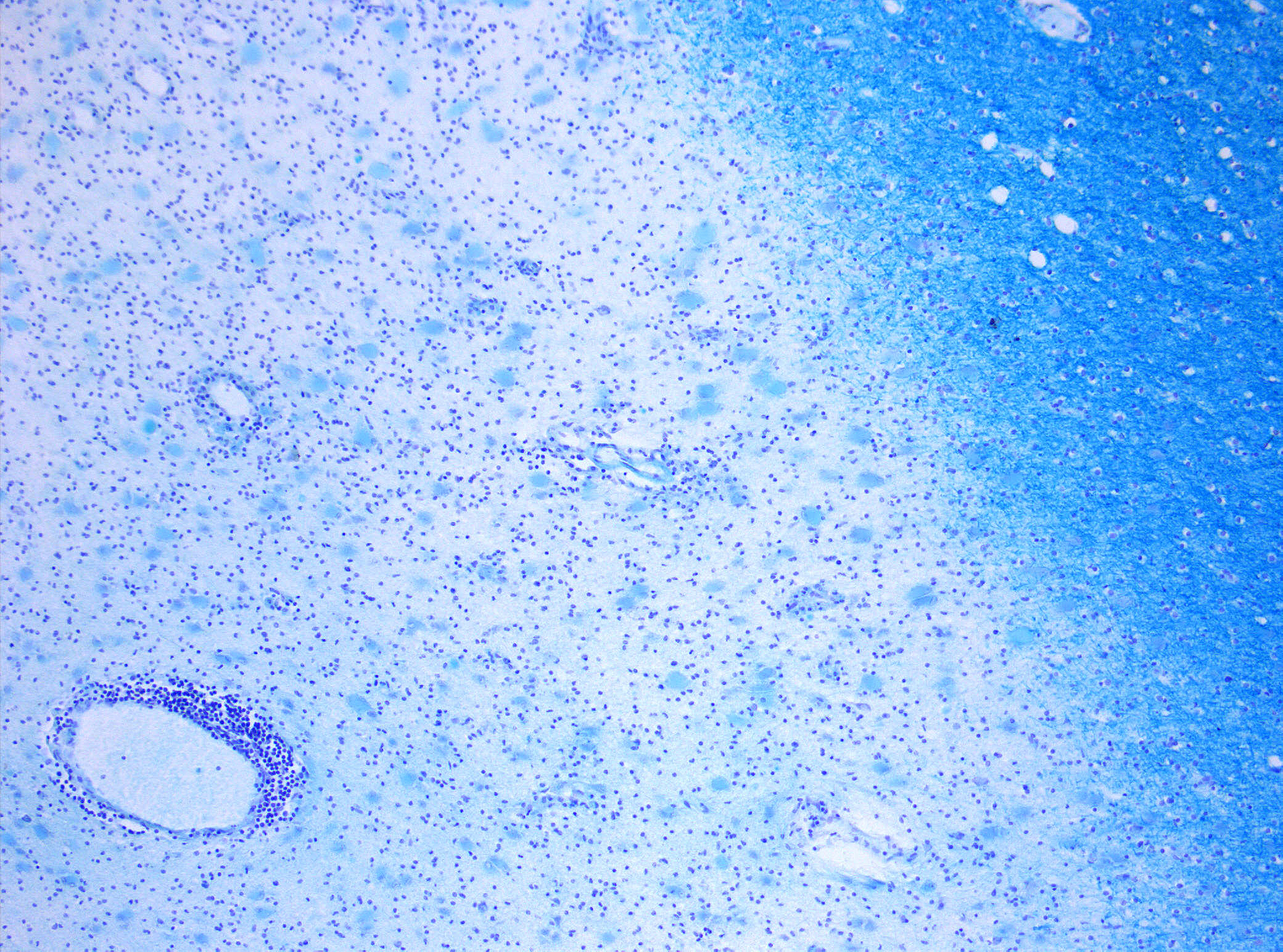
Demyelinization in MS. On Klüver-Barrera myelin staining, decoloration in the area of the lesion can be appreciated (Original scale 1:100).
-
Main symptoms of multiple sclerosis.
-
World map showing that risk for MS increases with greater distance from the equator
-
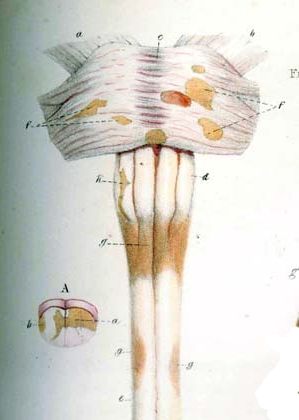
Detail of drawing from Carswell book depicting multiple sclerosis lesions in the brain stem and spinal cord (1838)
-
Chemical structure of alemtuzumab.
References
- ↑ Rosati G (2001). "The prevalence of multiple sclerosis in the world: an update". Neurol. Sci. 22 (2): 117–39. PMID 11603614.
- ↑ Epidemiology and multiple sclerosis. a personal review
- ↑ Marrie, RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004 Dec;3(12):709-18. Review. PMID 15556803
- ↑ Hammond SR, English DR, McLeod JG (2000). "The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia". Brain. 123 ( Pt 5): 968–74. PMID 10775541.
- ↑ Rothwell PM, Charlton D (1998). "High incidence and prevalence of multiple sclerosis in south east Scotland: evidence of a genetic predisposition". J. Neurol. Neurosurg. Psychiatr. 64 (6): 730–5. PMID 9647300.
- ↑ Sadovnick, AD, Ebers, GC, Dyment, DA, Risch, NJ. Evidence for genetic basis of multiple sclerosis. The Canadian Collaborative Study Group. Lancet 1996; 347:1728. PMID 8656905
- ↑ Weinshenker B (2005). "Western vs optic-spinal MS: two diseases, one treatment?". Neurology. 64 (4): 594–5. PMID 15728277.
- ↑ Charcot, J. Histologie de la sclerose en plaques. Gazette des hopitaux, Paris, 1868; 41: 554–555.
- ↑ Poser C (1994). "The dissemination of multiple sclerosis: a Viking saga? A historical essay". Ann. Neurol. 36 Suppl 2: S231–43. PMID 7998792.
- ↑ Firth, D (1948). The Case of August D`Esté. Cambridge: Cambridge University Press.
- ↑ Rentzos M, Nikolaou C, Anagnostouli M, Rombos A, Tsakanikas K, Economou M, Dimitrakopoulos A, Karouli M, Vassilopoulos D (2006). "Serum uric acid and multiple sclerosis". Clinical neurology and neurosurgery. 108 (6): 527–31. PMID 16202511.
- ↑ Lucchinetti, C. Bruck, W. Parisi, J. Scherhauer, B. Rodriguez, M. Lassmann, H.Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination Ann Neurol, 2000; 47(6):707-17. PMID 10852536
- ↑ Pascual AM, Martínez-Bisbal MC, Boscá I; et al. (2007). "Axonal loss is progressive and partly dissociated from lesion load in early multiple sclerosis". Neurology. 69 (1): 63–7. doi:10.1212/01.wnl.0000265054.08610.12. PMID 17606882.
- ↑ Lassmann H (2007). "Experimental models of multiple sclerosis". Rev. Neurol. (Paris). 163 (6–7): 651–5. PMID 17607184.
- ↑ Peter Behan and Abhijit Chaudhuri (2002). "The pathogenesis of multiple sclerosis revisited" (PDF). J R Coll Physicians Edinb. 32: 244–265.
- ↑ Chaudhuri A, Behan P (2004). "Multiple sclerosis is not an autoimmune disease". Arch. Neurol. 61 (10): 1610–2. PMID 15477520.
- ↑ Altmann D (2005). "Evaluating the evidence for multiple sclerosis as an autoimmune disease". Arch. Neurol. 62 (4): 688, author reply 688-9. PMID 15824275.
- ↑ van der Mei IA, Ponsonby AL, Dwyer T; et al. (2003). "Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study". BMJ. 327 (7410): 316. doi:10.1136/bmj.327.7410.316. PMID 12907484.
- ↑ Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006). "Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis". JAMA. 296 (23): 2832–8. doi:10.1001/jama.296.23.2832. PMID 17179460.
- ↑ Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Talat Islam, MBBS, PhD, W. James Gauderman, PhD, Wendy Cozen, DO, MPH and Thomas M. Mack, MD, MPH. Neurology 2007;69:381-388
- ↑ Sunshine 'protective' against MS. BBC News, 28 July 2007, 23:40
- ↑ Levin LI, Munger KL, Rubertone MV; et al. (2005). "Temporal relationship between elevation of epstein-barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis". JAMA. 293 (20): 2496–500. doi:10.1001/jama.293.20.2496. PMID 15914750.
- ↑ Brorson O, Brorson SH, Henriksen TH, Skogen PR, Schøyen R (2001). "Association between multiple sclerosis and cystic structures in cerebrospinal fluid". Infection. 29 (6): 315–9. PMID 11787831.
- ↑ Yao SY, Stratton CW, Mitchell WM, Sriram S (2001). "CSF oligoclonal bands in MS include antibodies against Chlamydophila antigens". Neurology. 56 (9): 1168–76. PMID 11342681.
- ↑ Li J, Johansen C, Bronnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J (2004). "The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark". Neurology. 62 (5): 726–9. PMID 15007121.
- ↑ Franklin GM, Nelson L (2003). "Environmental risk factors in multiple sclerosis: causes, triggers, and patient autonomy". Neurology. 61 (8): 1032–4. PMID 14581658.
- ↑ "Risk Alleles for Multiple Sclerosis Identified by a Genomewide Study". N Engl J Med. 2007. doi:10.1056/NEJMoa073493. PMID 17660530.
- ↑ Kurtzke JF (1983). "Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS)". Neurology. 33 (11): 1444–52. PMID 6685237.
- ↑ Navarro S, Mondéjar-Marín B, Pedrosa-Guerrero A, Pérez-Molina I, Garrido-Robres J, Alvarez-Tejerina A. "[Aphasia and parietal syndrome as the presenting symptoms of a demyelinating disease with pseudotumoral lesions]". Rev Neurol. 41 (10): 601–3. PMID 16288423.
- ↑ Jongen P (2006). "Psychiatric onset of multiple sclerosis". J Neurol Sci. 245 (1–2): 59–62. PMID 16631798.
- ↑ Paty D, Studney D, Redekop K, Lublin F. MS COSTAR: a computerized patient record adapted for clinical research purposes. Ann Neurol 1994;36 Suppl:S134-5. PMID 8017875
- ↑ Hennessey A, Robertson NP, Swingler R, Compston DA (1999). "Urinary, faecal and sexual dysfunction in patients with multiple sclerosis". J. Neurol. 246 (11): 1027–32. PMID 10631634.
- ↑ Burguera-Hernández JA (2000). "[Urinary alterations in multiple sclerosis]". Revista de neurologia (in Spanish; Castilian). 30 (10): 989–92. PMID 10919202.
- ↑ Betts CD, D'Mellow MT, Fowler CJ (1993). "Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis". J. Neurol. Neurosurg. Psychiatr. 56 (3): 245–50. PMID 8459239.
- ↑ Nour S, Svarer C, Kristensen JK, Paulson OB, Law I (2000). "Cerebral activation during micturition in normal men". Brain. 123 ( Pt 4): 781–9. PMID 10734009.
- ↑ Ayuso-Peralta L, de Andrés C (2002). "[Symptomatic treatment of multiple sclerosis]". Revista de neurologia (in Spanish; Castilian). 35 (12): 1141–53. PMID 12497297.
- ↑ Information from the USA National library of medicine on alfuzosin [1]
- ↑ Information from the USA National library of medicine on trospium [2]
- ↑ Information from the USA National library of medicine on flavoxate [3]
- ↑ Bosma R, Wynia K, Havlíková E, De Keyser J, Middel B (2005). "Efficacy of desmopressin in patients with multiple sclerosis suffering from bladder dysfunction: a meta-analysis". Acta Neurol. Scand. 112 (1): 1–5. doi:10.1111/j.1600-0404.2005.00431.x. PMID 15932348.
- ↑ Information from the USA National library of medicine on desmopressin [4]
- ↑ Frances M Diro (2006) "Urological Management in Neurological Disease". [5]
- ↑ Rao S, Leo G, Bernardin L, Unverzagt F (1991). "Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction". Neurology. 41 (5): 685–91. PMID 2027484.
- ↑ "Attention impairment in recently diagnosed multiple sclerosis". Eur J Neurol. 5 (1): 61–66. 1998. PMID 10210813.
- ↑ Achiron A, Barak Y (2003). "Cognitive impairment in probable multiple sclerosis". J Neurol Neurosurg Psychiatry. 74 (4): 443–6. PMID 12640060.
- ↑ Bobholz J, Rao S (2003). "Cognitive dysfunction in multiple sclerosis: a review of recent developments". Curr Opin Neurol. 16 (3): 283–8. PMID 12858063.
- ↑ Amato M, Ponziani G, Siracusa G, Sorbi S (2001). "Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years". Arch Neurol. 58 (10): 1602–6. PMID 11594918.
- ↑ Shawaryn M, Schultheis M, Garay E, Deluca J (2002). "Assessing functional status: exploring the relationship between the multiple sclerosis functional composite and driving". Arch Phys Med Rehabil. 83 (8): 1123–9. PMID 12161835.
- ↑ Montalban X, Rio J (2006). "Interferons and cognition". J Neurol Sci. 245 (1–2): 137–40. PMID 16626757.
- ↑ Christodoulou C, Melville P, Scherl W, Macallister W, Elkins L, Krupp L (2006). "Effects of donepezil on memory and cognition in multiple sclerosis". J Neurol Sci. 245 (1–2): 127–36. PMID 16626752.
- ↑ Porcel J, Montalban X (2006). "Anticholinesterasics in the treatment of cognitive impairment in multiple sclerosis". J Neurol Sci. 245 (1–2): 177–81. PMID 16674980.
- ↑ Information from the USA National library of medicine on donepezil [6]
- ↑ Sadovnick A, Remick R, Allen J, Swartz E, Yee I, Eisen K, Farquhar R, Hashimoto S, Hooge J, Kastrukoff L, Morrison W, Nelson J, Oger J, Paty D (1996). "Depression and multiple sclerosis". Neurology. 46 (3): 628–32. PMID 8618657.
- ↑ Patten S, Beck C, Williams J, Barbui C, Metz L (2003). "Major depression in multiple sclerosis: a population-based perspective". Neurology. 61 (11): 1524–7. PMID 14663036.
- ↑ Siegert R, Abernethy D (2005). "Depression in multiple sclerosis: a review". J. Neurol. Neurosurg. Psychiatr. 76 (4): 469–75. PMID 15774430.
- ↑ Sadovnick A, Eisen K, Ebers G, Paty D (1991). "Cause of death in patients attending multiple sclerosis clinics". Neurology. 41 (8): 1193–6. PMID 1866003.
- ↑ Bakshi R (2003). "Fatigue associated with multiple sclerosis: diagnosis, impact and management". Mult. Scler. 9 (3): 219–27. PMID 12814166.
- ↑ Mohr DC, Hart SL, Goldberg A (2003). "Effects of treatment for depression on fatigue in multiple sclerosis". Psychosomatic medicine. 65 (4): 542–7. PMID 12883103.
- ↑ 59.0 59.1 59.2 The Royal College of Physicians (2004). Multiple Sclerosis. National clinical guideline for diagnosis and management in primary and secondary care. Salisbury, Wiltshire: Sarum ColourView Group. ISBN 1 86016 182 0.Free full text(2004-08-13). Retrieved on 2007-10-01.
- ↑ Pucci E, Branãs P, D'Amico R, Giuliani G, Solari A, Taus C (2007). "Amantadine for fatigue in multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD002818. doi:10.1002/14651858.CD002818.pub2. PMID 17253480.
- ↑ Amantadine. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-07.
- ↑ Weinshenker BG, Penman M, Bass B, Ebers GC, Rice GP (1992). "A double-blind, randomized, crossover trial of pemoline in fatigue associated with multiple sclerosis". Neurology. 42 (8): 1468–71. PMID 1641137.
- ↑ Pemoline. US National Library of Medicine (Medline) (2006-01-01). Retrieved on 2007-10-07.
- ↑ Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P (2005). "Randomized controlled trial of an energy conservation course for persons with multiple sclerosis". Mult. Scler. 11 (5): 592–601. PMID 16193899.
- ↑ Matuska K, Mathiowetz V, Finlayson M (2007). "Use and perceived effectiveness of energy conservation strategies for managing multiple sclerosis fatigue". The American journal of occupational therapy. : official publication of the American Occupational Therapy Association. 61 (1): 62–9. PMID 17302106.
- ↑ Leigh RJ, Averbuch-Heller L, Tomsak RL, Remler BF, Yaniglos SS, Dell'Osso LF (1994). "Treatment of abnormal eye movements that impair vision: strategies based on current concepts of physiology and pharmacology". Ann. Neurol. 36 (2): 129–41. PMID 8053648.
- ↑ Starck M, Albrecht H, Pöllmann W, Straube A, Dieterich M (1997). "Drug therapy for acquired pendular nystagmus in multiple sclerosis". J. Neurol. 244 (1): 9–16. PMID 9007739.
- ↑ Clanet MG, Brassat D (2000). "The management of multiple sclerosis patients". Curr. Opin. Neurol. 13 (3): 263–70. PMID 10871249.
- ↑ Menon GJ, Thaller VT (2002). "Therapeutic external ophthalmoplegia with bilateral retrobulbar botulinum toxin- an effective treatment for acquired nystagmus with oscillopsia". Eye (London, England). 16 (6): 804–6. PMID 12439689.
- ↑ Jain S, Proudlock F, Constantinescu CS, Gottlob I (2002). "Combined pharmacologic and surgical approach to acquired nystagmus due to multiple sclerosis". Am. J. Ophthalmol. 134 (5): 780–2. PMID 12429265.
- ↑ Weinshenker BG, Bass B, Rice GP; et al. (1989). "The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability". Brain. 112 ( Pt 1): 133–46. PMID 2917275.
- ↑ Freeman JA (2001). "Improving mobility and functional independence in persons with multiple sclerosis". J. Neurol. 248 (4): 255–9. PMID 11374088.
- ↑ Beck RW, Trobe JD (1995). "What we have learned from the Optic Neuritis Treatment Trial". Ophthalmology. 102 (10): 1504–8. PMID 9097798.
- ↑ "The 5-year risk of MS after optic neuritis: experience of the optic neuritis treatment trial. 1997". Neurology. 57 (12 Suppl 5): S36–45. 2001. PMID 11902594.
- ↑ Stenager E, Knudsen L, Jensen K (1995). "Acute and chronic pain syndromes in multiple sclerosis. A 5-year follow-up study". Italian journal of neurological sciences. 16 (9): 629–32. PMID 8838789.
- ↑ Archibald CJ, McGrath PJ, Ritvo PG; et al. (1994). "Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients". Pain. 58 (1): 89–93. PMID 7970843.
- ↑ Clanet MG, Brassat D (2000). "The management of multiple sclerosis patients". Curr. Opin. Neurol. 13 (3): 263–70. PMID 10871249.
- ↑ Pöllmann W, Feneberg W, Erasmus LP (2004). "[Pain in multiple sclerosis--a still underestimated problem. The 1 year prevalence of pain syndromes, significance and quality of care of multiple sclerosis inpatients]". Der Nervenarzt (in German). 75 (2): 135–40. doi:10.1007/s00115-003-1656-5. PMID 14770283.
- ↑ Kerns RD, Kassirer M, Otis J (2002). "Pain in multiple sclerosis: a biopsychosocial perspective". Journal of rehabilitation research and development. 39 (2): 225–32. PMID 12051466.
- ↑ Brisman R (1987). "Trigeminal neuralgia and multiple sclerosis". Arch. Neurol. 44 (4): 379–81. PMID 3493757.
- ↑ Bayer DB, Stenger TG (1979). "Trigeminal neuralgia: an overview". Oral Surg. Oral Med. Oral Pathol. 48 (5): 393–9. PMID 226915.
- ↑ Information from the USA National library of medicine on carbamazepine [7]
- ↑ Information from the USA National library of medicine on phenytoin [8]
- ↑ Information from the USA National library of medicine on gabapentin [9]
- ↑ Solaro C, Messmer Uccelli M, Uccelli A, Leandri M, Mancardi GL (2000). "Low-dose gabapentin combined with either lamotrigine or carbamazepine can be useful therapies for trigeminal neuralgia in multiple sclerosis". Eur. Neurol. 44 (1): 45–8. PMID 10894995.
- ↑ Kondziolka D, Lunsford LD, Bissonette DJ (1994). "Long-term results after glycerol rhizotomy for multiple sclerosis-related trigeminal neuralgia". The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 21 (2): 137–40. PMID 8087740.
- ↑ Athanasiou TC, Patel NK, Renowden SA, Coakham HB (2005). "Some patients with multiple sclerosis have neurovascular compression causing their trigeminal neuralgia and can be treated effectively with MVD: report of five cases". British journal of neurosurgery. 19 (6): 463–8. doi:10.1080/02688690500495067. PMID 16574557.
- ↑ Eldridge PR, Sinha AK, Javadpour M, Littlechild P, Varma TR (2003). "Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis". Stereotactic and functional neurosurgery. 81 (1–4): 57–64. doi:10.1159/000075105. PMID 14742965.
- ↑ Gutrecht JA, Zamani AA, Slagado ED (1993). "Anatomic-radiologic basis of Lhermitte's sign in multiple sclerosis". Arch. Neurol. 50 (8): 849–51. PMID 8352672.
- ↑ Al-Araji AH, Oger J (2005). "Reappraisal of Lhermitte's sign in multiple sclerosis". Mult. Scler. 11 (4): 398–402. PMID 16042221.
- ↑ Sandyk R, Dann LC (1995). "Resolution of Lhermitte's sign in multiple sclerosis by treatment with weak electromagnetic fields". Int. J. Neurosci. 81 (3–4): 215–24. PMID 7628912.
- ↑ Kanchandani R, Howe JG (1982). "Lhermitte's sign in multiple sclerosis: a clinical survey and review of the literature". J. Neurol. Neurosurg. Psychiatr. 45 (4): 308–12. PMID 7077340.
- ↑ Information from the USA National library of medicine on clonazepam[10]
- ↑ Information from the USA National library of medicine on amitriptyline[11]
- ↑ Moulin DE, Foley KM, Ebers GC (1988). "Pain syndromes in multiple sclerosis". Neurology. 38 (12): 1830–4. PMID 2973568.
- ↑ O'Leary, M., Heyman, R., Erickson, J., Chancellor, M.B.: Premature ejaculation and MS: A Review, Consortium of MS Centers, http://www.mscare.org, June 2007
- ↑ Cardini RG, Crippa AC, Cattaneo D (2000). "Update on multiple sclerosis rehabilitation". J. Neurovirol. 6 Suppl 2: S179–85. PMID 10871810.
- ↑ ; Baclofen oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.
- ↑ Dantrolene oral. US National Library of Medicine (Medline) (2003-04-01). Retrieved on 2007-10-17.
- ↑ Diazepam. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.
- ↑ Tizanidine. US National Library of Medicine (Medline) (2005-07-01). Retrieved on 2007-10-17.
- ↑ Beard S, Hunn A, Wight J (2003). "Treatments for spasticity and pain in multiple sclerosis: a systematic review". Health technology assessment (Winchester, England). 7 (40): iii, ix–x, 1–111. PMID 14636486.
- ↑ Paisley S, Beard S, Hunn A, Wight J (2002). "Clinical effectiveness of oral treatments for spasticity in multiple sclerosis: a systematic review". Mult. Scler. 8 (4): 319–29. PMID 12166503.
- ↑ Becker WJ, Harris CJ, Long ML, Ablett DP, Klein GM, DeForge DA (1995). "Long-term intrathecal baclofen therapy in patients with intractable spasticity". The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 22 (3): 208–17. PMID 8529173.
- ↑ Koch M, Mostert J, Heersema D, De Keyser J (2007). "Tremor in multiple sclerosis". J. Neurol. 254 (2): 133–45. doi:10.1007/s00415-006-0296-7. PMID 17318714.
- ↑ Bozek CB, Kastrukoff LF, Wright JM, Perry TL, Larsen TA (1987). "A controlled trial of isoniazid therapy for action tremor in multiple sclerosis". J. Neurol. 234 (1): 36–9. PMID 3546605.
- ↑ Duquette P, Pleines J, du Souich P (1985). "Isoniazid for tremor in multiple sclerosis: a controlled trial". Neurology. 35 (12): 1772–5. PMID 3906430.
- ↑ Hallett M, Lindsey JW, Adelstein BD, Riley PO (1985). "Controlled trial of isoniazid therapy for severe postural cerebellar tremor in multiple sclerosis". Neurology. 35 (9): 1374–7. PMID 3895037.
- ↑ Information from the USA National library of medicine on Isoniazid [12]
- ↑ Information from the USA National library of medicine on carbamazepine [13]
- ↑ Koller WC (1984). "Pharmacologic trials in the treatment of cerebellar tremor". Arch. Neurol. 41 (3): 280–1. PMID 6365047.
- ↑ Sechi GP, Zuddas M, Piredda M, Agnetti V, Sau G, Piras ML, Tanca S, Rosati G (1989). "Treatment of cerebellar tremors with carbamazepine: a controlled trial with long-term follow-up". Neurology. 39 (8): 1113–5. PMID 2668787.
- ↑ Information from the USA National library of medicine on propanolol [14]
- ↑ Aisen ML, Holzer M, Rosen M, Dietz M, McDowell F (1991). "Glutethimide treatment of disabling action tremor in patients with multiple sclerosis and traumatic brain injury". Arch. Neurol. 48 (5): 513–5. PMID 2021365.
- ↑ Mills RJ, Yap L, Young CA (2007). "Treatment for ataxia in multiple sclerosis". Cochrane database of systematic reviews (Online) (1): CD005029. doi:10.1002/14651858.CD005029.pub2. PMID 17253537.
- ↑ Aisen ML, Arnold A, Baiges I, Maxwell S, Rosen M (1993). "The effect of mechanical damping loads on disabling action tremor". Neurology. 43 (7): 1346–50. PMID 8327136.
- ↑ Bittar RG, Hyam J, Nandi D, Wang S, Liu X, Joint C, Bain PG, Gregory R, Stein J, Aziz TZ (2005). "Thalamotomy versus thalamic stimulation for multiple sclerosis tremor". Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 12 (6): 638–42. doi:10.1016/j.jocn.2004.09.008. PMID 16098758.
- ↑ Confavreux C (2002). "Infections and the risk of relapse in multiple sclerosis". Brain. 125 (Pt 5): 933–4. PMID 11960883.
- ↑ Buljevac D, Hop WC, Reedeker W; et al. (2003). "Self reported stressful life events and exacerbations in multiple sclerosis: prospective study". BMJ. 327 (7416): 646. doi:10.1136/bmj.327.7416.646. PMID 14500435.
- ↑ Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD (2006). "Relationship between stress and relapse in multiple sclerosis: Part I. Important features". Mult. Scler. 12 (4): 453–64. PMID 16900759.
- ↑ Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD (2006). "Relationship between stress and relapse in multiple sclerosis: Part II. Direct and indirect relationships". Mult. Scler. 12 (4): 465–75. PMID 16900760.
- ↑ Martinelli V (2000). "Trauma, stress and multiple sclerosis". Neurol. Sci. 21 (4 suppl 2): S849–52. PMID 11205361.
- ↑ Tataru N, Vidal C, Decavel P, Berger E, Rumbach L (2006). "Limited impact of the summer heat wave in France (2003) on hospital admissions and relapses for multiple sclerosis". Neuroepidemiology. 27 (1): 28–32. doi:10.1159/000094233. PMID 16804331.
- ↑ Worthington J, Jones R, Crawford M, Forti A (1994). "Pregnancy and multiple sclerosis--a 3-year prospective study". J. Neurol. 241 (4): 228–33. PMID 8195822.
- ↑ Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S (2001). "Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group". N. Engl. J. Med. 344 (5): 319–26. PMID 11172162.
- ↑ Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol 1994;36 Suppl:S6–11. PMID 8017890
- ↑ Stern M (2005). "Aging with multiple sclerosis". Physical medicine and rehabilitation clinics of North America. 16 (1): 219–34. PMID 15561552.
- ↑ Pittock SJ; McClelland RL; Mayr WT; Jorgensen NW; Weinshenker BG; Noseworthy J; Rodriguez M. Clinical implications of benign multiple sclerosis: a 20-year population-based follow-up study Ann Neurol 2004 Aug;56(2):303-6. PMID 15293286
- ↑ Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003 Jul 10;349(2):139-45. PMID 12853586
See also
- MS fundraisers include the MS Challenge Walk, MS Walk and MS Bike Tour.
- List of multiple sclerosis organizations
Template:Diseases of the nervous system
ar:تصلب الأنسجة المتعدد bg:Множествена склероза ca:Esclerosi múltiple cs:Roztroušená skleróza de:Multiple Sklerose el:Πολλαπλή σκλήρυνση eo:Sklerozo multiloka fa:اسکلروز چندگانه id:Sklerosis ganda it:Sclerosi multipla he:טרשת נפוצה hu:Sclerosis multiplex nl:Multiple sclerose no:Multippel sklerose sq:Multiple skleroza sr:Multipla skleroza fi:MS-tauti sv:Multipel skleros