Dantrolene
{{DrugProjectFormSinglePage |authorTag=Rabin Bista, M.B.B.S. [1] |genericName=Dantrolene sodium |aOrAn=a |drugClass=skeletal muscle relaxant |indicationType=treatment |indication=clinical spasticity resulting from upper motor neuron disorders like spinal cord injury, stroke, cerebral palsy, or multiple sclerosis, and malignant hyperthermia |hasBlackBoxWarning=Yes |adverseReactions=drowsiness, dizziness, weakness, general malaise, fatigue, and diarrhea |blackBoxWarningTitle=BOXED WARNING:
|blackBoxWarningBody=
- Dantrium (dantrolene sodium) has a potential for hepatotoxicity, and should not be used in conditions other than those recommended. Symptomatic hepatitis (fatal and non-fatal) has been reported at various dose levels of the drug. The incidence reported in patients taking up to 400 mg/day is much lower than in those taking doses of 800 mg or more per day. Even sporadic short courses of these higher dose levels within a treatment regimen markedly increased the risk of serious hepatic injury. Liver dysfunction as evidenced by blood chemical abnormalities alone (liver enzyme elevations) has been observed in patients exposed to Dantrium for varying periods of time. Overt hepatitis has occurred at varying intervals after initiation of therapy, but has been most frequently observed between the third and twelfth month of therapy. The risk of hepatic injury appears to be greater in females, in patients over 35 years of age, and in patients taking other medication(s) in addition to Dantrium (dantrolene sodium). Spontaneous reports suggest a higher proportion of hepatic events with fatal outcome in elderly patients receiving Dantrium. However, the majority of these cases were complicated with confounding factors such as intercurrent illnesses and/or concomitant potentially hepatotoxic medications. Dantrium should be used only in conjunction with appropriate monitoring of hepatic function including frequent determination of SGOT or SGPT. If no observable benefit is derived from the administration of Dantrium after a total of 45 days, therapy should be discontinued. The lowest possible effective dose for the individual patient should be prescribed.
|fdaLIADAdult=====Indications====
Oral Dantrium
- Dantrium is indicated in controlling the manifestations of clinical spasticity resulting from upper motor neuron disorders like :
- It is of particular benefit to the patient whose functional rehabilitation has been retarded by the sequelae of spasticity. Such patients must have presumably reversible spasticity where relief of spasticity will aid in restoring residual function. Dantrium is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
- If improvement occurs, it will ordinarily occur within the dosage titration, and will be manifested by a decrease in the severity of spasticity and the ability to resume a daily function not quite attainable without Dantrium.
- Occasionally, subtle but meaningful improvement in spasticity may occur with Dantrium therapy. In such instances, information regarding improvement should be solicited from the patient and those who are in constant daily contact and attendance with him. Brief withdrawal of Dantrium for a period of 2 to 4 days will frequently demonstrate exacerbation of the manifestations of spasticity and may serve to confirm a clinical impression.
- A decision to continue the administration of Dantrium on a long-term basis is justified if introduction of the drug into the patient's regimen:
- produces a significant reduction in painful and/or disabling spasticity such as clonus, or
- permits a significant reduction in the intensity and/or degree of nursing care required, or
- rids the patient of any annoying manifestation of spasticity considered important by the patient himself.
- Oral Dantrium is also indicated preoperatively to prevent or attenuate the development of signs of malignant hyperthermia in known, or strongly suspect, malignant hyperthermia susceptible patients who require anesthesia and/or surgery. Currently accepted clinical practices in the management of such patients must still be adhered to (careful monitoring for early signs of malignant hyperthermia, minimizing exposure to triggering mechanisms and prompt use of intravenous dantrolene sodium and indicated supportive measures should signs of malignant hyperthermia appear)
- Oral Dantrium should be administered following a malignant hyperthermic crisis to prevent recurrence of the signs of malignant hyperthermia.
Dantrium Intravenous
- [[malignant hyperthermia crises]]
- Dantrium Intravenous is indicated, along with appropriate supportive measures, for the management of the fulminant hypermetabolism of skeletal muscle characteristic of malignant hyperthermia crises in patients of all ages. Dantrium Intravenous should be administered by continuous rapid intravenous push as soon as the malignant hyperthermia reaction is recognized (i.e., tachycardia, tachypnea, central venous desaturation, hypercarbia, metabolic acidosis, skeletal muscle rigidity, increased utilization of anesthesia circuit carbon dioxide absorber, cyanosis and mottling of the skin, and, in many cases, fever).
- Dantrium Intravenous is also indicated preoperatively, and sometimes postoperatively, to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia in individuals judged to be malignant hyperthermia susceptible.
Dosage
For Use in Chronic Spasticity
- Prior to the administration of Dantrium, consideration should be given to the potential response to treatment. A decrease in spasticity sufficient to allow a daily function not otherwise attainable should be the therapeutic goal of treatment with Dantrium.
- It is important to establish a therapeutic goal (regain and maintain a specific function such as therapeutic exercise program, utilization of braces, transfer maneuvers, etc.) before beginning Dantrium therapy. Dosage should be increased until the maximum performance compatible with the dysfunction due to underlying disease is achieved. No further increase in dosage is then indicated.
Usual Dosage
- It is important that the dosage be titrated and individualized for maximum effect. The lowest dose compatible with optimal response is recommended.
- In view of the potential for liver damage in long-term Dantrium use, therapy should be stopped if benefits are not evident within 45 days.
Adults
- The following gradual titration schedule is suggested. Some patients will not respond until higher daily dosage is achieved. Each dosage level should be maintained for seven days to determine the patient's response. If no further benefit is observed at the next higher dose, dosage should be decreased to the previous lower dose.
- 25 mg once daily for seven days, then
- 25 mg t.i.d. for seven days
- 50 mg t.i.d. for seven days
- 100 mg t.i.d.
- Therapy with a dose four times daily may be necessary for some individuals. Doses higher than 100 mg four times daily should not be used.
For Malignant Hyperthermia
Preoperatively
- Administer 4 to 8 mg/kg/day of oral Dantrium in 3 or 4 divided doses for one or two days prior to surgery, with the last dose being given approximately 3 to 4 hours before scheduled surgery with a minimum of water.
- This dosage will usually be associated with skeletal muscle weakness and sedation (sleepiness or drowsiness); adjustment can usually be made within the recommended dosage range to avoid incapacitation or excessive gastrointestinal irritation (including nausea and/or vomiting).
Post Crisis Follow-up
- Oral Dantrium should also be administered following a malignant hyperthermia crisis, in doses of 4 to 8 mg/kg per day in four divided doses, for a one to three day period to prevent recurrence of the manifestations of malignant hyperthermia.
Dantrium Intravenous for Malignant Hyperthermia
- As soon as the malignant hyperthermia reaction is recognized, all anesthetic agents should be discontinued; the administration of 100% oxygen is recommended. Dantrium Intravenous should be administered by continuous rapid intravenous push beginning at a minimum dose of 1 mg/kg, and continuing until symptoms subside or the maximum cumulative dose of 10 mg/kg has been reached
- If the physiologic and metabolic abnormalities reappear, the regimen may be repeated. It is important to note that administration of Dantrium Intravenous should be continuous until symptoms subside. The effective dose to reverse the crisis is directly dependent upon the individual's degree of susceptibility to malignant hyperthermia, the amount and time of exposure to the triggering agent, and the time elapsed between onset of the crisis and initiation of treatment.
Preoperatively
- Dantrium Intravenous and/or Dantrium Capsules may be administered preoperatively to patients judged malignant hyperthermia susceptible as part of the overall patient management to prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia.
Dantrium Intravenous
- The recommended prophylactic dose of Dantrium Intravenous is 2.5 mg/kg, starting approximately 1-1/4 hours before anticipated anesthesia and infused over approximately 1 hour. This dose should prevent or attenuate the development of clinical and laboratory signs of malignant hyperthermia provided that the usual precautions, such as avoidance of established malignant hyperthermia triggering agents, are followed.
- Additional Dantrium Intravenous may be indicated during anesthesia and surgery because of the appearance of early clinical and/or blood gas signs of malignant hyperthermia or because of prolonged surgery.Additional doses must be individualized.
Post Crisis Follow-Up
- Intravenous Dantrium may be used postoperatively to prevent or attenuate the recurrence of signs of malignant hyperthermia when oral Dantrium administration is not practical. The i.v. dose of Dantrium in the postoperative period must be individualized, starting with 1 mg/kg or more as the clinical situation dictates.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Dantrolene in adult patients. |offLabelAdultNoGuideSupport=* Neuroleptic malignant syndrome[1][2][3]
|fdaLIADPed======Chronic Spasticity=====
- The following gradual titration schedule is suggested. Some patients will not respond until higher daily dosage is achieved. Each dosage level should be maintained for seven days to determine the patient's response. If no further benefit is observed at the next higher dose, dosage should be decreased to the previous lower dose.
- 0.5 mg/kg once daily for seven days, then
- 0.5 mg/kg t.i.d. for seven days
- 1 mg/kg t.i.d. for seven days
- 2 mg/kg t.i.d.
- Therapy with a dose four times daily may be necessary for some individuals. Doses higher than 100 mg four times daily should not be used.
Malignant Hyperthermia
- Experience to date indicates that the dose of Dantrium Intravenous for pediatric patients is the same as for adults.
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Dantrolene in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Dantrolene in pediatric patients.
|contraindications=* Active hepatic disease
- Dantrium is contraindicated where spasticity is utilized to sustain upright posture and balance in locomotion or whenever spasticity is utilized to obtain or maintain increased function.
|warnings=* It is important to recognize that fatal and non-fatal liver disorders of an idiosyncratic or hypersensitivity type may occur with Dantrium therapy.
- At the start of Dantrium therapy, it is desirable to do liver function studies (SGOT, SGPT, alkaline phosphatase, total bilirubin) for a baseline or to establish whether there is pre-existing liver disease. If baseline liver abnormalities exist and are confirmed, there is a clear possibility that the potential for Dantrium hepatotoxicity could be enhanced, although such a possibility has not yet been established.
- Liver function studies (e.g., SGOT or SGPT) should be performed at appropriate intervals during Dantrium therapy. If such studies reveal abnormal values, therapy should generally be discontinued. Only where benefits of the drug have been of major importance to the patient, should reinitiation or continuation of therapy be considered. Some patients have revealed a return to normal laboratory values in the face of continued therapy while others have not.
- If symptoms compatible with hepatitis, accompanied by abnormalities in liver function tests or jaundice appear, Dantrium should be discontinued. If caused by Dantrium and detected early, the abnormalities in liver function characteristically have reverted to normal when the drug was discontinued.
- Dantrium therapy has been reinstituted in a few patients who have developed clinical and/or laboratory evidence of hepatocellular injury. If such reinstitution of therapy is done, it should be attempted only in patients who clearly need Dantrium and only after previous symptoms and laboratory abnormalities have cleared. The patient should be hospitalized and the drug should be restarted in very small and gradually increasing doses. Laboratory monitoring should be frequent and the drug should be withdrawn immediately if there is any indication of recurrent liver involvement. Some patients have reacted with unmistakable signs of liver abnormality upon administration of a challenge dose, while others have not.
- Dantrium should be used with particular caution in females and in patients over 35 years of age in view of apparent greater likelihood of drug-induced, potentially fatal, hepatocellular disease in these groups. Spontaneous reports suggest a higher proportion of hepatic events with fatal outcome in elderly patients receiving Dantrium. However, the majority of these cases were complicated with confounding factors such as intercurrent illnesses and/or concomitant potentially hepatotoxic medications
- The use of Dantrium Intravenous in the management of malignant hyperthermia crisis is not a substitute for previously known supportive measures. These measures must be individualized, but it will usually be necessary to discontinue the suspect triggering agents, attend to increased oxygen requirements, manage the metabolic acidosis, institute cooling when necessary, monitor urinary output, and monitor for electrolyte imbalance.
- Since the effect of disease state and other drugs on Dantrium related skeletal muscle weakness, including possible respiratory depression, cannot be predicted, patients who receive i.v. Dantrium preoperatively should have vital signs monitored.
- If patients judged malignant hyperthermia susceptible are administered intravenous or oral Dantrium preoperatively, anesthetic preparation must still follow a standard malignant hyperthermia susceptible regimen, including the avoidance of known triggering agents. Monitoring for early clinical and metabolic signs of malignant hyperthermia is indicated because attenuation of malignant hyperthermia, rather than prevention, is possible. These signs usually call for the administration of additional i.v. dantrolene.
Precautions
- Dantrium should be used with caution in patients with impaired pulmonary function, particularly those with obstructive pulmonary disease, and in patients with severely impaired cardiac function due to myocardial disease. Dantrium is associated with pleural effusion with associated eosinophilia. It should be used with caution in patients with a history of previous liver disease or dysfunction
- Dantrium might possibly evoke a photosensitivity reaction; patients should be cautioned about exposure to sunlight while taking it.
General
- Care must be taken to prevent extravasation of Dantrium solution into the surrounding tissues due to the high pH of the intravenous formulation and potential for tissue necrosis.
- When mannitol is used for prevention or treatment of late renal complications of malignant hyperthermia, the 3 g of mannitol needed to dissolve each 20 mg vial of i.v. Dantrium should be taken into consideration.
Hepatotoxicity seen with Dantrium Capsules
- Dantrium (dantrolene sodium) has a potential for hepatotoxicity, and should not be used in conditions other than those recommended. Symptomatic hepatitis (fatal and non-fatal) has been reported at various dose levels of the drug. The incidence reported in patients taking up to 400 mg/day is much lower than in those taking doses of 800 mg or more per day. Even sporadic short courses of these higher dose levels within a treatment regimen markedly increased the risk of serious hepatic injury. Liver dysfunction as evidenced by blood chemical abnormalities alone (liver enzyme elevations) has been observed in patients exposed to Dantrium for varying periods of time. Overt hepatitis has occurred at varying intervals after initiation of therapy, but has been most frequently observed between the third and twelfth month of therapy. The risk of hepatic injury appears to be greater in females, in patients over 35 years of age, and in patients taking other medication(s) in addition to Dantrium (dantrolene sodium). Dantrium should be used only in conjunction with appropriate monitoring of hepatic function including frequent determination of SGOT or SGPT.
- Fatal and non-fatal liver disorders of an idiosyncratic or hypersensitivity type may occur with Dantrium therapy.
|clinicalTrials======Body as a Whole=====
- These are generally transient, occurring early in treatment, and can often be obviated by beginning with a low dose and increasing dosage gradually until an optimal regimen is established. Diarrhea may be severe and may necessitate temporary withdrawal of Dantrium therapy. If diarrhea recurs upon readministration of Dantrium, therapy should probably be withdrawn permanently.
- Other less frequent side effects, listed according to system, are:
Cardiovascular
Digestive
- Constipation, rarely progressing to signs of intestinal obstruction
- GI bleeding
- anorexia
- swallowing difficulty
- gastric irritation
- abdominal cramps
- nausea
- vomiting
Hepatobiliary
Hematologic and Lymphatic
Musculoskeletal
Neurologic
- Speech disturbance
- seizure
- headache
- light-headedness
- visual disturbance
- diplopia
- alteration of taste
- insomnia
- drooling
Psychiatric
Respiratory
- Feeling of suffocation
- respiratory depression
Integumentary
- Abnormal hair growth
- acne-like rash
- pruritus
- urticaria
- eczematoid eruption
- sweating
Hypersensitivity
- Pleural effusion with pericarditis
- Pleural effusion with associated eosinophilia
- anaphylaxis
Special Senses
- Excessive tearing
Urogenital
- Increased urinary frequency
- crystalluria
- hematuria
- difficult erection
- urinary incontinence
- nocturia
- difficult urination
- urinary retention
Miscellaneous
IV Dantrolene
- There have been occasional reports of death following malignant hyperthermia crisis even when treated with intravenous dantrolene; incidence figures are not available (the pre-dantrolene mortality of malignant hyperthermia crisis was approximately 50%). Most of these deaths can be accounted for by late recognition, delayed treatment, inadequate dosage, lack of supportive therapy, intercurrent disease and/or the development of delayed complications such as renal failure or disseminated intravascular coagulopathy. In some cases there are insufficient data to completely rule out therapeutic failure of dantrolene.
- There are reports of fatality in malignant hyperthermia crisis, despite initial satisfactory response to i.v. dantrolene, which involve patients who could not be weaned from dantrolene after initial treatment.
- The administration of intravenous Dantrium to human volunteers is associated with loss of grip strength and weakness in the legs, as well as drowsiness and dizziness.
- The following adverse reactions are in approximate order of severity:
- There are rare reports of pulmonary edema developing during the treatment of malignant hyperthermia crisis in which the diluent volume and mannitol needed to deliver i.v. dantrolene possibly contributed.
- There have been reports of thrombophlebitis following administration of intravenous dantrolene; actual incidence figures are not available. Tissue necrosis secondary to extravasation has been reported.
- There have been rare reports of urticaria and erythema possibly associated with the administration of i.v. Dantrium. There has been one case of anaphylaxis.
- Injection site reactions (pain, erythema, swelling), commonly due to extravasation, have been reported.
- None of the serious reactions occasionally reported with long-term oral Dantrium use, such as hepatitis, seizures, and pleural effusion with pericarditis, have been reasonably associated with short-term Dantrium Intravenous therapy.
|postmarketing=There is limited information regarding Postmarketing Experience of Dantrolene in the drug label. |drugInteractions=* CNS Depressants
- Drowsiness may occur with Dantrium therapy, and the concomitant administration of CNS depressants such as sedatives and tranquilizing agents may result in further drowsiness.
- While a definite drug interaction with estrogen therapy has not yet been established, caution should be observed if the two drugs are to be given concomitantly. Hepatotoxicity has occurred more often in women over 35 years of age receiving concomitant estrogen therapy.
- Cardiovascular collapse in patients treated simultaneously with verapamil and dantrolene sodium is rare. The combination of therapeutic doses of intravenous dantrolene sodium and verapamil in halothane/α-chloralose anesthetized swine has resulted in ventricular fibrillation and cardiovascular collapse in association with marked hyperkalemia. Until the relevance of these findings to humans is established, the combination of dantrolene sodium and calcium channel blockers is not recommended during the management of malignant hyperthermia.
- Administration of Dantrium may potentiate vecuronium-induced neuromuscular block.
- Dantrium is metabolized by the liver, and it is theoretically possible that its metabolism may be enhanced by drugs known to induce hepatic microsomal enzymes. However, neither phenobarbital nor diazepam appears to affect Dantrium metabolism. Binding to plasma protein is not significantly altered by diazepam, diphenylhydantoin, or phenylbutazone. Binding to plasma proteins is reduced by warfarin and clofibrate and increased by tolbutamide.
|FDAPregCat=C |useInPregnancyFDA=* Adequate animal reproduction studies have not been conducted with Dantrium. It is also not known whether Dantrium can cause fatal harm when administered to a pregnant woman or can affect reproduction capacity. Dantrium should be given to a pregnant woman only if clearly needed. |useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dantrolene in women who are pregnant. |useInLaborDelivery=* In one non-randomized open-label study, 21 term pregnant patients received prophylactic oral Dantrium 100 mg per day for 2 to 10 days prior to delivery. Dantrolene readily crossed the placenta with maternal and fetal whole blood levels approximately equal at delivery; neonatal levels then fell approximately 50% per day for 2 days before declining sharply. No neonatal respiratory and neuromuscular side effects were detected at low dose. More data, at higher doses, are needed before more definitive conclusions can be made. |useInNursing=* Dantrolene has been detected in human milk at low concentrations (less than 2 micrograms per mL) during repeat intravenous administration over 3 days. Because of the potential for serious adverse reactions in nursing infants from dantrolene, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=* The long-term safety of Dantrium in pediatric patients under the age of 5 years has not been established. Because of the possibility that adverse effects of the drug could become apparent only after many years, a benefit-risk consideration of the long-term use of Dantrium is particularly important in pediatric patients. |useInGeri=* Clinical studies of Dantrium did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience in the literature has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. As with all patients receiving Dantrium, it is recommended that elderly patients receive the lowest dose compatible with the optimal response. Spontaneous reports suggest a higher proportion of hepatic events with fatal outcome in elderly patients receiving Dantrium. However, the majority of these cases were complicated with confounding factors such as intercurrent illnesses and/or concomitant potentially hepatotoxic medications |useInGender=There is no FDA guidance on the use of Dantrolene with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Dantrolene with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of Dantrolene in patients with renal impairment. |useInHepaticImpair=There is no FDA guidance on the use of Dantrolene in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of Dantrolene in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Dantrolene in patients who are immunocompromised.
|administration=* Oral
- Intravenous
Preaparation
- Each vial of Dantrium Intravenous should be reconstituted by adding 60 mL of sterile water for injection USP (without a bacteriostatic agent), and the vial shaken until the solution is clear. 5% Dextrose Injection USP, 0.9% Sodium Chloride Injection USP, and other acidic solutions are not compatible with Dantrium Intravenous and should not be used. The contents of the vial must be protected from direct light and used within 6 hours after reconstitution. Store reconstituted solutions between 15° to 30°C (59° to 86°F).
- Reconstituted Dantrium Intravenous should not be transferred to large glass bottles for prophylactic infusion due to precipitate formation observed with the use of some glass bottles as reservoirs.
- For prophylactic infusion, the required number of individual vials of Dantrium Intravenous should be reconstituted as outlined above. The contents of individual vials are then transferred to a larger volume sterile intravenous plastic bag. Stability data on file at JHP Pharmaceuticals indicate commercially available sterile plastic bags are acceptable drug delivery devices. However, it is recommended that the prepared infusion be inspected carefully for cloudiness and/or precipitation prior to dispensing and administration. Such solutions should not be used. While stable for 6 hours, it is recommended that the infusion be prepared immediately prior to the anticipated dosage administration time.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
|monitoring=* Liver function studies (e.g., SGOT or SGPT) should be performed at appropriate intervals during Dantrium therapy. If such studies reveal abnormal values, therapy should generally be discontinued. |IVCompat=There is limited information regarding IV Compatibility of Dantrolene in the drug label.
|overdose=* Symptoms which may occur in case of overdose include, but are not limited to, muscular weakness and alterations in the state of consciousness (e.g. lethargy, coma), vomiting, diarrhea, and crystalluria. For acute overdose, general supportive measures should be employed along with immediate gastric lavage.
- Intravenous fluids should be administered in fairly large quantities to avert the possibility of crystalluria. An adequate airway should be maintained and artificial resuscitation equipment should be at hand. Electrocardiographic monitoring should be instituted, and the patient carefully observed. To date, no experience has been reported with dialysis and its value in Dantrium overdose is not known.
|drugBox={{Drugbox2
| Verifiedfields = changed
| verifiedrevid = 459436942
| IUPAC_name = 1-{[5-(4-nitrophenyl)-2-furyl]methylideneamino}
imidazolidine-2,4-dione
| image = Dantrolene Wiki str.png
| tradename = Dantrium | Drugs.com = Monograph | pregnancy_US = C | legal_status = | routes_of_administration = Oral, intravenous
| bioavailability = 70% | protein_bound = | metabolism = Liver | elimination_half-life = | excretion = Biliary, renal
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 7261-97-4
| ATC_prefix = M03
| ATC_suffix = CA01
| ATC_supplemental =
| PubChem = 2952
| IUPHAR_ligand = 4172
| DrugBank_Ref =
| DrugBank = DB01219
| ChemSpiderID_Ref =
| ChemSpiderID = 2847
| UNII_Ref =
| UNII = F64QU97QCR
| KEGG_Ref =
| KEGG = D02347
| ChEBI_Ref =
| ChEBI = 4317
| ChEMBL_Ref =
| ChEMBL = 1201288
| C=14 | H=10 | N=4 | O=5
| molecular_weight = 314.253 g/mol
| smiles = [O-][N+](=O)c3ccc(c2oc(C=NN1C(=O)NC(=O)C1)cc2)cc3
| InChI = 1/C14H10N4O5/c19-13-8-17(14(20)16-13)15-7-11-5-6-12(23-11)9-1-3-10(4-2-9)18(21)22/h1-7H,8H2,(H,16,19,20)
| InChIKey = OZOMQRBLCMDCEG-UHFFFAOYAA
| StdInChI_Ref =
| StdInChI = 1S/C14H10N4O5/c19-13-8-17(14(20)16-13)15-7-11-5-6-12(23-11)9-1-3-10(4-2-9)18(21)22/h1-7H,8H2,(H,16,19,20)
| StdInChIKey_Ref =
| StdInChIKey = OZOMQRBLCMDCEG-UHFFFAOYSA-N
}}
|mechAction=* In skeletal muscle, Dantrium dissociates the excitation-contraction coupling, probably by interfering with the release of Ca++ from the sarcoplasmic reticulum. This effect appears to be more pronounced in fast muscle fibers as compared to slow ones, but generally affects both.
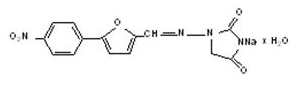
|structure=* The chemical formula of Dantrium (dantrolene sodium) is hydrated 1-[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2, 4-imidazolidinedione sodium salt. It is an orange powder, slightly soluble in water, but due to its slightly acidic nature the solubility increases somewhat in alkaline solution. The anhydrous salt has a molecular weight of 336. The hydrated salt contains approximately 15% water (3-1/2 moles) and has a molecular weight of 399. The structural formula for the hydrated salt is:
|PD=There is limited information regarding Pharmacodynamics of Dantrolene in the drug label.
|PK=* In isolated nerve-muscle preparation, Dantrium has been shown to produce relaxation by affecting the contractile response of the muscle at a site beyond the myoneural junction. In skeletal muscle, Dantrium dissociates the excitation-contraction coupling, probably by interfering with the release of Ca++ from the sarcoplasmic reticulum. The administration of intravenous Dantrium to human volunteers is associated with loss of grip strength and weakness in the legs, as well as subjective CNS complaints. Information concerning the passage of Dantrium across the blood-brain barrier is not available.
- In the anesthetic-induced malignant hyperthermia syndrome, evidence points to an intrinsic abnormality of skeletal muscle tissue. In affected humans, it has been postulated that "triggering agents" (e.g., general anesthetics and depolarizing neuromuscular blocking agents) produce a change within the cell which results in an elevated myoplasmic calcium. This elevated myoplasmic calcium activates acute cellular catabolic processes that cascade to the malignant hyperthermia crisis.
- It is hypothesized that addition of Dantrium to the "triggered" malignant hyperthermic muscle cell reestablishes a normal level of ionized calcium in the myoplasm. Inhibition of calcium release from the sarcoplasmic reticulum by Dantrium reestablishes the myoplasmic calcium equilibrium, increasing the percentage of bound calcium. In this way, physiologic, metabolic, and biochemical changes associated with the malignant hyperthermia crisis may be reversed or attenuated. Experimental results in malignant hyperthermia susceptible swine show that prophylactic administration of intravenous or oral dantrolene prevents or attenuates the development of vital sign and blood gas changes characteristic of malignant hyperthermia in a dose related manner. The efficacy of intravenous dantrolene in the treatment of human and porcine malignant hyperthermia crisis, when considered along with prophylactic experiments in malignant hyperthermia susceptible swine, lends support to prophylactic use of oral or intravenous dantrolene in malignant hyperthermia susceptible humans. When prophylactic intravenous dantrolene is administered as directed, whole blood concentrations remain at a near steady state level for 3 or more hours after the infusion is completed. Clinical experience has shown that early vital sign and/or blood gas changes characteristic of malignant hyperthermia may appear during or after anesthesia and surgery despite the prophylactic use of dantrolene and adherence to currently accepted patient management practices. These signs are compatible with attenuated malignant hyperthermia and respond to the administration of additional i.v. dantrolene. The administration of the recommended prophylactic dose of intravenous dantrolene to healthy volunteers was not associated with clinically significant cardiorespiratory changes.
- Specific metabolic pathways for the degradation and elimination of Dantrium in humans have been established. Dantrolene is found in measurable amounts in blood and urine. Its major metabolites in body fluids are 5-hydroxy dantrolene and an acetylamino metabolite of dantrolene. Another metabolite with an unknown structure appears related to the latter. Dantrium may also undergo hydrolysis and subsequent oxidation forming nitrophenylfuroic acid.
- The mean biologic half-life of Dantrium after intravenous administration is variable, between 4 to 8 hours under most experimental conditions. Based on assays of whole blood and plasma, slightly greater amounts of dantrolene are associated with red blood cells than with the plasma fraction of blood. Significant amounts of dantrolene are bound to plasma proteins, mostly albumin, and this binding is readily reversible.
- Cardiopulmonary depression has not been observed in malignant hyperthermia susceptible swine following the administration of up to 7.5 mg/kg i.v. dantrolene. This is twice the amount needed to maximally diminish twitch response to single supramaximal peripheral nerve stimulation (95% inhibition). A transient, inconsistent, depressant effect on gastrointestinal smooth muscles has been observed at high doses.
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
- Long-term safety of Dantrium in humans has not been established. Chronic studies in rats, dogs, and monkeys at dosages greater than 30 mg/kg/day showed growth or weight depression and signs of hepatopathy and possible occlusion nephropathy, all of which were reversible upon cessation of treatment. Sprague-Dawley female rats fed dantrolene sodium for 18 months at dosage levels of 15, 30, and 60 mg/kg/day showed an increased incidence of benign and malignant mammary tumors compared with concurrent controls. At the highest dose level, there was an increase in the incidence of benign lymphatic neoplasms. In a 30-month study at the same dose levels also in Sprague-Dawley rats, dantrolene sodium produced a decrease in the time of onset of mammary neoplasms. Female rats at the highest dose level showed an increased incidence of hepatic lymphangiomas and hepatic angiosarcomas.
- The only drug-related effect seen in a 30-month study in Fischer-344 rats was a dose-related reduction in the time of onset of mammary and testicular tumors. A 24-month study in HaM/ICR mice revealed no evidence of carcinogenic activity. Carcinogenicity in humans cannot be fully excluded, so that this possible risk of chronic administration must be weighed against the benefits of the drug (i.e., after a brief trial) for the individual patient.
- Dantrolene sodium has produced positive results in the Ames S. Typhimurium bacterial mutagenesis assay in the presence and absence of a liver activating system.
|clinicalStudies=There is limited information regarding Clinical Studies of Dantrolene in the drug label.
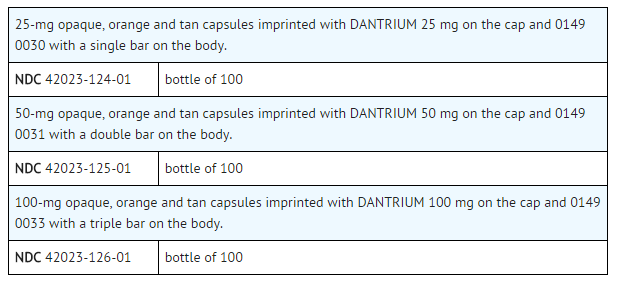
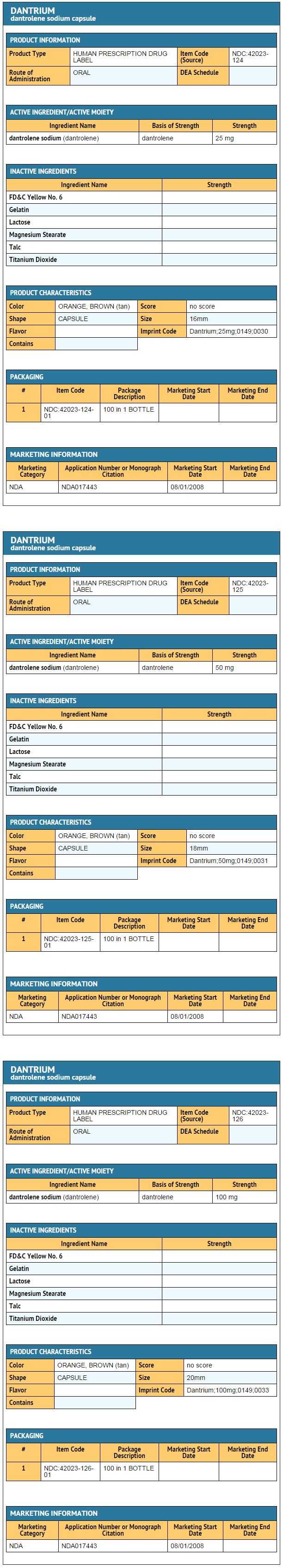
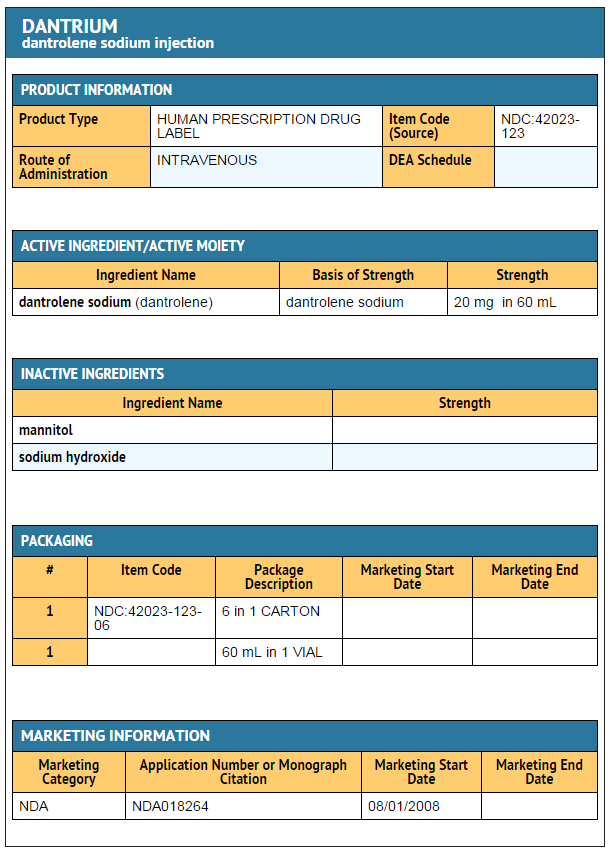
|howSupplied=* Dantrium (dantrolene sodium) is available in:
- Dantrium Intravenous (NDC 42023-123-06) is available in vials containing a sterile lyophilized mixture of 20 mg dantrolene sodium, 3000 mg mannitol, and sufficient sodium hydroxide to yield a pH of approximately 9.5 when reconstituted with 60 mL sterile water for injection USP (without a bacteriostatic agent).
|storage=* Store between 20° to 25°C (68° to 77°F). |packLabel======PRINCIPAL DISPLAY PANEL - 25 MG BOTTLE LABEL=====
PRINCIPAL DISPLAY PANEL - 50 MG BOTTLE LABEL
PRINCIPAL DISPLAY PANEL - 100 MG BOTTLE LABEL
PRINCIPAL DISPLAY PANEL - 20 MG VIAL CARTON
Ingredients and Appearance
|fdaPatientInfo=* Patients should be cautioned against driving a motor vehicle or participating in hazardous occupations while taking Dantrium. Caution should be exercised in the concomitant administration of tranquilizing agents.
- Dantrium might possibly evoke a photosensitivity reaction; patients should be cautioned about exposure to sunlight while taking it.
|alcohol=* Alcohol-Dantrolene interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=* Dantrium®[6]
- Dantrium intravenous®[7]
}}
- ↑ Tsutsumi Y, Yamamoto K, Matsuura S, Hata S, Sakai M, Shirakura K (1998). "The treatment of neuroleptic malignant syndrome using dantrolene sodium". Psychiatry Clin Neurosci. 52 (4): 433–8. doi:10.1046/j.1440-1819.1998.00416.x. PMID 9766694.
- ↑ Tsujimoto S, Maeda K, Sugiyama T, Yokochi A, Chikusa H, Maruyama K (1998). "Efficacy of prolonged large-dose dantrolene for severe neuroleptic malignant syndrome". Anesth Analg. 86 (5): 1143–4. PMID 9585314.
- ↑ Kaplan RF, Feinglass NG, Webster W, Mudra S (1986). "Phenelzine overdose treated with dantrolene sodium". JAMA. 255 (5): 642–4. PMID 3944965.
- ↑ Farquhar I, Hutchinson A, Curran J (1988). "Dantrolene in severe tetanus". Intensive Care Med. 14 (3): 249–50. PMID 3379189.
- ↑ Tidyman M, Prichard JG, Deamer RL, Mac N (1985). "Adjunctive use of dantrolene in severe tetanus". Anesth Analg. 64 (5): 538–40. PMID 3922250.
- ↑ "Dantrolene".
- ↑ "Dantrolene".