Magnetic resonance imaging
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor-In-Chief: Anand Patel, MD [2]
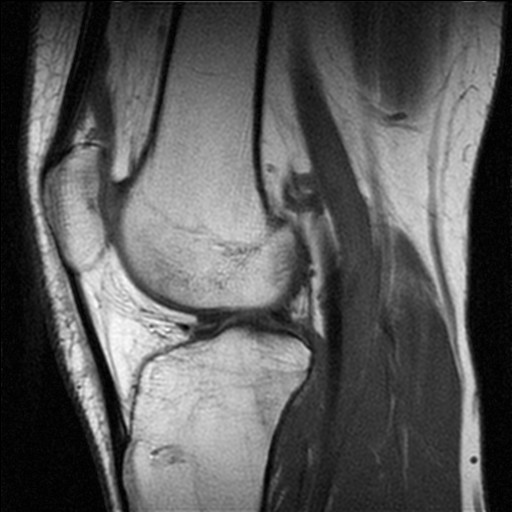
Overview
Magnetic resonance imaging (MRI), formerly referred to as magnetic resonance tomography (MRT) and, in scientific circles and as originally marketed by companies such as General Electric, nuclear magnetic resonance imaging (NMRI) or NMR zeugmatography imaging, is a non-invasive method using nuclear magnetic resonance to render images of the inside of an object. It is primarily used in medical imaging to demonstrate pathological or other physiological alterations of living tissues. MRI also has uses outside of the medical field, such as detecting rock permeability to hydrocarbons and as a non-destructive testing method to characterize the quality of products such as produce and timber.[1]
MRI should not be confused with the NMR spectroscopy technique used in chemistry, although both are based on the same principles of nuclear magnetic resonance. In fact MRI is a series of NMR experiments applied to the signal from nuclei (typified by the hydrogen nuclei in water) used to acquire spatial information in place of chemical information about molecules. The same equipment, provided suitable probes and magnetic gradients are available, can be used for both imaging and spectroscopy.[2]
The scanners used in medicine have a typical magnetic field strength of 0.2 to 3 Teslas. Construction costs approximately US$ 1 million per Tesla and maintenance an additional several hundred thousand dollars per year.
MRI units can operate up to 21.1 Teslas (in the case of a 900 MHz unit; see the magnetogyric ratio and Larmor frequency).
Background
Nomenclature
Magnetic resonance imaging was developed from knowledge gained in the study of nuclear magnetic resonance. In its early years MRI was referred to as nuclear magnetic resonance imaging (NMRI), but the word nuclear has been associated with ionizing radiation exposure, which is not used in an MRI, so to prevent patients from making a negative association between MRI and ionizing radiation, the word has been almost universally removed. Scientists still use the term NMR when discussing non-medical devices operating on the same principles.
One of the inventors of MRI, Paul Lauterbur, originally named the technique zeugmatography, a Greek term meaning "that which is used for joining".[3] The term referred to the interaction between the static and the gradient magnetic fields necessary to create an image, but the nomenclature never caught on.
Principle
Medical MRI most frequently relies on the relaxation properties of excited hydrogen nuclei in water and lipids. When the object to be imaged is placed in a powerful, uniform magnetic field, the spins of atomic nuclei with a resulting non-zero spin have to arrange in a particular manner with the applied magnetic field according to quantum mechanics. Nuclei of hydrogen atoms (protons) have a simple spin 1/2 and therefore align either parallel or antiparallel to the magnetic field.
The spin polarization determines the basic MRI signal strength. For protons, it refers to the population difference of the two energy states that are associated with the parallel and antiparallel alignment of the proton spins in the magnetic field and governed by Boltzmann statistics. In a 1.5 T magnetic field (at room temperature) this difference refers to only about one in a million nuclei since the thermal energy far exceeds the energy difference between the parallel and antiparallel states. Yet the vast quantity of nuclei in a small volume sum to produce a detectable change in field. Most basic explanations of MRI will say that the nuclei align parallel or anti-parallel with the static magnetic field; however, because of quantum mechanical reasons, the individual nuclei are actually set off at an angle from the direction of the static magnetic field. The bulk collection of nuclei can be partitioned into a set whose sum spin are aligned parallel and a set whose sum spin are anti-parallel.
The magnetic dipole moment of the nuclei then precesses around the axial field. While the proportion is nearly equal, slightly more are oriented at the low energy angle. The frequency with which the dipole moments precess is called the Larmor frequency. The tissue is then briefly exposed to pulses of electromagnetic energy (radiofrequency pulses) in a plane perpendicular to the magnetic field, causing some of the magnetically aligned hydrogen nuclei to assume a temporary non-aligned high-energy state. Or in other words, the steady-state equilibrium established in the static magnetic field becomes perturbed and the population difference of the two energy levels is altered. The frequency of the pulses is governed by the Larmor equation to match the required energy difference between the two spin states.
Image formation
In order to selectively image different voxels (volume picture elements) of the subject, orthogonal magnetic gradients are applied. Although it is relatively common to apply gradients in the principal axes of a patient (so that the patient is imaged in x, y, and z from head to toe), MRI allows completely flexible orientations for images. All spatial encoding is obtained by applying magnetic field gradients which encode position within the phase of the signal. In one dimension, a linear phase with respect to position can be obtained by collecting data in the presence of a magnetic field gradient. In three dimensions (3D), a plane can be defined by "slice selection", in which an RF pulse of defined bandwidth is applied in the presence of a magnetic field gradient in order to reduce spatial encoding to two dimensions (2D). Spatial encoding can then be applied in 2D after slice selection, or in 3D without slice selection. Spatially-encoded phases are recorded in a 2D or 3D matrix; this data represents the spatial frequencies of the image object. Images can be created from the matrix using the discrete Fourier transform (DFT). Typical medical resolution is about 1 mm³, while research models can exceed 1 µm³.
Scanner construction and operation
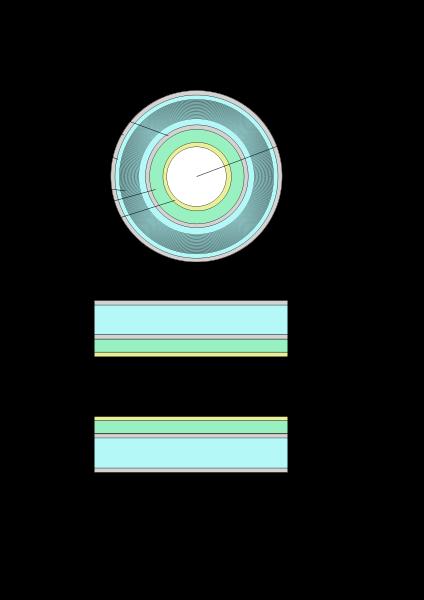
The three systems described above form the major components of an MRI scanner: a static magnetic field, an RF transmitter and receiver, and three orthogonal, controllable magnetic gradients.
Magnet
The magnet is the largest and most expensive component of the scanner, and the remainder of the scanner is built around it. Just as important as the strength of the main magnet is its precision. The straightness of magnet lines within the centre or, as it is known as, the iso-centre of the magnet, need to be almost perfect. This is known as homogeneity. Fluctuations or, non-homogeneities in the field strength, within the scan region, should be less than three parts-per-million (3 PPM). Three types of magnet have been used:
- Permanent magnet: Conventional magnets made from ferromagnetic materials (e.g., steel) can be used to provide the static magnetic field. These are extremely bulky (the magnet can weigh in excess of 100 tonnes), but once installed require little costly maintenance. Permanent magnets can only achieve limited field strength (usually < 0.4 T) and have limited stability and precision. There are also potential safety issues, as the magnetic field cannot be removed in case of entrapment.
- Resistive electromagnet: A solenoid wound from copper wire is an alternative to a permanent magnet. The advantages are low cost, but field strength is limited, and stability is poor. The electromagnet requires considerable electrical energy during operation which can make it expensive to operate. This design is essentially obsolete.
- Superconducting electromagnet: When a niobium-titanium alloy is cooled by liquid helium at 4K (-269°C, -452°F) it becomes superconducting where it loses all resistance to flow of electrical current. By building an electromagnet from superconducting wire, it is possible to develop extremely high field strengths, with very high stability. The construction of such magnets is extremely costly, and the cryogenic helium is expensive and difficult to handle. However, despite its cost, helium cooled superconducting magnets are the most common type found in MRI scanners today.
Most superconducting magnets have their coils of superconductive wire immersed in liquid helium, inside a vessel called a Cryostat. Despite thermal insulation, ambient heat causes the helium to slowly boil off. Such magnets, therefore, require regular topping-up with helium. Generally a Cryocooler, also known as a Coldhead is used to recondense some helium vapour back into the liquid helium bath. Several manufacturers now offer 'cryogenless' scanners, where instead of being immersed in liquid helium the magnet wire is cooled directly by a cryocooler.
Magnets are available in a variety of shapes. However, permanent magnets are most frequently 'C' shaped, and superconducting magnets most frequently cylindrical. However, C-shaped superconducting magnets and box-shaped permanent magnets have also been used.
Magnetic field strength is an important factor determining image quality. Higher magnetic fields increase signal-to-noise ratio, permitting higher resolution or faster scanning. However, higher field strengths require more costly magnets with higher maintenance costs, and have increased safety concerns. 1.0 - 1.5 T field strengths are a good compromise between cost and performance for general medical use. However, for certain specialist uses (e.g., brain imaging), field strengths up to 3.0T may be desirable.
RF system
The RF transmission system consists of a RF synthesizer, power amplifier and transmitting coil. This is usually built into the body of the scanner. The power of the transmitter is variable, but high-end scanners may have a peak output power of up to 35 kW, and be capable of sustaining average power of 1 kW. The receiver consists of the coil, pre-amplifier and signal processing system. While it is possible to scan using the integrated coil for transmitting and receiving, if a small region is being imaged then better image quality is obtained by using a close-fitting smaller coil. A variety of coils are available which fit around parts of the body, e.g., the head, knee, wrist, or internally, e.g., the rectum.
A recent development in MRI technology has been the development of sophisticated multi-element phased array coils which are capable of acquiring multiple channels of data in parallel. This 'parallel imaging' technique uses unique acquisition schemes that allow for accelerated imaging, by replacing some of the spatial coding originating from the magnetic gradients with the spatial sensitivity of the different coil elements. However the increased acceleration also reduces the signal-to-noise ratio and can create residual artifacts in the image reconstruction. Two frequently used parallel acquisition and reconstruction schemes are SENSE[4] and GRAPPA[5]. A detailed review of parallel imaging techniques can be found here: [6]
Gradients
Magnetic gradients are generated by three orthogonal coils, oriented in the x, y and z directions of the scanner. These are usually resistive electromagnets powered by sophisticated amplifiers which permit rapid and precise adjustments to their field strength and direction. Typical gradient systems are capable of producing gradients from 20 mT/m to 100 mT/m (i.e. in a 1.5 T magnet, when a maximal z-axis gradient is applied the field strength may be 1.45 T at one end of a 1m long bore, and 1.55 T at the other). It is the magnetic gradients that determine the plane of imaging - because the orthogonal gradients can be combined freely, any plane can be selected for imaging.
Scan speed is dependent on performance of the gradient system. Stronger gradients allow for faster imaging, or for higher resolution; similarly, gradients systems capable of faster switching can also permit faster scanning. However, gradient performance is limited by safety concerns over nerve stimulation.
In order to understand MRI contrast, it is important to have some understanding of the time constants involved in relaxation processes that establish equilibrium following RF excitation. As the high-energy nuclei relax and realign they emit energy at rates which are recorded to provide information about the material they are in. The realignment of nuclear spins with the magnetic field is termed longitudinal relaxation and the time required for a certain percentage of the tissue's nuclei to realign is termed "Time 1" or T1, which is typically about 1 second. T2-weighted imaging relies upon local dephasing of spins following the application of the transverse energy pulse; the transverse relaxation time is termed "Time 2" or T2, typically < 100 ms for tissue. A subtle but important variant of the T2 technique is called T2* imaging. T2 imaging employs a spin echo technique, in which spins are refocused to compensate for local magnetic field inhomogeneities. T2* imaging is performed without refocusing. This sacrifices some image integrity (resolution) but provides additional sensitivity to relaxation processes that cause incoherence of transverse magnetization. Applications of T2* imaging include functional MRI (fMRI) or evaluation of baseline vascular perfusion (e.g. cerebral blood flow (CBF)) and cerebral blood volume (CBV) using injected agents; in these cases, there is an inherent trade-off between image quality and detection sensitivity. Because T2*-weighted sequences are sensitive to magnetic inhomogeneity (as can be caused by deposition of iron-containing blood-degradation products), such sequences are utilized to detect subtle areas of recent or chronic intracranial hemorrhage ("Heme sequence").
Image contrast is created by using a selection of image acquisition parameters that weights signal by T1, T2 or T2*, or no relaxation time ("proton-density images"). In the brain, T1-weighting causes the nerve connections of white matter to appear white, and the congregations of neurons of gray matter to appear gray, while cerebrospinal fluid appears dark. The contrast of "white matter," "gray matter'" and "cerebrospinal fluid" is reversed using T2 or T2* imaging, whereas proton-weighted imaging provides little contrast in normal subjects. Additionally, functional information (CBF, CBV, blood oxygenation) can be encoded within T1, T2, or T2*.
Diffusion weighted imaging (DWI) [7] uses very fast scans with an additional series of gradients (diffusion gradients) rapidly turned on and off. Protons from water diffusing randomly within the brain, via Brownian motion, lose phase coherence and, thus signal during application of diffusion gradients. In a brain with an acute infarction water diffusion is impaired, and signal loss on DWI sequences is less than in normal brain. DWI is the most sensitive method of detecting cerebral infarction (stroke) and works within 30 minutes of the ictus.
Contrast enhancement
Both T1-weighted and T2-weighted images are acquired for most medical examinations; However they do not always adequately show the anatomy or pathology. The first option is to use a more sophisticated image acquisition technique such as fat suppression or chemical-shift imaging.[8] The other is to administer a contrast agent to delineate areas of interest.
A contrast agent may be as simple as water, taken orally, for imaging the stomach and small bowel although substances with specific magnetic properties may be used. Most commonly, a paramagnetic contrast agent (usually a gadolinium compound[9][10]) is given. Gadolinium-enhanced tissues and fluids appear extremely bright on T1-weighted images. This provides high sensitivity for detection of vascular tissues (e.g. tumors) and permits assessment of brain perfusion (e.g. in stroke). There have been concerns raised recently regarding the toxicity of gadolinium-based contrast agents and their impact on persons with impaired kidney function. Special actions may be taken, such as hemodialysis following a contrast MRI scan for renally-impaired patients.
More recently, superparamagnetic contrast agents (e.g. iron oxide nanoparticles[11][12]) have become available. These agents appear very dark on T2*-weighted images and may be used for liver imaging - normal liver tissue retains the agent, but abnormal areas (e.g. scars, tumors) do not. They can also be taken orally, to improve visualisation of the gastrointestinal tract, and to prevent water in the gastrointestinal tract from obscuring other organs (e.g. pancreas).
Diamagnetic agents such as barium sulfate have been studied for potential use in the gastrointestinal tract, but are less frequently used.
MRI vs CT
A computed tomography (CT) scanner uses X-rays, a type of ionizing radiation, to acquire its images, making it a good tool for examining tissue composed of elements of a relatively higher atomic number than the tissue surrounding them, such as bone and calcifications (calcium based) within the body (carbon based flesh), or of structures (vessels, bowel). MRI, on the other hand, uses non-ionizing radio frequency (RF) signals to acquire its images and is best suited for non-calcified tissue.
CT may be enhanced by use of contrast agents containing elements of a higher atomic number than the surrounding flesh (iodine, barium). Contrast agents for MRI are those which have paramagnetic properties. One example is gadolinium.
Both CT and MRI scanners can generate multiple two-dimensional cross-sections (slices) of tissue and three-dimensional reconstructions. Unlike CT, which uses only X-ray attenuation to generate image contrast, MRI has a long list of properties that may be used to generate image contrast. By variation of scanning parameters, tissue contrast can be altered and enhanced in various ways to detect different features. (See Application below.)
MRI can generate cross-sectional images in any plane (including oblique planes). CT was limited to acquiring images in the axial (or near axial) plane in the past. The scans used to be called Computed Axial Tomography scans (CAT scans). However, the development of multi-detector CT scanners with near-isotropic resolution, allows the CT scanner to produce data that can be retrospectively reconstructed in any plane with minimal loss of image quality.
For purposes of tumor detection and identification, MRI is generally superior.[13][14][15] However, CT usually is more widely available, faster, much less expensive, and may be less likely to require the person to be sedated or anesthetized.
The k-space formalism
- See main article K-space
In 1983 Ljunggren[16] and Tweig[17] independently introduced the k-space formalism, a technique that proved invaluable in unifying different MR imaging techniques. They showed that the demodulated MR signal <math>S(t)</math> generated by freely precessing nuclear spins in the presence of a linear magnetic field gradient <math>G</math> equals the Fourier transform of the effective spin density <math>\rho_\mathrm{eff}\ </math> i.e.
<math>S(t) = {\tilde \rho}_{\mathrm{effective}}( {\vec k}(t) ) \equiv \int d^3x \ \rho( {\vec x} ) \cdot e^{2 \pi \imath \ {\vec k}(t) \cdot {\vec x} } </math>
where:
<math>{\vec k}(t) \equiv \int_0^t {\vec G}(t')\ dt' </math>
In other words, as time progresses the signal traces out a trajectory in k-space with the velocity vector of the trajectory proportional to the vector of the applied magnetic field gradient. By the term effective spin density we mean the true spin density <math>\rho({\vec x})</math> corrected for the effects of <math>T_1</math> preparation, <math>T_2</math> decay, dephasing due to field inhomogeneity, flow, diffusion, etc. and any other phenomena that affect that amount of transverse magnetization available to induce signal in the RF probe.
From the basic k-space formula, it follows immediately that we reconstruct an image <math>I({\vec x})</math> simply by taking the inverse Fourier transform of the sampled data viz.
<math>I({\vec x}) = \int d^3 k \ S( {\vec k}(t) ) \cdot e^{-2 \pi \imath \ {\vec k}(t) \cdot {\vec x} } </math>
Using the k-space formalism, a number of seemingly complex ideas become simple. For example, it becomes very easy to understand the role of phase encoding (the so-called spin-warp method). In a standard spin echo or gradient echo scan, where the readout (or view) gradient is constant (e.g. <math>G_x</math>), a single line of k-space is scanned per RF excitation. When the phase encoding gradient is zero, the line scanned is the <math>k_x</math> axis. When a non-zero phase-encoding pulse is added in between the RF excitation and the commencement of the readout gradient, this line moves up or down in k-space i.e. we scan the line <math>k_y</math>=constant.
The k-space formalism also makes it very easy to compare different scanning techniques. In single-shot EPI, all of k-space is scanned in a single shot, following either a sinusoidal or zig-zag trajectory. Since alternating lines of k-space are scanned in opposite directions, this must be taken into account in the reconstruction. Multi-shot EPI and fast spin echo techniques acquire only part of k-space per excitation. In each shot, a different interleaved segment is acquired, and the shots are repeated until k-space is sufficiently well-covered. Since the data at the center of k-space represent lower spatial frequencies than the data at the edges of k-space, the <math>T_E</math> value for the center of k-space determines the image's <math>T_2</math> contrast.
The importance of the center of k-space in determining image contrast can be exploited in more advanced imaging techniques. One such technique is spiral acquisition - a rotating magnetic field gradient is applied, causing the trajectory in k-space to trace out spiral out from the center to the edge. Due to <math>T_2</math> and <math>T_2*</Math> decay the signal is greatest at the start of the acquisition, hence acquiring the center of k-space first improves contrast to noise ratio (CNR) when compared to conventional zig-zag acquisitions, especially in the presence of rapid movement.
Since <math>\vec x</math> and <math>\vec k</math> are conjugate variables (with respect to the Fourier transform) we can use the Nyquist theorem to show that the step in k-space determines the field of view of the image (maximum frequency that is correctly sampled) and the maximum value of k sampled determines the resolution i.e.
<math>FOV \propto \frac{1}{\Delta k} \qquad \mathrm{Resolution} \propto |k_{\max}|</math>
(these relationships apply to each axis (X, Y, and Z) independently).
Application
In clinical practice, MRI is used to distinguish pathologic tissue (such as a brain tumor) from normal tissue. One advantage of an MRI scan is that it is thought to be harmless to the patient. It uses strong magnetic fields and non-ionizing radiation in the radio frequency range. Compare this to CT scans and traditional X-rays which involve doses of ionizing radiation and may increase the risk of malignancy, especially in a fetus.
While CT provides good spatial resolution (the ability to distinguish two structures an arbitrarily small distance from each other as separate), MRI provides comparable resolution with far better contrast resolution (the ability to distinguish the differences between two arbitrarily similar but not identical tissues). The basis of this ability is the complex library of pulse sequences that the modern medical MRI scanner includes, each of which is optimized to provide image contrast based on the chemical sensitivity of MRI.
For example, with particular values of the echo time (TE) and the repetition time (TR), which are basic parameters of image acquisition, a sequence will take on the property of T2-weighting. On a T2-weighted scan, fat-, water- and fluid-containing tissues are bright (most modern T2 sequences are actually fast T2 sequences). Damaged tissue tends to develop edema, which makes a T2-weighted sequence sensitive for pathology, and generally able to distinguish pathologic tissue from normal tissue. With the addition of an additional radio frequency pulse and additional manipulation of the magnetic gradients, a T2-weighted sequence can be converted to a FLAIR sequence, in which free water is now dark, but edematous tissues remain bright. This sequence in particular is currently the most sensitive way to evaluate the brain for demyelinating diseases, such as multiple sclerosis.
The typical MRI examination consists of 5-20 sequences, each of which are chosen to provide a particular type of information about the subject tissues. This information is then synthesized by the interpreting physician.
Specialized MRI scans
Diffusion MRI
Diffusion MRI measures the diffusion of water molecules in biological tissues.[18] In an isotropic medium (inside a glass of water for example) water molecules naturally move randomly according to Brownian motion. In biological tissues however, the diffusion may be anisotropic. For example a molecule inside the axon of a neuron has a low probability of crossing the myelin membrane. Therefore the molecule will move principally along the axis of the neural fiber. If we know that molecules in a particular voxel diffuse principally in one direction we can make the assumption that the majority of the fibers in this area are going parallel to that direction.
The recent development of diffusion tensor imaging (DTI) enables diffusion to be measured in multiple directions and the fractional anisotropy in each direction to be calculated for each voxel. This enables researchers to make brain maps of fiber directions to examine the connectivity of different regions in the brain (using tractography) or to examine areas of neural degeneration and demyelination in diseases like Multiple Sclerosis.
Another application of diffusion MRI is diffusion-weighted imaging (DWI). Following an ischemic stroke, DWI is highly sensitive to the changes occurring in the lesion (Moseley ME et al., Magn Reson Med 1990;14:330–346). It is speculated that increases in restriction (barriers) to water diffusion, as a result of cytotoxic edema (cellular swelling), is responsible for the increase in signal on a DWI scan. Other theories, including acute changes in cellular permeability and loss of energy-dependent (ATP) cytoplastic streaming, have been proposed to explain the phenomena. The DWI enhancement appears within 5-10 minutes of the onset of stroke symptoms (as compared with computed tomography, which often does not detect changes of acute infarct for up to 4-6 hours) and remains for up to two weeks. CT, due to its insensitivity to acute ischemia, is typically employed to rule out hemorragic stroke, which would entirely prevent the use of tissue plasminogen activator (tPA). Further, coupled with scans sensitized to cerebral perfusion, researchers can highlight regions of "perfusion/diffusion mismatch" that may indicate regions capable of salvage by reperfusion therapy.
Finally, it has been proposed that diffusion MRI may be able to detect minute changes in extracellular water diffusion and therefore could be used as a tool for fMRI. The nerve cell body enlarges when it conducts an action potential, hence restricting extracellular water molecules from diffusing naturally. Although this process works in theory, evidence is only moderately convincing.
Like many other specialized applications, this technique is usually coupled with a fast image acquisition sequence, such as echo planar imaging sequence.
Magnetic resonance angiography
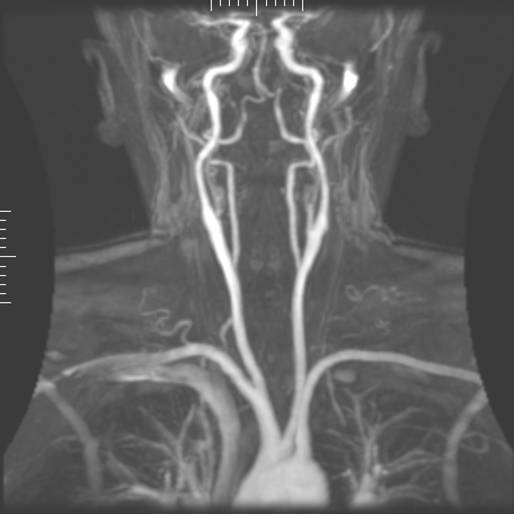
Magnetic resonance angiography (MRA) is used to generate pictures of the arteries in order to evaluate them for stenosis (abnormal narrowing) or aneurysms (vessel wall dilatations, at risk of rupture). MRA is often used to evaluate the arteries of the neck and brain, the thoracic and abdominal aorta, the renal arteries, and the legs (called a "run-off"). A variety of techniques can be used to generate the pictures, such as administration of a paramagnetic contrast agent (gadolinium) or using a technique known as "flow-related enhancement" (e.g. 2D and 3D time-of-flight sequences), where most of the signal on an image is due to blood which has recently moved into that plane, see also FLASH MRI. Magnetic resonance venography (MRV) is a similar procedure that is used to image veins. In this method the tissue is now excited inferiorly while signal is gathered in the plane immediately superior to the excitation plane, and thus imaging the venous blood which has recently moved from the excited plane.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS), also known as MRSI (MRS Imaging) and volume selective NMR spectroscopy, is a technique which combines the spatially-addressable nature of MRI with the spectroscopically-rich information obtainable from NMR. That is to say, MRI allows one to study a particular region within an organism or sample, but gives relatively little information about the chemical or physical nature of that region--its chief value is in being able to distinguish the properties of that region relative to those of surrounding regions. MR spectroscopy, however, provides a wealth of chemical information about that region, as would an NMR spectrum of that region.
Functional MRI
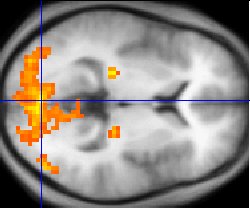
Functional MRI (fMRI) measures signal changes in the brain that are due to changing neural activity. The brain is scanned at low resolution but at a rapid rate (typically once every 2-3 seconds). Increases in neural activity cause changes in the MR signal via T2* changes[19]; this mechanism is referred to as the BOLD (blood-oxygen-level dependent) effect. Increased neural activity causes an increased demand for oxygen, and the vascular system actually overcompensates for this, increasing the amount of oxygenated hemoglobin (haemoglobin) relative to deoxygenated hemoglobin. Because deoxygenated hemoglobin attenuates the MR signal, the vascular response leads to a signal increase that is related to the neural activity. The precise nature of the relationship between neural activity and the BOLD signal is a subject of current research. The BOLD effect also allows for the generation of high resolution 3D maps of the venous vasculature within neural tissue.
While BOLD signal is the most common method employed for neuroscience studies in human subjects, the flexible nature of MR imaging provides means to sensitize the signal to other aspects of the blood supply. Alternative techniques employ arterial spin labeling (ASL) or weight the MRI signal by cerebral blood flow (CBF) and cerebral blood volume (CBV). The CBV method requires injection of a class of MRI contrast agents that are now in human clinical trials. Because this method has been shown to be far more sensitive than the BOLD technique in pre-clinical studies, it may potentially expand the role of fMRI in clinical applications. The CBF method provides more quantitative information than the BOLD signal, albeit at a significant loss of detection sensitivity.
Interventional MRI
The lack of harmful effects on the patient and the operator make MRI well-suited for "interventional radiology", where the images produced by a MRI scanner are used to guide minimally-invasive procedures.
Radiation therapy simulation
Because of MRI's superior imaging of soft tissues, it is now being utilized to specifically locate tumors within the body in preparation for radiation therapy treatments. For therapy simulation, a patient is placed in specific, reproducible, body position and scanned. The MRI system then computes the precise location, shape and orientation of the tumor mass, correcting for any spatial distortion inherent in the system. The patient is then marked or tattooed with points which, when combined with the specific body position, will permit precise triangulation for radiation therapy.
Current density imaging
Current density imaging (CDI) endeavors to use the phase information from images to reconstruct current densities within a subject. Current density imaging works because electrical currents generate magnetic fields, which in turn affect the phase of the magnetic dipoles during an imaging sequence. To date no successful CDI has been performed using biological currents, but several studies have been published which involve applied currents through a pair of electrodes.
Magnetic resonance guided focused ultrasound
In MRgFUS therapy, ultrasound beams are focused on a tissue - guided and controlled using MR thermal imaging - and due to the significant energy deposition at the focus, temperature within the tissue rises to more than 65°C, completely destroying it. This technology can achieve precise "ablation" of diseased tissue. MR imaging provides a three-dimensional view of the target tissue, allowing for precise focusing of ultrasound energy. The MR imaging provides quantitative, real-time, thermal images of the treated area. This allows the physician to ensure that the temperature generated during each cycle of ultrasound energy is sufficient to cause thermal ablation within the desired tissue and if not, to adapt the parameters to ensure effective treatment.
Multinuclear imaging
Hydrogen is the most frequently imaged nucleus in MRI because it is present in biological tissues in great abundance. However, any nucleus which has a net nuclear spin could potentially be imaged with MRI. Such nuclei include helium-3, carbon-13, oxygen-17, sodium-23, phosphorus-31 and xenon-129. 23Na and 31P are naturally abundant in the body, so can be imaged directly. Gaseous isotopes such as 3He or 129Xe must be hyperpolarized and then inhaled as their nuclear density is too low to yield a useful signal under normal conditions. 17O and 13C can be administered in sufficient quantities in liquid form (e.g. 17O-water, or 13C-glucose solutions) that hyperpolarization is not a necessity.
Multinuclear imaging is primarily a research technique at present. However, potential applications include functional imaging and imaging of organs poorly seen on 1H MRI (e.g. lungs and bones) or as alternative contrast agents. Inhaled hyperpolarized ³He can be used to image the distribution of air spaces within the lungs. Injectable solutions containing 13C or stabilized bubbles of hyperpolarized 129Xe have been studied as contrast agents for angiography and perfusion imaging. 31P can potentially provide information on bone density and structure, as well as functional imaging of the brain.
Experimental MRI techniques
Currently there is active research in several new MRI technologies like magnetization transfer MRI (MT-MRI), diffusion tensor MRI (DT-MRI), and proton MR spectroscopy, plus recent research in to Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure.
Safety
Implants and foreign bodies: Pacemakers are generally considered an absolute contraindication towards MRI scanning, though highly specialized protocols have been developed to permit scanning of select pacing devices. Several cases of arrhythmias or death have been reported in patients with pacemakers who have undergone MRI scanning without appropriate precautions. Other electronic implants have varying contraindications, depending upon scanner technology, implant properties, scanning protocols and anatomy being imaged.
Though pacemakers receive significant attention, it should also be noted that many other forms of medical or biostimulation implants may be contraindicated for MRI scans. These may include Vagus nerve stimulators, implantable cardioverter-defibrillators (ICD), loop recorders, insulin pumps, cochlear implants, deep brain stimulators and many others. Medical device patients should always present complete information (manufacturer, model, serial number and date of implantation) about all implants to both the referring physician and to the radiologist or technologist before entering the room for the MRI scan.
While these implants pose a current problem, scientist are working on a nano coating for implants. This will screen the implants from the radio frequency waves and thus patients with future implants will be able to use MRI scanners. The current article for this is from the new scientist.
Ferromagnetic foreign bodies (e.g. shell fragments), or metallic implants (e.g. surgical prostheses, aneurysm clips) are also potential risks, and safety aspects need to be considered on an individual basis. Interaction of the magnetic and radiofrequency fields with such objects can lead to: trauma due to movement of the object in the magnetic field, thermal injury from radio-frequency induction heating of the object, or failure of an implanted device. These issues are especially problematic when dealing with the eye. Most MRI centers require an orbital x-ray be performed on anyone who suspects they may have small metal fragments in their eyes, perhaps from a previous accident, something not uncommon in metalworking.
Because of its non-ferromagnetic nature and poor electrical conductivity, titanium and its alloys are useful for long term implants and surgical instruments intended for use in image-guided surgery. In particular, not only is titanium safe from movement from the magnetic field, but artifacts around the implant are less frequent and less severe than with more ferromagnetic materials e.g. stainless steel. Artifacts from metal frequently appear as regions of empty space around the implant - frequently called 'black-hole artifact' e.g. a 3mm titanium alloy coronary stent may appear as a 5mm diameter region of empty space on MRI, whereas around a stainless steel stent, the artifact may extend for 10-20 mm or more.
In 2006, a new classification system for implants and ancillary clinical devices has been developed by ASTM International and is now the standard supported by the US Food and Drug Administration:
- MR-Safe: The device or implant is completely non-magnetic, non-electrically conductive, and non-RF reactive, eliminating all of the primary potential threats during an MRI procedure.
- MR-Conditional: A device or implant that may contain magnetic, electrically conductive or RF-reactive components that is safe for operations in proximity to the MRI, provided the conditions for safe operation are defined and observed (such as 'tested safe to 1.5 Teslas' or 'safe in magnetic fields below 500 gauss in strength').
- MR-Unsafe: Nearly self-explanatory, this category is reserved for objects that are significantly ferromagnetic and pose a clear and direct threat to persons and equipment within the magnet room.
In the case of pacemakers, the risk is thought to be primarily RF induction in the pacing electrodes/wires causing inappropriate pacing of the heart, rather than the magnetic field affecting the pacemaker itself. Much research and development is being undertaken, and many tools are being developed in order to predict the effects of the RF fields inside the body.
Other significant safety issues include:
- Projectiles: As a result of the very high strength of the magnetic field needed to produce scans (frequently up to 60,000 times the earth's own magnetic field effects), there are several incidental safety issues addressed in MRI facilities. Missile-effect accidents, where ferromagnetic objects are attracted to the center of the magnet, have resulted in injury and death.[20] It is for this reason that ferrous objects and devices are prohibited in proximity to the MRI scanner, with non ferro-magnetic versions of many of these objects typically retained by the scanning facility. The magnetic field remains a permanent hazard — the superconductive MRI magnet retains its magnetic field at all times. The proliferation of ferromagnetic materials makes screening them out a significant challenge. New ferromagnetic-only detection devices are supplementing conventional screening techniques in many leading hospitals and imaging centers. A video of what happens when a ferromagnetic bottle of oxygen enters the vicinity of an MRI magnet can be viewed here [3], the bottle is violently sucked into the bore of the magnet and oscillates rapidly in midair until coming to rest at the center.
- Radio frequency energy: A powerful radio transmitter is needed for excitation of proton spins. This can heat the body significantly, with the risk of hyperthermia in patients, particularly the obese or patients with thermoregulation disorders. Several countries have issued restrictions on the maximum specific absorption rate that a scanner may produce.
- Peripheral nerve stimulation (PNR): The rapid switching (on and off) of the magnetic field gradients needed for imaging is capable of causing nerve stimulation. Volunteers report a twitching sensation when exposed to rapidly switched fields, particularly in their extremities. The reason the peripheral nerves are stimulated is that the changing field increases with distance from the center of the gradient coils (which more or less coincides with the center of the magnet). Note however that when imaging the head, the heart is far off-center and induction of even a tiny current into the heart must be avoided at all costs. Although PNR was not a problem for the slow, weak gradients used in the early days of MRI, the strong, rapidly-switched gradients used in techniques such as EPI, fMRI, diffusion MRI, etc. are indeed capable of inducing PNR. American and European regulatory agencies insist that manufacturers stay below specified dB/dt limits (dB/dt is the change in field per unit time) or else prove (via clinical studies) that no PNR is induced for any imaging sequence. As a result of dB/dt limitation software and/or hardware, commercial MRI systems cannot use the full rated power of their gradient amplifiers.
- Acoustic noise: Loud noises and vibrations are produced by forces resulting from rapidly switched magnetic gradients interacting with the main magnetic field, in turn causing minute expansions and contractions of the coil itself. This is most marked with high-field machines and rapid-imaging techniques in which sound intensity can reach 130 dB (equivalent to a jet engine at take-off). Appropriate use of ear protection is essential. Manufacturers are now incorporating noise insulation and active noise cancellation systems on their equipment.
- Cryogens: An emergency shut-down of a superconducting electromagnet, an operation known as "quenching", involves the rapid boiling of liquid helium from the device. If the rapidly expanding helium cannot be dissipated though external vents, it may be released into the scanner room where it may cause displacement of the oxygen and present a risk of asphyxiation. Since a quench results in immediate loss of all cryogens in the magnet, recommissioning the magnet is extremely expensive and time-consuming. Spontaneous quenches are uncommon, but can occur at any time.
Contrast agents
The most frequently used intravenous contrast agents are based on chelates of gadolinium. In general, these agents have proved safer than the iodinated contrast agents used in X-ray radiography or CT. Anaphylactoid reactions are rare occurring in approx 0.03-0.1%. [21] Of particular interest is the lower incidence of nephrotoxicity, compared with iodinated agents, when given at usual doses—this has made contrast-enhanced MRI scanning an option for patients with renal impairment, who would otherwise not be able to undergo contrast-enhanced CT. [22]
Although gadolinium agents have proved useful for patients with renal impairment, there has been a newly identified risk described in patients with severe renal failure requiring dialysis. A rare, but serious, illness affecting dialysis patients, nephrogenic systemic fibrosis, has been linked to the use of certain gadolinium containing agents: the most frequently associated is gadodiamide association with some other agents has been reported. [23] Although a causal link has not been definitively established, current guidelines in the United States are that dialysis patients should only receive gadolinium agents where essential, and that dialysis should be performed as soon as possible after the scan is complete, in order to remove the agent from the body promptly. [24] In Europe where more gadolinium-containing agents are avaliable, a classification of agents according to potential risks has been released.[25][26]
Pregnancy
No harmful effects of MRI on the fetus have been demonstrated. In particular, MRI avoids the use of ionizing radiation, to which the fetus is particularly sensitive. However, as a precaution, current guidelines recommend that pregnant women undergo MRI only when essential. This is particularly the case during the first trimester of pregnancy, as organogenesis takes place during this period. The concerns in pregnancy are the same as for MRI in general, but the fetus may be more sensitive to the effects—particularly to heating and to noise. However, one additional concern is the use of contrast agents; gadolinium compounds are known to cross the placenta and enter the fetal bloodstream, and it is recommended that their use be avoided.
Despite these concerns, MRI is rapidly growing in importance as a way of diagnosing and monitoring congenital defects of the fetus because it can provide more diagnostic information than ultrasound and it lacks the ionizing radiation of CT. MRI without contrast is the imaging mode of choice for pre-surgical, in-utero diagnosis and evaluation of fetal tumors, primarily teratomas, facilitating open fetal surgery, other fetal interventions, and planning for procedures (such as the EXIT procedure) to safely deliver and treat babies who have defects otherwise incompatible with life.
Claustrophobia and discomfort
Due to the construction of MRI scanners, they are potentially unpleasant to lie in. The part of the body being imaged needs to lie at the center of the magnet (which is often a long, narrow tube). Because scan times may be long (perhaps one hour), people with even mild claustrophobia are often unable to tolerate an MRI scan without management.
Management may include:
- Advance preparation
- visiting the scanner to see the room and practice lying on the table
- visualization techniques
- chemical sedation
- general anesthesia
- Coping while inside the scanner
- holding a "panic button"
- listening to music on headphones or watching a movie with a head-mounted display while in the machine
- Modified scanner designs
- open-bore design scanners.
Though open MRIs have increased in popularity as of late, they produce inferior scan quality because they operate at lower magnetic fields than closed MRIs.
- upright MRIs (made exclusively by FONAR)
For babies and children, chemical sedation or general anesthesia are the norm. These MRI subjects are too young to be instructed to hold still during the scanning session. Obese patients and pregnant women may find the MRI machine to be a tight fit, and even someone without a history of claustrophobia may find the experience intolerable without sedation. Pregnant women may also have difficulty lying on their back without moving for an hour or more.
The noise associated with the operation of an MRI scanner (especially the audible noise associated with the gradient pulses applied to the subject) can also exacerbate the discomfort associated with the procedure.
Guidance
Safety issues, including the potential for biostimulation device interference, movement of ferromagnetic bodies, and incidental localized heating, have been addressed in the American College of Radiology's White Paper on MR Safety which was originally published in 2002 and expanded in 2004. The ACR White Paper on MR Safety has been rewritten and was released early in 2007 under the new title ACR Guidance Document for Safe MR Practices.
The European Physical Agents Directive
The European Physical Agents (Electromagnetic Fields) Directive is European legislation that has been adopted in European legislature. By 2008 each individual state within the European Union must include this directive in its own law.
The directive applies to occupational exposure to electromagnetic fields (not medical exposure) and was intended to limit workers’ acute exposure to strong electromagnetic fields, as may be found near electricity substations, radio or television transmitters or industrial equipment. However, the regulations impact significantly on MRI, with separate sections of the regulations limiting exposure to static magnetic fields, changing magnetic fields and radio frequency energy. Field strength limits are given which may not be exceeded for any period of time. An employer may commit a criminal offence by allowing a worker to exceed an exposure limit if that is how the Directive is implemented in a particular Member State.
The Directive is based on the international consensus of established effects of exposure to electromagnetic fields, and in particular the advice of the European Commissions's advisor, the International Commission on Non-Ionizing Radiation Protection (ICNIRP). The aims of the Directive, and the ICNIRP guidelines upon which it is based, are to prevent exposure to potentially harmful fields. The actual limits in the Directive are very similar to the limits advised by the Institute of Electrical and Electronics Engineers, with the exception of the frequencies produced by the gradient coils, where the IEEE limits are significantly higher.
Many Member States of the EU already have either specific EMF regulations or (as in the UK) a general requirement under workplace health and safety legislation to protect workers against electromagnetic fields. In almost all cases the existing regulations are aligned with the ICNIRP limits so that the Directive should, in theory, have little impact on any employer already meeting their legal responsibilities.
The introduction of the Directive has brought to light an existing potential issue with occupational exposures to MRI fields. There are at present very few data on the number or types of MRI practice that might lead to exposures in excess of the levels of the Directive. There is a justifiable concern amongst MRI practitioners that if the Directive were to be enforced more vigorously than existing legislation, the use of MRI might be restricted, or working practices of MRI personnel might have to change.
In the initial draft a limit of static field strength to 2 T was given. This has since been removed from the regulations, and whilst it is unlikely to be restored as it was without a strong justification, some restriction on static fields may be reintroduced after the matter has been considered more fully by ICNIRP. The effect of such a limit might be to restrict the installation, operation and maintenance of MRI scanners with magnets of 2 T and stronger. As the increase in field strength has been instrumental in developing higher resolution and higher performance scanners, this would be a significant step back. This is why it is unlikely to happen without strong justification.
Individual government agencies and the European Commission have now formed a working group to examine the implications on MRI and to try to address the issue of occupational exposures to electromagnetic fields from MRI.
2003 Nobel Prize
Reflecting the fundamental importance and applicability of MRI in the medical field, Paul Lauterbur of the University of Illinois, Urbana-Champaign and Sir Peter Mansfield of the University of Nottingham were awarded the 2003 Nobel Prize in Medicine or Physiology for their "discoveries concerning magnetic resonance imaging". The Nobel Prize committee acknowledged Lauterbur's insight of using magnetic field gradients to introduce spatial localization, a discovery that allowed rapid acquisition of 2D images. Sir Peter Mansfield was credited with introducing the mathematical formalism and developing techniques for efficient gradient utilization and fast imaging.
Controversy
The 2003 Nobel Prize in Medicine award was vigorously protested by Raymond Vahan Damadian, who claimed that he was the inventor of MRI, and that Paul Lauterbur and Sir Peter Mansfield had merely refined the technology. An ad hoc group, called "The Friends of Raymond Damadian", took out full-page advertisements in New York Times and The Washington Post entitled "The Shameful Wrong That Must Be Righted", demanding that he be awarded at least a share of the Nobel Prize.[27] The Nobel Assembly at Karolinska Institutet, which picks the winner in medicine, refused, as is their custom, to comment on Damadian's claims or change the awardees.
In a letter to Physics Today, Herman Carr pointed out his own early use of field gradients for 1D MR imaging.[28] The contribution by John Mallard and colleagues at the University of Aberdeen, who developed the spin-warp technology, as well as producing the first clinically useful images in patients, is also often overlooked. [29] [30] [31]
See also
- History of brain imaging
- Nobel Prize controversies
- Raymond Damadian
- FLASH MRI
- Functional magnetic resonance imaging
- High intensity focused ultrasound
- Relaxation and relaxometry
- Robinson oscillator
- Signal-to-noise ratio
- Contrast to noise ratio
- Contrast resolution
- Spatial resolution
- Rabi cycle
- InVesalius
- Specific absorption rate
- Neuroimaging software
- Electron-spin resonance (spin physics)
- Pneumoencephalogram
- Nephrogenic fibrosing dermopathy
References
- ↑ http://www.mri.cl/index.pl/industrial_stud#355
- ↑ Wayne E. Steinmetz, Cyrus R. Maher, Magnetic resonance imaging on an NMR spectrometer. An experiment for the physical chemistry laboratory, Concepts in Magnetic Resonance Part A, 2007; 3:133-139
- ↑ Lauterbur, P.C., Nature, 1973; 242:190-191.
- ↑ Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. "SENSE: sensititivy encoding for fast MRI." Magn Reson Med. 1999 Nov;42(5):952-62. PMID 10542355
- ↑ Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. "Generalized autocalibrating partially parallel acquisitions (GRAPPA)." Magn Reson Med. 2002 Jun;47(6):1202-10. PMID 12111967
- ↑ http://cfmriweb.ucsd.edu/ttliu/be280a_05/blaimer05.pdf
- ↑ Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986 Nov;161(2):401-7 PMID 3763909
- ↑ Haase A., Frahm J., Hanicke W., Matthaei D. "1H NMR chemical shift selective (CHESS) imaging." Phys Med Biol. 1985 Apr;30(4):341-4. PMID 4001160
- ↑ Weinmann HJ, Brasch RC, Press WR, Wesbey GE. "Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent." AJR Am J Roentgenol. 1984 Mar;142(3):619-24. PMID 6607655
- ↑ Laniado M, Weinmann HJ, Schorner W, Felix R, Speck U. "First use of GdDTPA/dimeglumine in man." Physiol Chem Phys Med NMR. 1984;16(2):157-65. PMID 6505042
- ↑ Widdler DJ, Greif WL, Widdler KJ, Edelman RR, Brady TJ. "Magnetite Albumin Microspheres: A New MR Contrast Material." AJR Am J Roentgenol. 1987;148(2):399-404. PMID 3492120
- ↑ Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. "Ultrasmall Superparamagnetic Iron Oxide: Characterization of a New Class of Contrast Agents for MR Imaging." Radiology. 1990;175(2):489-93. PMID 2326474
- ↑ Magnetic resonance and computerized tomography of posterior cranial fossa tumors in childhood. Differential diagnosis and assessment of lesion extent][Article in Italian] Colosimo C, Celi G, Settecasi C, Tartaglione T, Di Rocco C, Marano P. (1995) Radiol Med (Torino) 90(4):386-395
- ↑ The clinical and radiological evaluation of primary brain neoplasms in children, Part II: Radiological evaluation. Allen ED, Byrd SE, Darling CF, Tomita T, Wilczynski MA. (1993) J Natl Med Assoc. 85(7):546-553
- ↑ Computed tomography versus magnetic resonance imaging of the brain. A collaborative interinstitutional study. Deck MD, Henschke C, Lee BC, Zimmerman RD, Hyman RA, Edwards J, Saint Louis LA, Cahill PT, Stein H, Whalen JP. (1989) Clin Imaging 13(1):2-15
- ↑ Ljunggren S. J Magn Reson 1983; 54:338.
- ↑ Twieg D (1983). "The k-trajectory formulation of the NMR imaging process with applications in analysis and synthesis of imaging methods". Med Phys. 10 (5): 610–21. PMID 6646065.
- ↑ Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986 Nov;161(2):401-7 PMID 3763909
- ↑ Thulborn KR, Waterton JC, Matthews PM, Radda GK. "Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field." Biochim Biophys Acta. 1982 Feb 2;714(2):265-70. PMID 6275909
- ↑ Randal C. Archibold, "Hospital Details Failures Leading to M.R.I. Fatality", The New York Times, 2001 August 22.
- ↑ Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR 1996; 167:847-849
- ↑ "ACR guideline, 2005"
- ↑ H.S. Thomsen, S.K. Morcos and P. Dawson, Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)?, Clinical Radiology, Volume 61 (11), Nov 2006, pp. 905-906.
- ↑ "FDA Public Health Advisory: Gadolinium-containing Contrast Agents for Magnetic Resonance Imaging"
- ↑ http://www.mhra.gov.uk/home/idcplg?IdcService=GET_FILE&dID=35149&noSaveAs=0&Rendition=WEB
- ↑ http://www.ismrm.org/special/EMEA2.pdf
- ↑ H.F. Judson, "No Nobel Prize for whining", New York Times, 20 October 2003. Accessed 2006-11-02.
- ↑ Carr, Herman. "Letter: Field Gradients in Early MRI". Physics Today. 57 (7): 83. Unknown parameter
|month=ignored (help) - ↑ Hutchison JMS, Mallard JR, Goll CC (1974). "In-vivo imaging of body structures using proton resonance". Proceedings of the 18th Ampère Congress: Magnetic resonance and related phenomena. Oxford, Amsterdam: North-Holland Publishing Company. pp. 283–284.
- ↑ Edelstein WA, Hutchison JMS, Johnson G, Redpath TW (1980). "Spin-warp NMR imaging and applications to human whole-body imaging". Phys Med Biol. 25: 751–756. PMID 7454767.
- ↑ Mallard J (2006). "Magnetic resonance imaging—the Aberdeen perspective on developments in the early years". Phys Med Biol. 51: R45–R60. PMID 16790917.
- James Mattson and Merrill Simon. The Pioneers of NMR and Magnetic Resonance in Medicine: The Story of MRI. Jericho & New York: Bar-Ilan University Press, 1996. ISBN 0-9619243-1-4.
- E. M. Haacke, R.W. Brown, M.L. Thompson, R. Venkatesan, Magnetic Resonance Imaging: Physical Principles and Sequence Design, John Wiley, 1999. ISBN 0471351288
bs:Magnetska rezonanca de:Magnetresonanztomographie et:Magnetresonantskuvamine el:Μαγνητική τομογραφία eo:Magneta resonanca bildigo fa:امآرآی hr:Magnetna rezonancija it:Imaging a risonanza magnetica he:דימות תהודה מגנטית lb:Magnéitresonanztomographie ms:MRI nl:MRI-scanner no:Magnetresonanstomografi nn:Magnetresonanstomografi sk:Zobrazovanie magnetickou rezonanciou sl:Slikanje z magnetno resonanco fi:Magneettikuvaus