Multiple sclerosis pathophysiology
| https://https://www.youtube.com/watch?v=yzH8ul5PSZ8 |350}} |
|
Multiple sclerosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Multiple sclerosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Multiple sclerosis pathophysiology |
|
Risk calculators and risk factors for Multiple sclerosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Fahimeh Shojaei, M.D.,
Overview
Multiple sclerosis is a disease of the central nervous system and it’s known to be multi factorial. Whatever the trigger is, it will lead to an acquired immune response followed by inflammatory reactions. These reactions lead to secretion of cytokines in the CNS parenchyma and activation of resident microglia. Microglia cells activate astrocytes to release more inflammatory cytokines, leading to recruitment and infiltration of circulatory leukocytes. This burst events cause destruction of myelin sheath and forms focal sclerotic white matter plaques, which are characteristic of multiple sclerotic disease. There is some evidence proving genetic involvement in onset of MS so that it increases the risk of developing MS from 0.1% in general population to 3% in those who have siblings with MS and 25% in those with a monozygote twin affected. Based on studies performed on post mortem brain tissue of patients with multiple sclerosis, there are four types of white matter lesion pathology. Damage to myelin sheath is prominent in type 1 and 2 while type 3 and 4 characteristic is dying oligodendrocytes. the etiology of oligodendrocytes death known to be multifactorial or followed by hypoxia, mitochondrial dysfunction and macrophages.
Pathophysiology
Physiology
- Soma is the neuronal cell body which is a closed area with cell membrane.[1]
- Myelin sheath is the oligodendrocyte membrane which wraps around the axons.
- Myelin sheath is insulated against electrical impulses and is separated by nodes of Ranvier which can transfer the electrical impulse.
- This structure leads to fast traveling of electrical impulses.
Pathogenesis
- Multiple sclerosis is a disease of the central nervous system and it’s known to be multi factorial.[2]
- There are both inflammation and degeneration in the course of the disease, but as it progress, degeneration becomes more prominent.
- There are variety of different cells participating in MS pathophysiology. Whatever the trigger is, it will lead to an acquired immune response followed by inflammatory reactions.
- These reactions lead to secretion of cytokines in CNS parenchyma and activation of resident microglia. Microglia cells activate astrocytes to release more inflammatory cytokines leading to recruitment and infiltration of circulatory leukocytes.[3][4][5]
- This burst events cause destruction of myelin sheath and CNS tissue and releasing more auto antigens including myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), proteolipid protein (PLP).[6][7]
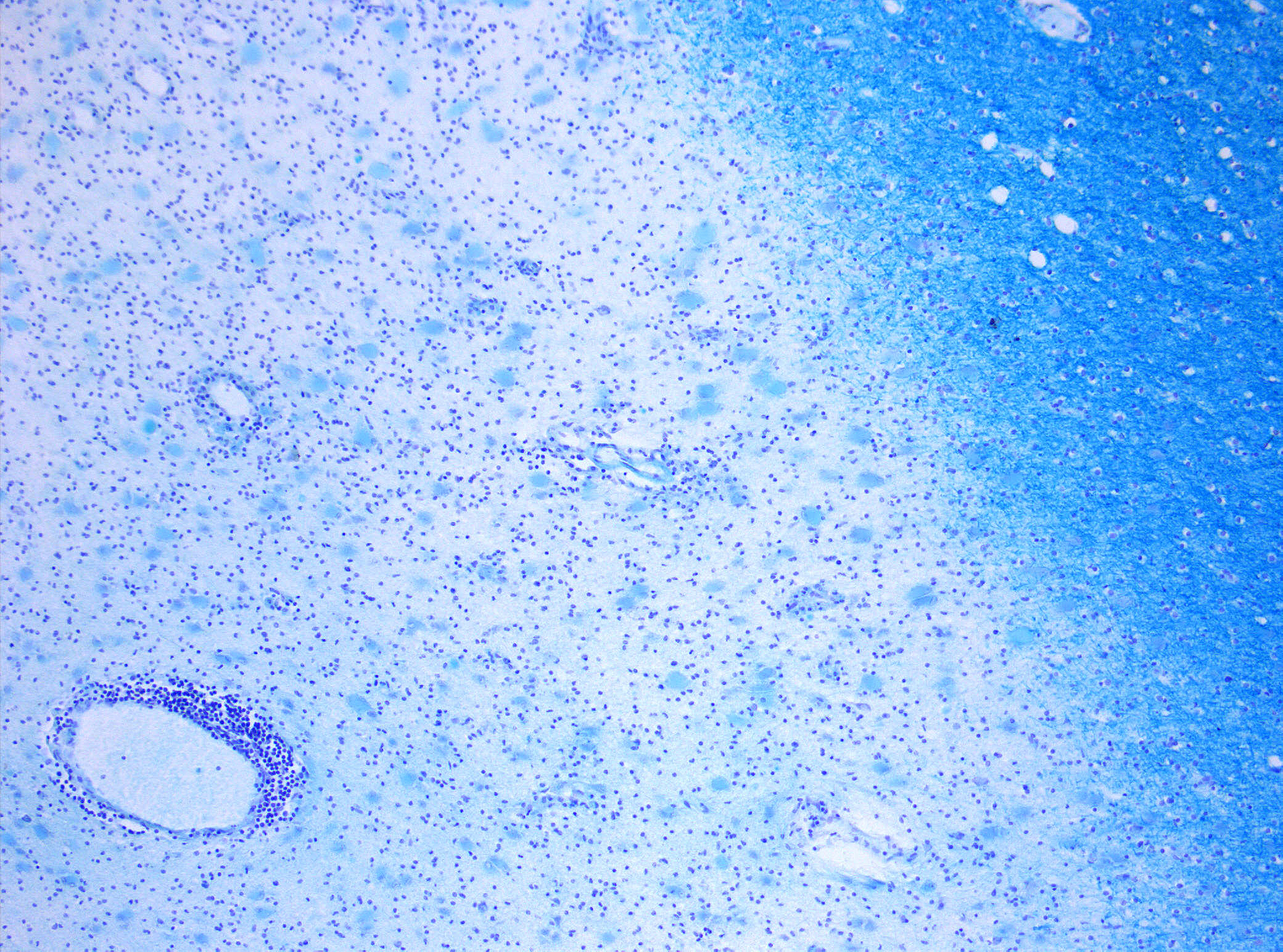
- Focal sclerotic white matter plaques, which are characteristic of multiple sclerotic disease, are mostly located in the optic nerve, periventricular white matter, juxtacortical border, cerebellum, brain stem, and cervical spine.[8] This pattern of lesion formation is specific for MS.[9]
- Appearing of new white matter lesions is a way to estimate the efficacy of our therapy since it is an indicator of continued inflammation.[10]
- In the acute phase of the disease there is several evidence of blood brain barrier disruption.[11]
- Formation of white matter lesions is started by CD8+ T cells and then, CD4+ T cells, B cells, plasma cells and macrophages but the most common cells in lesions are macrophages and microglial cells.[12][13][14]
- There is some evidence of cortical (gray matter) demyelination in MS patients.[15][16] It correlates with cognitive deficits and seizures in patients.[17][18] It is not clear yet that whether the pathphysiology of cortical demyelination is similar to white matter demyelination and is a consequence of it or it is a completely different phenomenon.
- Cortical demyelination tends to be global in contrast with focal white matter lesions.[19]
- In post mortem brain tissue of patients with MS, gray matter lesions show blood brain barrier dysfunction, macrophages filled with myelin, T cells, B cells and meningeal inflammation. These findings are suggestive of inflammation as an underlying cause of these lesions.[20]
- Cortical demyelination is more prominent in PPMS and SPMS but it can also be seen in RRMS.[15]
- There are some lesions called "shadow plaques". Remyelination occurs in these lesions and they have a large number of oligodendrocyte precursor cells (OPC) and mature oligodendrocytes.[21][22]
- It may be because of more permissive environment that this event occurs mostly in cortical lesions rather than white matter lesions.[21]
- Remyelination occurs equally among patients with RRMS, SPMS and PPMS.[23]
- The loss of mature oligodendrocytes in chronic MS is a sign of failure in the course of maturation.
- Several inhibitory mediators have been found to have a role in this and prevent the axonal attachment and expressing myelin-specific genes.[24][25]
- There are no imaging techniques which can differentiate remyelinated plaques from early demyelinating lesions. It seems that remyelinated plaques are more susceptible to demyelination attacks.
Genetics
- There is some evidence proving genetic involvement in onset of MS so that it increases the risk of developing MS from 0.1% in general population to 3% in those who have siblings with MS and 25% in those with a monozygote twin affected.[26]
- HLA alleles seems to have a huge relationship with MS susceptibility.[27]
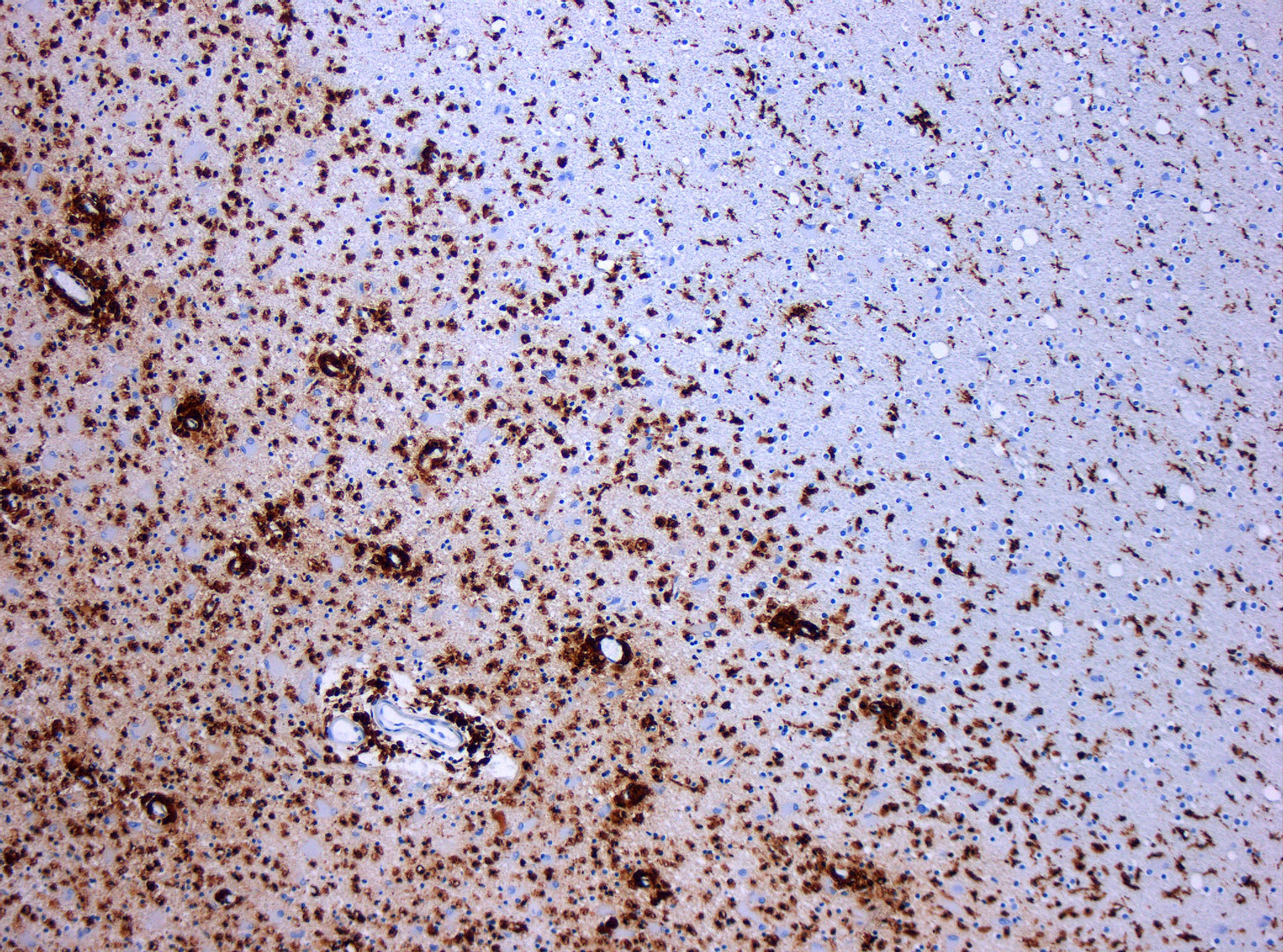
Microscopic Pathology
Based on studies performed on post mortem brain tissue of patients with multiple sclerosis, there are four types of white matter lesion pathology:[28][8]
- Microscopic pathology type 1: Found in 10% of patients especially those with less than 1 year of disease history. In this type, the lesions have sharp borders and perivascular T cell infiltration. Demyelination process is still active and microglia cells and macrophages are full of myelin.
- Microscopic pathology type 2: Found in 55% of patients. IgG and complement (C9neo) deposition with sever macrophage and T cell infiltration.
- Microscopic pathology type 3: Found in 30% of patients. The borders of lesion in this type are not sharply defined. There are evidences of vessel inflammation and dying oligodendrocytes.
- Microscopic pathology type 4: Found in 5% of patients with PPMS. Degeneration of oligodendrocytes and infiltration of T cells and macrophages are seen in this type of lesions.[29]
NOTE: Damage to myelin sheath is prominent in type 1 and 2 while type 3 and 4 characteristic is dying oligodendrocytes.[8][30] the etiology of oligodendrocytes death known to be multifactorial or followed by hypoxia, mitochondrial dysfunction and macrophages.[31][32]
References
- ↑ Mattle, Heinrich (2017). Fundamentals of neurology : an illustrated guide. Stuttgart New York: Thieme. ISBN 9783131364524.
- ↑ Fiorini A, Koudriavtseva T, Bucaj E, Coccia R, Foppoli C, Giorgi A, Schininà ME, Di Domenico F, De Marco F, Perluigi M (2013). "Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: the spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis". PLoS ONE. 8 (6): e65184. doi:10.1371/journal.pone.0065184. PMC 3676399. PMID 23762311.
- ↑ John GR, Lee SC, Song X, Rivieccio M, Brosnan CF (2005). "IL-1-regulated responses in astrocytes: relevance to injury and recovery". Glia. 49 (2): 161–76. doi:10.1002/glia.20109. PMID 15472994.
- ↑ Kawakami N, Nägerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A (2005). "Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion". J. Exp. Med. 201 (11): 1805–14. doi:10.1084/jem.20050011. PMC 2213265. PMID 15939794.
- ↑ Sofroniew MV (2015). "Astrocyte barriers to neurotoxic inflammation". Nat. Rev. Neurosci. 16 (5): 249–63. doi:10.1038/nrn3898. PMC 5253239. PMID 25891508.
- ↑ McCarthy DP, Richards MH, Miller SD (2012). "Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease". Methods Mol. Biol. 900: 381–401. doi:10.1007/978-1-60761-720-4_19. PMC 3583382. PMID 22933080.
- ↑ Pirko I, Johnson AJ (2008). "Neuroimaging of demyelination and remyelination models". Curr. Top. Microbiol. Immunol. 318: 241–66. PMID 18219821.
- ↑ 8.0 8.1 8.2 Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S (2015). "The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis". Prog. Neurobiol. 127-128: 1–22. doi:10.1016/j.pneurobio.2015.02.003. PMC 4578232. PMID 25802011.
- ↑ Katz Sand I (2015). "Classification, diagnosis, and differential diagnosis of multiple sclerosis". Curr. Opin. Neurol. 28 (3): 193–205. doi:10.1097/WCO.0000000000000206. PMID 25887774.
- ↑
- ↑ Silver NC, Tofts PS, Symms MR, Barker GJ, Thompson AJ, Miller DH (2001). "Quantitative contrast-enhanced magnetic resonance imaging to evaluate blood-brain barrier integrity in multiple sclerosis: a preliminary study". Mult. Scler. 7 (2): 75–82. doi:10.1177/135245850100700201. PMID 11424635.
- ↑ van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L, Gerritsen W, Kooi EJ, Witte ME, Geurts JJ, de Vries HE, Peferoen-Baert R, van den Elsen PJ, van der Valk P, Amor S (2012). "Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation". J Neuroinflammation. 9: 156. doi:10.1186/1742-2094-9-156. PMC 3411485. PMID 22747960.
- ↑ Johnson AJ, Suidan GL, McDole J, Pirko I (2007). "The CD8 T cell in multiple sclerosis: suppressor cell or mediator of neuropathology?". Int. Rev. Neurobiol. 79: 73–97. doi:10.1016/S0074-7742(07)79004-9. PMID 17531838.
- ↑ Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH (2008). "B-cell depletion with rituximab in relapsing-remitting multiple sclerosis". N. Engl. J. Med. 358 (7): 676–88. doi:10.1056/NEJMoa0706383. PMID 18272891.
- ↑ 15.0 15.1 Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005). "Cortical demyelination and diffuse white matter injury in multiple sclerosis". Brain. 128 (Pt 11): 2705–12. doi:10.1093/brain/awh641. PMID 16230320.
- ↑ De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, Guidi L, Ghezzi A, Montanari E, Cifelli A, Federico A, Smith SM (2003). "Evidence of early cortical atrophy in MS: relevance to white matter changes and disability". Neurology. 60 (7): 1157–62. PMID 12682324.
- ↑ Dehmeshki J, Chard DT, Leary SM, Watt HC, Silver NC, Tofts PS, Thompson AJ, Miller DH (2003). "The normal appearing grey matter in primary progressive multiple sclerosis: a magnetisation transfer imaging study". J. Neurol. 250 (1): 67–74. doi:10.1007/s00415-003-0955-x. PMID 12527995.
- ↑ Martínez-Lapiscina EH, Ayuso T, Lacruz F, Gurtubay IG, Soriano G, Otano M, Bujanda M, Bacaicoa MC (2013). "Cortico-juxtacortical involvement increases risk of epileptic seizures in multiple sclerosis". Acta Neurol. Scand. 128 (1): 24–31. doi:10.1111/ane.12064. PMID 23289848.
- ↑ Haider L, Simeonidou C, Steinberger G, Hametner S, Grigoriadis N, Deretzi G, Kovacs GG, Kutzelnigg A, Lassmann H, Frischer JM (2014). "Multiple sclerosis deep grey matter: the relation between demyelination, neurodegeneration, inflammation and iron". J. Neurol. Neurosurg. Psychiatry. 85 (12): 1386–95. doi:10.1136/jnnp-2014-307712. PMC 4251183. PMID 24899728.
- ↑ Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Brück W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM (2011). "Inflammatory cortical demyelination in early multiple sclerosis". N. Engl. J. Med. 365 (23): 2188–97. doi:10.1056/NEJMoa1100648. PMC 3282172. PMID 22150037.
- ↑ 21.0 21.1 Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sørensen PS, Laursen H (2010). "Demyelination versus remyelination in progressive multiple sclerosis". Brain. 133 (10): 2983–98. doi:10.1093/brain/awq250. PMID 20855416.
- ↑ Kuhlmann T, Miron V, Cui Q, Cuo Q, Wegner C, Antel J, Brück W (2008). "Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis". Brain. 131 (Pt 7): 1749–58. doi:10.1093/brain/awn096. PMID 18515322.
- ↑ Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Brück W, Lucchinetti C, Lassmann H (2006). "Remyelination is extensive in a subset of multiple sclerosis patients". Brain. 129 (Pt 12): 3165–72. doi:10.1093/brain/awl217. PMID 16921173.
- ↑ Bin JM, Rajasekharan S, Kuhlmann T, Hanes I, Marcal N, Han D, Rodrigues SP, Leong SY, Newcombe J, Antel JP, Kennedy TE (2013). "Full-length and fragmented netrin-1 in multiple sclerosis plaques are inhibitors of oligodendrocyte precursor cell migration". Am. J. Pathol. 183 (3): 673–80. doi:10.1016/j.ajpath.2013.06.004. PMID 23831296.
- ↑ Franklin RJ, Gallo V (2014). "The translational biology of remyelination: past, present, and future". Glia. 62 (11): 1905–15. doi:10.1002/glia.22622. PMID 24446279.
- ↑ Dessa Sadovnick A (July 2002). "The genetics of multiple sclerosis". Clin Neurol Neurosurg. 104 (3): 199–202. PMID 12127654.
- ↑ Ramagopalan SV, Dyment DA (March 2011). "What is Next for the Genetics of Multiple Sclerosis?". Autoimmune Dis. 2011: 519450. doi:10.4061/2011/519450. PMC 3085300. PMID 21541245.
- ↑ Kutzelnigg A, Lassmann H (2014). "Pathology of multiple sclerosis and related inflammatory demyelinating diseases". Handb Clin Neurol. 122: 15–58. doi:10.1016/B978-0-444-52001-2.00002-9. PMID 24507512.
- ↑ Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O (2011). "The neuropathological basis of clinical progression in multiple sclerosis". Acta Neuropathol. 122 (2): 155–70. doi:10.1007/s00401-011-0840-0. PMID 21626034.
- ↑ Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000). "Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination". Ann. Neurol. 47 (6): 707–17. PMID 10852536.
- ↑ Lassmann H, Brück W, Lucchinetti C (2001). "Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy". Trends Mol Med. 7 (3): 115–21. PMID 11286782.
- ↑ Ziabreva I, Campbell G, Rist J, Zambonin J, Rorbach J, Wydro MM, Lassmann H, Franklin RJ, Mahad D (2010). "Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes". Glia. 58 (15): 1827–37. doi:10.1002/glia.21052. PMC 3580049. PMID 20665559.