Mevalonate kinase: Difference between revisions
m (Bot: HTTP→HTTPS (v475)) |
imported>Jsamwrites (Added free to read link in citations with OAbot #oabot) |
||
| Line 11: | Line 11: | ||
{{Infobox_gene}} | {{Infobox_gene}} | ||
'''Mevalonate kinase''' is an [[enzyme]] that in humans is encoded by the ''MVK'' [[gene]].<ref name="entrez">{{cite web | title = Entrez Gene: mevalonate kinase| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4598| accessdate = }}</ref><ref name="pmid1377680">{{cite journal | vauthors = Schafer BL, Bishop RW, Kratunis VJ, Kalinowski SS, Mosley ST, Gibson KM, Tanaka RD | title = Molecular cloning of human mevalonate kinase and identification of a missense mutation in the genetic disease mevalonic aciduria | journal = J. Biol. Chem. | volume = 267 | issue = 19 | pages = 13229–38 |date=July 1992 | pmid = 1377680 | doi = | url = | issn = }}</ref> Mevalonate kinases are found in a wide variety of organisms from bacteria to mammals. This enzyme catalyzes the following reaction: | '''Mevalonate kinase''' is an [[enzyme]] (specifically a [[kinase]]) that in humans is encoded by the ''MVK'' [[gene]].<ref name="entrez">{{cite web | title = Entrez Gene: mevalonate kinase| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4598| accessdate = }}</ref><ref name="pmid1377680">{{cite journal | vauthors = Schafer BL, Bishop RW, Kratunis VJ, Kalinowski SS, Mosley ST, Gibson KM, Tanaka RD | title = Molecular cloning of human mevalonate kinase and identification of a missense mutation in the genetic disease mevalonic aciduria | journal = J. Biol. Chem. | volume = 267 | issue = 19 | pages = 13229–38 |date=July 1992 | pmid = 1377680 | doi = | url = | issn = }}</ref> Mevalonate kinases are found in a wide variety of organisms from bacteria to mammals. This enzyme catalyzes the following reaction: | ||
[[Image:Mevalonate kinase reaction.svg|650px|thumb|center|[[Adenosine triphosphate|ATP]] + ''(R)''-[[mevalonic acid|mevalonate]] <math>\rightleftharpoons</math> [[Adenosine diphosphate|ADP]] + ''(R)''-5-[[phosphomevalonic acid|phosphomevalonate]]]]. | [[Image:Mevalonate kinase reaction.svg|650px|thumb|center|[[Adenosine triphosphate|ATP]] + ''(R)''-[[mevalonic acid|mevalonate]] <math>\rightleftharpoons</math> [[Adenosine diphosphate|ADP]] + ''(R)''-5-[[phosphomevalonic acid|phosphomevalonate]]]]. | ||
| Line 38: | Line 38: | ||
{{refbegin | 2}} | {{refbegin | 2}} | ||
*{{cite journal |vauthors=Ma J, Dempsey AA, Stamatiou D, etal |title=Identifying leukocyte gene expression patterns associated with plasma lipid levels in human subjects. |journal=Atherosclerosis |volume=191 |issue= 1 |pages= 63–72 |year= 2007 |pmid= 16806233 |doi= 10.1016/j.atherosclerosis.2006.05.032 }} | *{{cite journal |vauthors=Ma J, Dempsey AA, Stamatiou D, etal |title=Identifying leukocyte gene expression patterns associated with plasma lipid levels in human subjects. |journal=Atherosclerosis |volume=191 |issue= 1 |pages= 63–72 |year= 2007 |pmid= 16806233 |doi= 10.1016/j.atherosclerosis.2006.05.032 }} | ||
*{{cite journal |vauthors=Willer CJ, Sanna S, Jackson AU, etal |title=Newly identified loci that influence lipid concentrations and risk of coronary artery disease. |journal=Nat. Genet. |volume=40 |issue= 2 |pages= 161–9 |year= 2008 |pmid= 18193043 |doi= 10.1038/ng.76 }} | *{{cite journal |vauthors=Willer CJ, Sanna S, Jackson AU, etal |title=Newly identified loci that influence lipid concentrations and risk of coronary artery disease. |journal=Nat. Genet. |volume=40 |issue= 2 |pages= 161–9 |year= 2008 |pmid= 18193043 |doi= 10.1038/ng.76 |pmc=5206900 }} | ||
*{{cite journal |vauthors=Naruto T, Nakagishi Y, Mori M, etal |title=Hyper-IgD syndrome with novel mutation in a Japanese girl. |journal=Mod Rheumatol |volume=19 |issue= 1 |pages= 96–9 |year= 2009 |pmid= 18941711 |doi= 10.1007/s10165-008-0130-4 }} | *{{cite journal |vauthors=Naruto T, Nakagishi Y, Mori M, etal |title=Hyper-IgD syndrome with novel mutation in a Japanese girl. |journal=Mod Rheumatol |volume=19 |issue= 1 |pages= 96–9 |year= 2009 |pmid= 18941711 |doi= 10.1007/s10165-008-0130-4 }} | ||
*{{cite journal |author=Krisans SK |title=The role of peroxisomes in cholesterol metabolism. |journal=Am. J. Respir. Cell Mol. Biol. |volume=7 |issue= 4 |pages= 358–64 |year= 1992 |pmid= 1356376 |doi= 10.1165/ajrcmb/7.4.358}} | *{{cite journal |author=Krisans SK |title=The role of peroxisomes in cholesterol metabolism. |journal=Am. J. Respir. Cell Mol. Biol. |volume=7 |issue= 4 |pages= 358–64 |year= 1992 |pmid= 1356376 |doi= 10.1165/ajrcmb/7.4.358}} | ||
*{{cite journal |vauthors=Houten SM, Wanders RJ, Waterham HR |title=Biochemical and genetic aspects of mevalonate kinase and its deficiency. |journal=Biochim. Biophys. Acta |volume=1529 |issue= 1-3 |pages= 19–32 |year= 2000 |pmid= 11111075 |doi= 10.1016/s1388-1981(00)00135-9}} | *{{cite journal |vauthors=Houten SM, Wanders RJ, Waterham HR |title=Biochemical and genetic aspects of mevalonate kinase and its deficiency. |journal=Biochim. Biophys. Acta |volume=1529 |issue= 1-3 |pages= 19–32 |year= 2000 |pmid= 11111075 |doi= 10.1016/s1388-1981(00)00135-9|url=https://pure.uva.nl/ws/files/3751864/23070_Thesis.pdf }} | ||
*{{cite journal |vauthors=Fu Z, Voynova NE, Herdendorf TJ, etal |title=Biochemical and structural basis for feedback inhibition of mevalonate kinase and isoprenoid metabolism. |journal=Biochemistry |volume=47 |issue= 12 |pages= 3715–24 |year= 2008 |pmid= 18302342 |doi= 10.1021/bi7024386 }} | *{{cite journal |vauthors=Fu Z, Voynova NE, Herdendorf TJ, etal |title=Biochemical and structural basis for feedback inhibition of mevalonate kinase and isoprenoid metabolism. |journal=Biochemistry |volume=47 |issue= 12 |pages= 3715–24 |year= 2008 |pmid= 18302342 |doi= 10.1021/bi7024386 }} | ||
*{{cite journal |vauthors=Kathiresan S, Willer CJ, Peloso GM, etal |title=Common variants at 30 loci contribute to polygenic dyslipidemia. |journal=Nat. Genet. |volume=41 |issue= 1 |pages= 56–65 |year= 2009 |pmid= 19060906 |doi= 10.1038/ng.291 |pmc=2881676}} | *{{cite journal |vauthors=Kathiresan S, Willer CJ, Peloso GM, etal |title=Common variants at 30 loci contribute to polygenic dyslipidemia. |journal=Nat. Genet. |volume=41 |issue= 1 |pages= 56–65 |year= 2009 |pmid= 19060906 |doi= 10.1038/ng.291 |pmc=2881676}} | ||
Latest revision as of 20:12, 31 October 2018
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Mevalonate kinase is an enzyme (specifically a kinase) that in humans is encoded by the MVK gene.[2][3] Mevalonate kinases are found in a wide variety of organisms from bacteria to mammals. This enzyme catalyzes the following reaction:
.
Function
Mevalonate is a key intermediate, and mevalonate kinase a key early enzyme, in isoprenoid and sterol synthesis.[2]
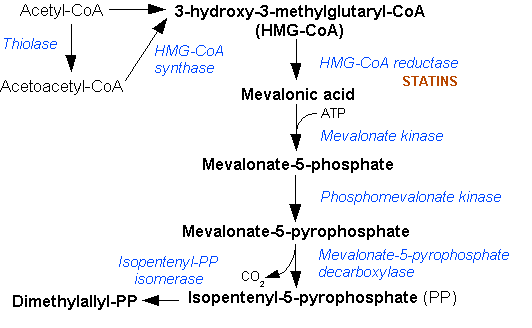 |
Clinical significance
Defects can be associated with hyperimmunoglobulinemia D with recurrent fever.[4]
Mevalonate kinase deficiency caused by mutation of this gene results in mevalonic aciduria, a disease characterized psychomotor retardation, failure to thrive, hepatosplenomegaly, anemia and recurrent febrile crises. Defects in this gene also cause hyperimmunoglobulinaemia D and periodic fever syndrome, a disorder characterized by recurrent episodes of fever associated with lymphadenopathy, arthralgia, gastrointestinal dismay and skin rash.[2]
See also
References
- ↑ PDB: 2X7I; Oke M, Carter LG, Johnson KA, Liu H, McMahon SA, Yan X, Kerou M, Weikart ND, Kadi N, Sheikh MA, Schmelz S, Dorward M, Zawadzki M, Cozens C, Falconer H, Powers H, Overton IM, van Niekerk CA, Peng X, Patel P, Garrett RA, Prangishvili D, Botting CH, Coote PJ, Dryden DT, Barton GJ, Schwarz-Linek U, Challis GL, Taylor GL, White MF, Naismith JH (June 2010). "The Scottish Structural Proteomics Facility: targets, methods and outputs". J. Struct. Funct. Genomics. 11 (2): 167–80. doi:10.1007/s10969-010-9090-y. PMC 2883930. PMID 20419351.
- ↑ 2.0 2.1 2.2 "Entrez Gene: mevalonate kinase".
- ↑ Schafer BL, Bishop RW, Kratunis VJ, Kalinowski SS, Mosley ST, Gibson KM, Tanaka RD (July 1992). "Molecular cloning of human mevalonate kinase and identification of a missense mutation in the genetic disease mevalonic aciduria". J. Biol. Chem. 267 (19): 13229–38. PMID 1377680.
- ↑ Online Mendelian Inheritance in Man (OMIM) 260920
Further reading
- Ma J, Dempsey AA, Stamatiou D, et al. (2007). "Identifying leukocyte gene expression patterns associated with plasma lipid levels in human subjects". Atherosclerosis. 191 (1): 63–72. doi:10.1016/j.atherosclerosis.2006.05.032. PMID 16806233.
- Willer CJ, Sanna S, Jackson AU, et al. (2008). "Newly identified loci that influence lipid concentrations and risk of coronary artery disease". Nat. Genet. 40 (2): 161–9. doi:10.1038/ng.76. PMC 5206900. PMID 18193043.
- Naruto T, Nakagishi Y, Mori M, et al. (2009). "Hyper-IgD syndrome with novel mutation in a Japanese girl". Mod Rheumatol. 19 (1): 96–9. doi:10.1007/s10165-008-0130-4. PMID 18941711.
- Krisans SK (1992). "The role of peroxisomes in cholesterol metabolism". Am. J. Respir. Cell Mol. Biol. 7 (4): 358–64. doi:10.1165/ajrcmb/7.4.358. PMID 1356376.
- Houten SM, Wanders RJ, Waterham HR (2000). "Biochemical and genetic aspects of mevalonate kinase and its deficiency" (PDF). Biochim. Biophys. Acta. 1529 (1–3): 19–32. doi:10.1016/s1388-1981(00)00135-9. PMID 11111075.
- Fu Z, Voynova NE, Herdendorf TJ, et al. (2008). "Biochemical and structural basis for feedback inhibition of mevalonate kinase and isoprenoid metabolism". Biochemistry. 47 (12): 3715–24. doi:10.1021/bi7024386. PMID 18302342.
- Kathiresan S, Willer CJ, Peloso GM, et al. (2009). "Common variants at 30 loci contribute to polygenic dyslipidemia". Nat. Genet. 41 (1): 56–65. doi:10.1038/ng.291. PMC 2881676. PMID 19060906.
- Hager EJ, Gibson KM (2007). "Mevalonate kinase deficiency and autoinflammation". N. Engl. J. Med. 357 (18): 1871–2. doi:10.1056/NEJMc072799. PMID 17978300.
- Marques-Vidal P, Bochud M, Paccaud F, et al. (2010). "No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels". Atherosclerosis. 211 (2): 551–7. doi:10.1016/j.atherosclerosis.2010.04.001. PMID 20430392.
- Lu Y, Dollà ME, Imholz S, et al. (2008). "Multiple genetic variants along candidate pathways influence plasma high-density lipoprotein cholesterol concentrations". J. Lipid Res. 49 (12): 2582–9. doi:10.1194/jlr.M800232-JLR200. PMID 18660489.
- Samkari A, Borzutzky A, Fermo E, et al. (2010). "A novel missense mutation in MVK associated with MK deficiency and dyserythropoietic anemia". Pediatrics. 125 (4): e964–8. doi:10.1542/peds.2009-1774. PMID 20194276.
- Nakayama K, Bayasgalan T, Yamanaka K, et al. (2009). "Large scale replication analysis of loci associated with lipid concentrations in a Japanese population". J. Med. Genet. 46 (6): 370–4. doi:10.1136/jmg.2008.064063. PMID 19487539.
- Koné-Paut I, Sanchez E, Le Quellec A, et al. (2007). "Autoinflammatory gene mutations in Behçet's disease". Ann. Rheum. Dis. 66 (6): 832–4. doi:10.1136/ard.2006.068841. PMC 1954666. PMID 17213252.
- Weissglas-Volkov D, Aguilar-Salinas CA, Sinsheimer JS, et al. (2010). "Investigation of variants identified in caucasian genome-wide association studies for plasma high-density lipoprotein cholesterol and triglycerides levels in Mexican dyslipidemic study samples". Circ Cardiovasc Genet. 3 (1): 31–8. doi:10.1161/CIRCGENETICS.109.908004. PMC 2827864. PMID 20160193.
- Simon A, van der Meer JW, Vesely R, et al. (2006). "Approach to genetic analysis in the diagnosis of hereditary autoinflammatory syndromes". Rheumatology (Oxford). 45 (3): 269–73. doi:10.1093/rheumatology/kei138. PMID 16234278.
- Gattorno M, Sormani MP, D'Osualdo A, et al. (2008). "A diagnostic score for molecular analysis of hereditary autoinflammatory syndromes with periodic fever in children". Arthritis Rheum. 58 (6): 1823–32. doi:10.1002/art.23474. PMID 18512793.
- Fogarty MP, Xiao R, Prokunina-Olsson L, et al. (2010). "Allelic expression imbalance at high-density lipoprotein cholesterol locus MMAB-MVK". Hum. Mol. Genet. 19 (10): 1921–9. doi:10.1093/hmg/ddq067. PMC 2860891. PMID 20159775.
- Nair AK, Young MA, Menon KM (2008). "Regulation of luteinizing hormone receptor mRNA expression by mevalonate kinase--role of the catalytic center in mRNA recognition". FEBS J. 275 (13): 3397–407. doi:10.1111/j.1742-4658.2008.06490.x. PMID 18494797.
- Deo RC, Reich D, Tandon A, et al. (2009). "Genetic differences between the determinants of lipid profile phenotypes in African and European Americans: the Jackson Heart Study". PLoS Genet. 5 (1): e1000342. doi:10.1371/journal.pgen.1000342. PMC 2613537. PMID 19148283.
- Junyent M, Parnell LD, Lai CQ, et al. (2009). "Novel variants at KCTD10, MVK, and MMAB genes interact with dietary carbohydrates to modulate HDL-cholesterol concentrations in the Genetics of Lipid Lowering Drugs and Diet Network Study". Am. J. Clin. Nutr. 90 (3): 686–94. doi:10.3945/ajcn.2009.27738. PMC 2728650. PMID 19605566.
External links
- mevalonate+kinase at the US National Library of Medicine Medical Subject Headings (MeSH)
| Stub icon | This biochemistry article is a stub. You can help Wikipedia by expanding it. |