Hydroxocobalamin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hydroxocobalamin is a vitamin from the vitamin B complex and a Cyanide Antidote that is FDA approved for the treatment of known or suspected cyanide poisoning.. Common adverse reactions include transient chromaturia, erythema, rash, increased blood pressure, nausea, headache, and injection site reactions..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cyanide Poisoning
Recommended Dosing
The starting dose of hydroxocobalamin for adults is 5 g administered as an intravenous infusion over 15 minutes (approximately 15 mL/min). Administration of the entire vial constitutes a complete starting dose. Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by intravenous infusion for a total dose of 10 g. The rate of infusion for the second dose may range from 15 minutes (for patients in extremis) to two hours, as clinically indicated.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroxocobalamin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxocobalamin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Hydroxocobalamin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroxocobalamin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxocobalamin in pediatric patients.
Contraindications
There is limited information regarding Hydroxocobalamin Contraindications in the drug label.
Warnings
Emergency Patient Management
In addition to Cyanokit, treatment of cyanide poisoning must include immediate attention to airway patency, adequacy of oxygenation and hydration, cardiovascular support, and management of any seizure activity. Consideration should be given to decontamination measures based on the route of exposure.
Allergic Reactions
Use caution in the management of patients with known anaphylactic reactions to hydroxocobalamin or cyanocobalamin. Consideration should be given to use of alternative therapies, if available.
- Allergic reactions may include: anaphylaxis, chest tightness, edema, urticaria, pruritus, dyspnea, and rash.
- Allergic reactions including angioneurotic edema have also been reported in postmarketing experience.
Blood Pressure Increase
Many patients with cyanide poisoning will be hypotensive; however, elevations in blood pressure have also been observed in known or suspected cyanide poisoning victims. Elevations in blood pressure (≥180 mmHg systolic or ≥110 mmHg diastolic) were observed in approximately 18% of healthy subjects (not exposed to cyanide) receiving hydroxocobalamin 5 g and 28% of subjects receiving 10 g. Increases in blood pressure were noted shortly after the infusions were started; the maximal increase in blood pressure was observed toward the end of the infusion. These elevations were generally transient and returned to baseline levels within 4 hours of dosing.
Use of Blood Cyanide Assay
While determination of blood cyanide concentration is not required for management of cyanide poisoning and should not delay treatment with Cyanokit, collecting a pretreatment blood sample may be useful for documenting cyanide poisoning as sampling post-Cyanokit use may be inaccurate.
Interference with Clinical Laboratory Evaluations and Clinical Methods
Clinical Laboratory Evaluations
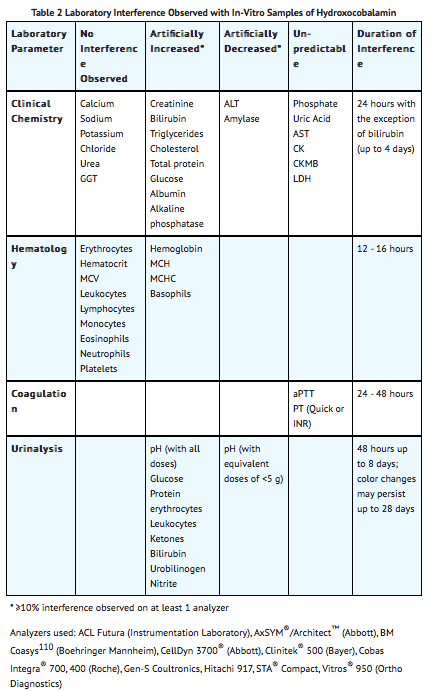
Because of its deep red color, hydroxocobalamin has been found to interfere with colorimetric determination of certain laboratory parameters (e.g., clinical chemistry, hematology, coagulation, and urine parameters). In-vitro tests indicated that the extent and duration of the interference are dependent on numerous factors such as the dose of hydroxocobalamin, analyte, methodology, analyzer, hydroxocobalamin concentration, and partially on the time between sampling and measurement.
Based on in-vitro studies and pharmacokinetic data obtained in healthy volunteers, the following table (TABLE 2) describes laboratory interference that may be observed following a 5 g dose of hydroxocobalamin. Interference following a 10 g dose can be expected to last up to an additional 24 hours. The extent and duration of interference in cyanide-poisoned patients may differ. Results may vary substantially from one analyzer to another; therefore, caution should be used when reporting and interpreting laboratory results.
Photosensitivity
Hydroxocobalamin absorbs visible light in the UV spectrum. It therefore has potential to cause photosensitivity. While it is not known if the skin redness predisposes to photosensitivity, patients should be advised to avoid direct sun while their skin remains discolored.
Adverse Reactions
Clinical Trials Experience
Clinical Studies Experience
Because clinical trials were conducted under widely varying conditions, adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice.
Experience in Healthy Subjects
A double-blind, randomized, placebo-controlled, single-ascending-dose (2.5, 5, 7.5, and 10 g) study was conducted to assess the safety, tolerability, and pharmacokinetics of hydroxocobalamin in 136 healthy adult subjects. Because of the dark red color of hydroxocobalamin, the two most frequently occurring adverse reactions were chromaturia (red-colored urine) which was reported in all subjects receiving a 5 g dose or greater; and erythema (skin redness), which occurred in most subjects receiving a 5 g dose or greater. Adverse reactions reported in at least 5% of the 5 g dose group and corresponding rates in the 10 g and placebo groups are shown in TABLE 3.

In this study, the following adverse reactions were reported to have occurred in a dose-dependent fashion and with greater frequency than observed in placebo-treated cohorts: increased blood pressure (particularly diastolic blood pressure), rash, nausea, headache and infusion site reactions. All were mild to moderate in severity and resolved spontaneously when the infusion was terminated or with standard supportive therapies.
Other adverse reactions reported in this study and considered clinically relevant were:
- Eye disorders: swelling, irritation, redness
- Gastrointestinal disorders: dysphagia, abdominal discomfort, vomiting, diarrhea, dyspepsia, hematochezia
- General disorders and administration site conditions: peripheral edema, chest discomfort
- Immune system disorders: allergic reaction
- Nervous system disorders: memory impairment, dizziness
- Psychiatric disorders: restlessness
- Respiratory, thoracic and mediastinal disorders: dyspnea, throat tightness, dry throat
- Skin and subcutaneous tissue disorders: urticaria, pruritus
- Vascular disorders: hot flush
Experience in Known or Suspected Cyanide Poisoning Victims
Four open-label, uncontrolled, clinical studies (one of which was prospective and three of which were retrospective) were conducted in known or suspected cyanide-poisoning victims. A total of 245 patients received hydroxocobalamin treatment in these studies. Systematic collection of adverse events was not done in all of these studies and interpretation of causality is limited due to the lack of a control group and due to circumstances of administration (e.g., use in fire victims).
Adverse reactions reported in these studies listed by system organ class included:
- Cardiac disorders: ventricular extrasystoles
- Investigations: electrocardiogram depolarization abnormality, heart rate increased
- Respiratory, thoracic, and mediastinal disorders: pleural effusion
- Adverse reactions common to both the studies in known or suspected cyanide poisoning victims and the study in healthy volunteers are listed in the healthy volunteer section only and are not duplicated in this list.
Postmarketing Experience
There is limited information regarding Hydroxocobalamin Postmarketing Experience in the drug label.
Drug Interactions
No formal drug interaction studies have been conducted with Cyanokit.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well controlled studies of Cyanokit in pregnant women. In animal studies, hydroxocobalamin caused skeletal and visceral (soft tissue) abnormalities at exposures (based on AUC) similar to human exposures at the therapeutic dose. Cyanokit should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because cyanide readily crosses the placenta, maternal cyanide poisoning results in fetal cyanide poisoning. Timely treatment of the pregnant mother may be lifesaving for both mother and fetus.
In animal studies, pregnant rats and rabbits received Cyanokit (75, 150, or 300 mg/kg/d) during the period of organogenesis. Following intraperitoneal dosing in rats and intravenous dosing in rabbits, maternal exposures were equivalent to 0.5, 1, or 2 times the human exposure at the therapeutic dose (based on AUC). In the high dose groups for both species, maternal toxicity occurred, and there was a reduced number of live fetuses due to embryofetal resorptions. In addition, decreased live fetal weight occurred in high dose rats, but not in rabbits. Incomplete skeletal ossification occurred in both rats and rabbits. In rats, two fetuses of the high dose group and two fetuses of the mid dose group (each from a different litter) had short, rudimentary or small front or hind legs. Rabbit litters and fetuses exhibited a dose dependant increase in various gross soft tissue and skeletal anomalies. The main findings in rabbits were flexed, rigid flexor or medially rotated forelimbs or hindlimbs and domed heads at external examination; enlarged anterior or posterior fontanelles of the ventricles of the brain and flat, bowed or large ribs at skeletal examination; and dilated ventricles of the brain, and thick wall of the stomach at visceral examination.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydroxocobalamin in women who are pregnant.
Labor and Delivery
The effect of Cyanokit on labor and delivery is unknown.
Nursing Mothers
It is not known whether hydroxocobalamin is excreted in human milk. Cyanokit may be administered in life-threatening situations, and therefore, breast-feeding is not a contraindication to its use. Because of the unknown potential for adverse reactions in nursing infants, the patient should discontinue nursing after receiving Cyanokit.
Pediatric Use
Safety and effectiveness of Cyanokit have not been established in this population. In non-US marketing experience, a dose of 70 mg/kg has been used to treat pediatric patients.
Geriatic Use
Approximately 50 known or suspected cyanide poisoning victims aged 65 or older received hydroxocobalamin in clinical studies. In general, the safety and effectiveness of hydroxocobalamin in these patients was similar to that of younger patients. No adjustment of dose is required in elderly patients.
Gender
There is no FDA guidance on the use of Hydroxocobalamin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydroxocobalamin with respect to specific racial populations.
Renal Impairment
The safety and effectiveness of Cyanokit have not been studied in patients with renal impairment.
Hydroxocobalamin and cyanocobalamin are eliminated unchanged by the kidneys. Oxalate crystals have been observed in the urine of both healthy subjects given hydroxocobalamin and patients treated with hydroxocobalamin following suspected cyanide poisoning.
Hepatic Impairment
The safety and effectiveness of Cyanokit have not been studied in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydroxocobalamin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydroxocobalamin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Hydroxocobalamin Administration in the drug label.
Monitoring
There is limited information regarding Hydroxocobalamin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hydroxocobalamin and IV administrations.
Overdosage
No data are available about overdose with Cyanokit in adults. Should overdose occur, treatment should be directed to the management of symptoms. Hemodialysis may be effective in such a circumstance, but is only indicated in the event of significant hydroxocobalamin-related toxicity. Because of its deep red color, hydroxocobalamin may interfere with the performance of hemodialysis machines.
Pharmacology
Mechanism of Action
Cyanide is an extremely toxic poison. In the absence of rapid and adequate treatment, exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well. Signs and symptoms of acute systemic cyanide poisoning may develop rapidly within minutes, depending on the route and extent of cyanide exposure.
The action of Cyanokit in the treatment of cyanide poisoning is based on its ability to bind cyanide ions. Each hydroxocobalamin molecule can bind one cyanide ion by substituting it for the hydroxo ligand linked to the trivalent cobalt ion, to form cyanocobalamin, which is then excreted in the urine.
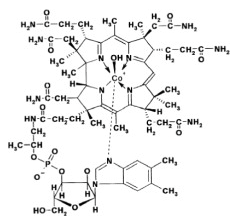
Structure
Hydroxocobalamin has a molecular weight of 1346.36 atomic mass units, an empirical formula of C62H89CoN13O15P and the following structural formula:
Pharmacodynamics
Administration of Cyanokit to cyanide-poisoned patients with the attendant formation of cyanocobalamin resulted in increases in blood pressure and variable changes in heart rate upon initiation of hydroxocobalamin infusions.
Pharmacokinetics
Following intravenous administration of hydroxocobalamin significant binding to plasma proteins and low molecular weight physiological compounds occurs, forming various cobalamin-(III) complexes by replacing the hydroxo ligand. The low molecular weight cobalamins-(III) formed, including hydroxocobalamin, are termed “free cobalamins-(III)”; the sum of free and protein-bound cobalamins is termed “total cobalamins-(III)”. In order to reflect the exposure to the sum of all derivatives, pharmacokinetics of cobalamins-(III) (i.e. cobalamin-(III) entity without specific ligand) were investigated instead of hydroxocobalamin alone, using the concentration unit μg eq/mL.
Dose-proportional pharmacokinetics were observed following single dose intravenous administration of 2.5 to 10 g of hydroxocobalamin in healthy volunteers. Mean free and total cobalamins-(III) Cmax values of 113 and 579 μg eq/mL, respectively, were determined following a dose of 5 g of hydroxocobalamin. Similarly, mean free and total cobalamins-(III) Cmax values of 197 and 995 μg eq/mL, respectively, were determined following the dose of 10 g of hydroxocobalamin. The predominant mean half-life of free and total cobalamins-(III) was found to be approximately 26 to 31 hours at both the 5 g and 10 g dose level.
The mean total amount of cobalamins-(III) excreted in urine during the collection period of 72 hours was about 60% of a 5 g dose and about 50% of a 10 g dose of hydroxocobalamin. Overall, the total urinary excretion was calculated to be at least 60 to 70% of the administered dose. The majority of the urinary excretion occurred during the first 24 hours, but red-colored urine was observed for up to 35 days following the intravenous infusion.
When normalized for body weight, male and female subjects revealed no major differences in pharmacokinetic parameters of free and total cobalamins-(III) following the administration of 5 and 10 g of hydroxocobalamin.
Nonclinical Toxicology
There is limited information regarding Hydroxocobalamin Nonclinical Toxicology in the drug label.
Clinical Studies
Due to ethical considerations, no controlled human efficacy studies have been performed. A controlled animal study demonstrated efficacy in cyanide-poisoned adult dogs [see Animal Pharmacology (13.2)].
Smoke Inhalation Victims
A prospective, uncontrolled, open-label study was carried out in 69 subjects who had been exposed to smoke inhalation from fires. Subjects had to be over 15 years of age, present with soot in the mouth and expectoration (to indicate significant smoke exposure), and have altered neurological status. The median hydroxocobalamin dose was 5 g with a range from 4 to 15 g.
Fifty of 69 subjects (73%) survived following treatment with hydroxocobalamin. Nineteen subjects treated with hydroxocobalamin did not survive. Fifteen patients treated with hydroxocobalamin were in cardiac arrest initially at the scene; 13 of these subjects died and 2 survived.
Of the 42 subjects with pretreatment cyanide levels considered to be potentially toxic, 28 (67%) survived. Of the 19 subjects whose pretreatment cyanide levels were considered potentially lethal, 11 (58%) survived. Of the 50 subjects who survived, 9 subjects (18%) had neurological sequelae at hospital discharge. These included dementia, confusion, psychomotor retardation, anterograde amnesia, intellectual deterioration moderate cerebellar syndrome, aphasia, and memory impairment.
Two additional retrospective, uncontrolled studies were carried out in subjects who had been exposed to cyanide from fire or smoke inhalation. Subjects were treated with up to 15 g of hydroxocobalamin. Survival in these two studies was 34 of 61 (56%) for one study, and 30 of 72 (42%) for the second.
Cyanide Poisoning by Ingestion or Inhalation
A retrospective, uncontrolled study was carried out in 14 subjects who had been exposed to cyanide from sources other than from fire or smoke (i.e., ingestion or inhalation). Subjects were treated with 5 to 20 g of hydroxocobalamin. Eleven of 12 subjects whose blood cyanide concentration was known had initial blood cyanide levels considered to be above the lethal threshold.
Ten of 14 subjects (71%) survived, following administration of hydroxocobalamin. One of the four subjects who died had presented in cardiac arrest. Of the 10 subjects who survived, only 1 subject had neurological sequelae at hospital discharge. This subject had post-anoxic encephalopathy, with memory impairment, considered to be due to cyanide poisoning.
Cross-Study Findings
Experience with Dosing Greater than 10 g of Hydroxocobalamin
Across all four uncontrolled studies, 10 patients who did not demonstrate a full response to 5 or 10 g-doses of hydroxocobalamin were treated with more than 10 g of hydroxocobalamin. One of these 10 patients survived with unspecified neurological sequelae.
Effects on Blood Pressure
Initiation of hydroxocobalamin infusion as part of the therapeutic interventions generally resulted in increases in blood pressure and variable changes in heart rate (often normalization).
Survival of Patients Presenting in Cardiac Arrest
Of the 245 patients across all four studies, 68 (28%) presented in cardiac arrest. While blood pressure and heart rate may have been restored in many of these 68 patients, only five (7%) survived.
How Supplied
Each Cyanokit carton (NDC 11704-370-01) consists of the following:
- One 250 mL glass vial, containing lyophilized hydroxocobalamin for injection, 5 g
- One sterile transfer spike
- One sterile intravenous infusion set
- One quick use reference guide
- One package insert
- Diluent is not included
Storage
- Lyophilized form: Store at 25°C (77°F); excursions permitted to 15-30°C (59 to 86°F) [see USP Controlled Room Temperature].
- Cyanokit may be exposed during short periods to the temperature variations of usual transport (15 days submitted to temperatures ranging from 5 to 40°C (41 to 104°F), transport in the desert (4 days submitted to temperatures ranging from 5 to 60°C (41 to 140°F)) and freezing/defrosting cycles (15 days submitted to temperatures ranging from -20 to 40°C (-4 to 104°F)).
- Reconstituted solution: Store up to 6 hours at a temperature not exceeding 40ºC (104°F). Do not freeze. Discard any unused portion after 6 hours.
Images
Drug Images
{{#ask: Page Name::Hydroxocobalamin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Hydroxocobalamin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Hydroxocobalamin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Hydroxocobalamin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Hydroxocobalamin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Hydroxocobalamin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Injectable (IM) |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Very high (90%) |
| Metabolism | Primarily hepatic. Cobalamins are absorbed in the ileum and stored in the liver. |
| Elimination half-life | ~6 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C64H93CoN13O17P |
| Molar mass | 1406.46 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
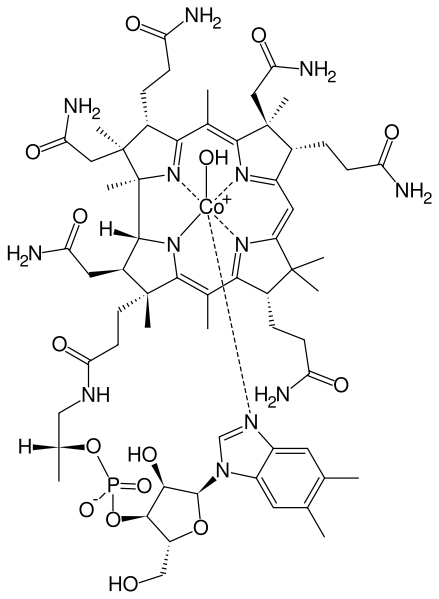
Hydroxocobalamin (OHCbl) is a natural analog of vitamin B12, a basic member of the cobalamin family of compounds. Once described as the most beautiful compound in the world[citation needed] since it has an intense red color. Vitamin B12 is a term that refers to a group of compounds called cobalamins that are available in the human body in a variety of mostly interconvertible forms. Together with folic acid, cobalamins are essential cofactors required for DNA synthesis in cells where chromosomal replication and division are occurring—most notably the bone marrow and myeloid cells. As a cofactor, cobalamins are essential for two cellular reactions: (1) the mitochondrial methylmalonylcoenzyme A mutase conversion of methylmalonic acid (MMA) to succinate, which links lipid and carbohydrate metabolism, and (2) activation of methionine synthase, which is the rate limiting step in the synthesis of methionine from homocysteine and tetrahydrofolate (Katzung, 1989).
Chemical characteristics
Description: OHCbl acetate occurs as an odorless, dark-red orthorhombic needles. The injection formulations appear as a clear, dark-red solution. Distribution Coefficient: 1.133 × 10-5 (octanol:acetate buffer pH 7.4) pKa: 7.65 Systematic Name: Cobinamide, Co-hydroxy-, dihydrogen phosphate (ester), inner salt, 3'- ester with (5,6-dimethyl-1-alpha-D-ribofuranosyl-1H-benzimidazole- kappaN3)
Dietary Deficiency
Hydroxocobalamin Injection USP, are as used to rectify the following causes of B12 deficiency (taken from the drug prescription label published by the FDA:
a. Pernicious anemia both uncomplicated and accompanied by nervous system involvement
b. Dietary deficiency of vitamin B12 occurring in strict vegetarians and in their breastfed infants. (Isolated vitamin B12 deficiency is very rare.)
c. Malabsorption of vitamin B12 resulting from structural or functional damage to the stomach where IF is secreted or to the ilium where IF facilitates vitamin B12 absorption (These conditions include tropical sprue and nontropical sprue [idiopathic steatorrhea, glutean-induced enterpathy]. Folate deficiency in these patients is usually more severe than vitamin B12 deficiency.)
d. Inadequate secretion of IF, resulting from lesions that destroy the gastric mucosa (ingestion of corrosives, extensive neoplasia, and a number of conditions associated with a variable degree of agastric atrophy, such as multiple sclerosis, certain endocrine disorders, iron deficiency, and subtotal gastrectomy). (Total gastrectomy always produces vitamin B12 deficiency.)
e. Structural lesions leading to vitamin B12 deficiency include regional ilultis, ileal reactions, malignancies, etc.
f. Competition for vitamin B12 by intestinal parasites or bacteria
g. The fish tapeworm (Diphyllobothrium latum) absorbs huge quantities of vitamin B12 and infested patients often have associated gastric atrophy. The blind loop syndrome may produce deficiency of vitamin B12 or folate.
h. Inadequate utilization of vitamin B12 (This may occur if antimetabolites for the vitamin are employed in the treatment of neoplasia.)
Indications
Vitamin B12 deficiency
Compound is used as prescription medicine (injection) for vitamin B12 replacement therapy usually at 100 mcg/dose.
For more , the standard therapy for treatment of vitamin B12 deficiency has been intramuscular (IM) injections of vitamin B12 in the form of cyanocobalamin (CNCbl) or hydroxocobalamin (OHCbl). CNCbl is traditionally prescribed in the United States. Outside of the United States, OHCbl is most generally used for vitamin B12 replacement therapy and is considered the “drug of choice” for vitamin B12 deficiency by the Martindale Extra Pharmacopoeia (Sweetman, 2002) and the World Health Organization (WHO) Model List of Essential Drugs. This preference for OHCbl in many countries is due to its long retention in the body and the need for less frequent IM injections in restoring vitamin B12 (cobalamin) serum levels. Furthermore, IM administration of OHCbl is also the preferred treatment for the following: pediatric patients with intrinsic cobalamin metabolic diseases; vitamin B12 deficient patients with tobacco amblyopia due to cyanide poisoning; and patients with pernicious anemia who have optic neuropathy (Carethers, 1988; Chisholm et al., 1967; Freeman, 1992; Markle, 1996).
In a newly-diagnosed vitamin B12-deficient patient, that is when serum cobalamin (vitamin B12) levels are less than 200 pg/mL, daily IM injections of OHCbl up to 1,000 μg per day are given to replenish the body’s depleted cobalamin stores. In the presence of neurological symptoms, following daily treatment, weekly or biweekly injections are indicated for 6 months before initiating monthly IM injections. Once clinical improvement is confirmed, maintenance IM injections of OHCbl or CNCbl must be given monthly for life. Damage that results from vitamin B12 deficiency can be prevented with early diagnosis and adequate treatment.
Cyanide poisoning
Recent regulatory approval for use for cyanide poisoning at doses from 2.5 to 10 gm per injection. Hydroxocobalamin will bind circulating and cellular cyanide molecules to form cyanocobalamin which is excreted in the urine.
Toxicity
The literature data on the acute toxicity profile of OHCbl show that it is generally regarded as safe with local and systemic exposure. The ability of OHCbl to rapidly scavenge and detoxify cyanide by chelation has resulted in several acute animal and human studies using systemic OHCbl doses at suprapharmacological doses as high as 140 mg/kg to support its use as an intravenous (IV) treatment for cyanide exposure (Forsyth et al., 1993; Riou et al., 1993). The US FDA at the end of 2006 approved the use OHCbl as an injection for the treatment of cyanide poisoning.
B12 group
Vitamin B12 is a term that refers to a group of compounds called cobalamins that are available in the human body in a variety of mostly interconvertible forms. Together with folic acid, cobalamins are essential cofactors required for DNA synthesis in cells where chromosomal replication and division are occurring—most notably the bone marrow and myeloid cells. As a cofactor, cobalamins are essential for two cellular reactions:
(1) the mitochondrial methylmalonylcoenzyme A mutase conversion of methylmalonic acid (MMA) to succinate, which links lipid and carbohydrate metabolism, and
(2) activation of methionine synthase, which is the rate limiting step in the synthesis of methionine from homocysteine and tetrahydrofolate (Katzung, 1989). Cobalamins are characterized by a porphyrin like corrin nucleus that contains a single cobalt atom bound to a benzimidazolyl nucleotide and a variable residue (R) group.
The variable R group gives rise to the four most commonly known cobalamins: CNCbl, methylcobalamin, 5-deoxyadenosylcobalamin, and OHCbl. In the serum, OHCbl and CNCbl are believed to function as storage or transport forms of the molecule; whereas, methylcobalamin and 5 deoxyadenosylcobalamin are the active forms of the coenzyme required for cell growth and replication (Katzung, 1989). CNCbl is usually converted to OHCbl in the serum, whereas OHCbl is converted to either methylcobalamin or 5 deoxyadenosyl cobalamin. Cobalamins circulate bound to serum proteins called transcobalamins (TC)and haptocorrins. OHCbl has a higher affinity to the TC II transport protein than CNCbl, or 5- deoxyadenosylcobalamin. From a biochemical point of view, two essential enzymatic reactions require vitamin B12 (cobalamin) (Katzung, 1989, Hardman, 2001).
Intracellular vitamin B12 is maintained in two active coenzymes, methylcobalamin and 5 deoxyadenosylcobalamin, which are both involved in specific enzymatic reactions. In the face of vitamin B12 deficiency, conversion of methylmalonyl-CoA to succinyl-CoA cannot take place, which results in accumulation of methylmalonyl CoA and aberrant fatty acid synthesis. In the other enzymatic reaction, methylcobalamin supports the methionine synthetase reaction, which is essential for normal metabolism of folate. The folate-cobalamin interaction is pivotal for normal synthesis of purines and pyrimidines and the transfer of the methyl group to cobalamin is essential for the adequate supply of tetrahydrofolate, the substrate for metabolic steps that require folate.
In a state of vitamin B12 deficiency, the cell responds by redirecting folate metabolic pathways to supply increasing amounts of methyltetrahydrofolate. The resulting elevated concentrations of homocysteine and MMA are often found in patients with low serum vitamin B12 and can usually be lowered with successful vitamin B12 replacement therapy. However, elevated MMA and homocysteine concentrations may persist in patients with cobalamin concentrations between 200 to 350 pg/mL (Lindenbaum et al 1994). Supplementation with vitamin B12 during conditions of deficiency restores the intracellular level of cobalamin and maintains a sufficient level of the two active coenzymes: methylcobalamin and deoxyadenosylcobalamin.
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from August 2007
- Articles with invalid date parameter in template
- B vitamins
- Cobalt compounds
- Organometallic chemistry