Gemcitabine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | <10% |
| Elimination half-life | Short infusions 32-94 minutes for long infusions 245-638 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
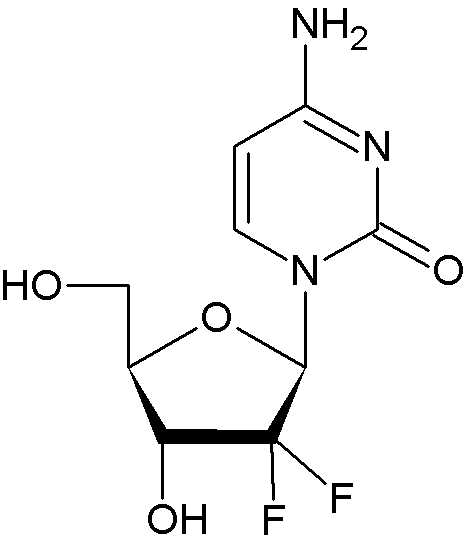
| Formula | C9H11F2N3O4 |
| Molar mass | 263.198 g/mol |
|
WikiDoc Resources for Gemcitabine |
|
Articles |
|---|
|
Most recent articles on Gemcitabine Most cited articles on Gemcitabine |
|
Media |
|
Powerpoint slides on Gemcitabine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Gemcitabine at Clinical Trials.gov Clinical Trials on Gemcitabine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Gemcitabine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Gemcitabine Discussion groups on Gemcitabine Patient Handouts on Gemcitabine Directions to Hospitals Treating Gemcitabine Risk calculators and risk factors for Gemcitabine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Gemcitabine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Gemcitabine is a nucleoside analog used as chemotherapy. It is marketed as Gemzar® by Eli Lilly and Company.
Pharmacology
Chemically gemcitabine is a nucleoside analog in which the hydrogens on the 2' carbons of deoxycytidine are replaced by fluorides.
As with fluorouracil and other analogues of pyrimidines, the drug replaces one of the building blocks of nucleic acids, in this case cytidine, during DNA replication. The process arrests tumor growth, as new nucleosides cannot be attached to the "faulty" nucleoside, resulting in apoptosis (cellular "suicide").
Indications
Gemcitabine is used in various carcinomas: non-small cell lung cancer, pancreatic cancer, bladder cancer and breast cancer. It is being investigated for use in oesophageal cancer, and is used experimentally in lymphomas and various other tumor types. Gemcitabine represents an advance in pancreatic cancer care. It is also not as debilitating as other forms of chemotherapy.
"Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Controlled Trial" reported in the Journal of the American Medical Association (JAMA. 2007;297:239.) suggest that gemcitabine shows benefit in patients with pancreatic cancer who were felt to have successful tumor resections.
Recently, research done at the Anderson Cancer Center in Texas on mice with pancreatic cancer has been done where cromolyn, an allergy and asthma drug with little side effects has been effective in binding with proteins that cancer cells produce. The long term prospects of this new development are very encouraging. Cromolyn is used for asthma, as an eye drop and can be taken orally to treat mastocytosis. The study was published on December 20, 2006 in a leading Cancer journal.
A protein produced by cancerous cells in the pancreas called S100P is found in excess among pancreatic cancer patients and is necessary for cancer cell growth and survival. In addition, S100P activates a cell surface protein receptor called RAGE.
Gemcitabine became first line treatment for bladder cancer Stage 4 with metastases in combination with Cisplatin after a study with 405 patients showed similar efficacy but less toxicity compared to the former MVAC regimen ( J Clin Oncol 2000;18:3068). This new CG-regimen is Cisplatin on day 2, Gemcitabine on days 1,8,15.
External links
de:Gemcitabin gemcitabine is a topo-isomerase tubule inhibitor REF: S. Hussein
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents
- Lilly