Caspase 8: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
imported>Rjwilmsi m (→Interactions: Journal cites: fix DOI, Added 1 doi to a journal cite) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Infobox gene}} | |||
{{ | |||
}} | |||
'''Caspase-8''' is a [[caspase]] protein, encoded by the ''CASP8'' gene. It most likely acts upon [[caspase-3]]. | |||
''CASP8'' [[orthologs]]<ref name="OrthoMaM">{{cite web | title = OrthoMaM phylogenetic marker: CASP8 coding sequence | url = http://www.orthomam.univ-montp2.fr/orthomam/data/cds/detailMarkers/ENSG00000064012_CASP8.xml }}</ref> have been identified in numerous [[mammals]] for which complete genome data are available. These unique orthologs are also present in [[birds]]. | |||
}} | |||
== Function == | |||
The ''CASP8'' gene encodes a member of the [[cysteine]]-[[aspartic acid]] [[protease]] ([[caspase]]) family. Sequential activation of caspases plays a central role in the execution-phase of cell [[apoptosis]]. Caspases exist as inactive [[proenzyme]]s composed of a [[prodomain]], a large protease [[Protein subunit|subunit]], and a small protease subunit. Activation of caspases requires [[proteolytic]] processing at conserved internal aspartic residues to generate a [[heterodimeric]] enzyme consisting of the large and small subunits. This protein is involved in the [[programmed cell death]] induced by [[Fas receptor|Fas]] and various apoptotic stimuli. The N-terminal [[FADD]]-like death effector domain of this protein suggests that it may interact with Fas-interacting protein FADD. This protein was detected in the insoluble fraction of the affected brain region from [[Huntington disease]] patients but not in those from normal controls, which implicated the role in [[neurodegenerative]] diseases. Many alternatively spliced transcript variants encoding different isoforms have been described, although not all variants have had their full-length sequences determined.<ref>{{cite web | title = Entrez Gene: CASP8 caspase 8, apoptosis-related cysteine peptidase| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=841| accessdate = }}</ref> | |||
''' | |||
== Clinical significance == | |||
[[ | A very rare genetic disorder of the immune system can also be caused by mutations in this gene. This disease, called CEDS, stands for “Caspase eight deficiency state.” CEDS has features similar to [[Autoimmune lymphoproliferative syndrome|ALPS]], another genetic disease of [[apoptosis]], with the addition of an [[immunodeficient]] phenotype. Thus, the clinical manifestations include [[splenomegaly]] and [[lymphadenopathy]], in addition to recurrent sinopulmonary infections, recurrent [[mucocutaneous]] [[herpesvirus]], persistent warts and [[molluscum contagiosum]] infections, and [[hypogammaglobulinemia]]. There is sometimes lymphocytic infiltrative disease in [[parenchymal]] organs, but [[autoimmunity]] is minimal and [[lymphoma]] has not been observed in the CEDS patients. CEDS is inherited in an autosomal recessive manner.<ref name="Chun_2002">{{cite journal | vauthors = Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ | title = Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency | journal = Nature | volume = 419 | issue = 6905 | pages = 395–9 | year = 2002 | pmid = 12353035 | doi = 10.1038/nature01063 }}</ref> | ||
= | The clinical phenotype of CEDS patients represented a [[paradox]] since caspase-8 was considered to be chiefly a [[proapoptotic]] [[protease]], that was mainly involved in signal transduction from [[Tumor necrosis factor receptor]] family death receptors such as Fas. The defect in lymphocyte activation and protective immunity suggested that caspase-8 had additional signaling roles in [[lymphocytes]]. Further work revealed that caspase-8 was essential for the induction of the transcription factor “nuclear factor κB” ([[NF-κB]]) after stimulation through [[antigen]] receptors, Fc receptors, or Toll-like receptor 4 in T, B, and [[natural killer cells]].<ref name="Chun_2002"/> | ||
==Further reading== | Biochemically, caspase-8 was found to enter the complex of the inhibitor of [[NF-κB]] [[kinase]] (IKK) with the upstream Bcl10-MALT1 (mucosa-associated lymphatic tissue) adapter complex which were crucial for the induction of nuclear translocation of NF-κB. Moreover, the biochemical form of caspase-8 differed in the two pathways. For the death pathway, the caspase-8 [[zymogen]] is cleaved into subunits that assemble to form the mature, highly active caspase heterotetramer whereas for the activation pathway, the zymogen appears to remain intact perhaps to limit its proteolytic function but enhance its capability as an adapter protein.<ref name="Chun_2002"/> | ||
== Interactions == | |||
Caspase-8 has been shown to [[Protein-protein interaction|interact]] with: | |||
{{div col|colwidth=20em}} | |||
* [[BCAP31]],<ref name = pmid9334338>{{cite journal | vauthors = Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC | title = p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum | journal = J. Cell Biol. | volume = 139 | issue = 2 | pages = 327–38 | date = October 1997 | pmid = 9334338 | pmc = 2139787 | doi = 10.1083/jcb.139.2.327 }}</ref> | |||
* [[BH3 interacting domain death agonist|BID]],<ref name = pmid15659383/><ref name = pmid11832478/> | |||
* [[Bcl-2]],<ref name = pmid11832478/><ref name = pmid11406564>{{cite journal | vauthors = Poulaki V, Mitsiades N, Romero ME, Tsokos M | title = Fas-mediated apoptosis in neuroblastoma requires mitochondrial activation and is inhibited by FLICE inhibitor protein and Bcl-2 | journal = Cancer Res. | volume = 61 | issue = 12 | pages = 4864–72 | date = June 2001 | pmid = 11406564 | doi = }}</ref> | |||
* [[CFLAR]],<ref name = pmid12887920>{{cite journal | vauthors = Micheau O, Tschopp J | title = Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes | journal = Cell | volume = 114 | issue = 2 | pages = 181–90 | date = July 2003 | pmid = 12887920 | doi = 10.1016/s0092-8674(03)00521-x }}</ref><ref name = pmid9208847>{{cite journal | vauthors = Shu HB, Halpin DR, Goeddel DV | title = Casper is a FADD- and caspase-related inducer of apoptosis | journal = Immunity | volume = 6 | issue = 6 | pages = 751–63 | date = June 1997 | pmid = 9208847 | doi = 10.1016/s1074-7613(00)80450-1 }}</ref><ref name = pmid9289491>{{cite journal | vauthors = Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D | title = CASH, a novel caspase homologue with death effector domains | journal = J. Biol. Chem. | volume = 272 | issue = 32 | pages = 19641–4 | date = August 1997 | pmid = 9289491 | doi = 10.1074/jbc.272.32.19641 }}</ref><ref name = pmid9228018>{{cite journal | vauthors = Srinivasula SM, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce CM, Litwack G, Tomaselli KJ, Armstrong RC, Alnemri ES | title = FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis | journal = J. Biol. Chem. | volume = 272 | issue = 30 | pages = 18542–5 | date = July 1997 | pmid = 9228018 | doi = 10.1074/jbc.272.30.18542 }}</ref><ref name = pmid12215447>{{cite journal | vauthors = Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grütter MG | title = The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex | journal = J. Biol. Chem. | volume = 277 | issue = 47 | pages = 45162–71 | date = November 2002 | pmid = 12215447 | doi = 10.1074/jbc.M206882200 }}</ref><ref name = pmid9326610>{{cite journal | vauthors = Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L | title = MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 94 | issue = 21 | pages = 11333–8 | date = October 1997 | pmid = 9326610 | pmc = 23459 | doi = 10.1073/pnas.94.21.11333 }}</ref><ref name = pmid11741985>{{cite journal | vauthors = Roth W, Stenner-Liewen F, Pawlowski K, Godzik A, Reed JC | title = Identification and characterization of DEDD2, a death effector domain-containing protein | journal = J. Biol. Chem. | volume = 277 | issue = 9 | pages = 7501–8 | date = March 2002 | pmid = 11741985 | doi = 10.1074/jbc.M110749200 }}</ref> | |||
* [[Caspase-10]],<ref name = pmid15659383>{{cite journal | vauthors = Gajate C, Mollinedo F | title = Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy | journal = J. Biol. Chem. | volume = 280 | issue = 12 | pages = 11641–7 | date = March 2005 | pmid = 15659383 | doi = 10.1074/jbc.M411781200 }}</ref><ref name = pmid11832478>{{cite journal | vauthors = Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES | title = Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria | journal = J. Biol. Chem. | volume = 277 | issue = 16 | pages = 13430–7 | date = April 2002 | pmid = 11832478 | doi = 10.1074/jbc.M108029200 }}</ref><ref name = pmid12887920/><ref name = pmid8962078>{{cite journal | vauthors = Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES | title = Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 93 | issue = 25 | pages = 14486–91 | date = December 1996 | pmid = 8962078 | pmc = 26159 | doi = 10.1073/pnas.93.25.14486 }}</ref> | |||
* [[Caspase-2]],<ref name = pmid11832478/><ref name = pmid8962078/> | |||
* [[Caspase-3]],<ref name = pmid11832478/><ref name = pmid8962078/> | |||
* [[Caspase-6]],<ref name = pmid11832478/><ref name = pmid8962078/><ref name = pmid12232792>{{cite journal | vauthors = Cowling V, Downward J | title = Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain | journal = Cell Death Differ. | volume = 9 | issue = 10 | pages = 1046–56 | date = October 2002 | pmid = 12232792 | doi = 10.1038/sj.cdd.4401065 }}</ref> | |||
* [[Caspase-7]],<ref name = pmid11832478/><ref name = pmid8962078/> | |||
* [[Caspase-9]],<ref name = pmid11832478/><ref name = pmid8962078/> | |||
* [[DEDD]],<ref name = pmid11965497>{{cite journal | vauthors = Zhan Y, Hegde R, Srinivasula SM, Fernandes-Alnemri T, Alnemri ES | title = Death effector domain-containing proteins DEDD and FLAME-3 form nuclear complexes with the TFIIIC102 subunit of human transcription factor IIIC | journal = Cell Death Differ. | volume = 9 | issue = 4 | pages = 439–47 | date = April 2002 | pmid = 11965497 | doi = 10.1038/sj.cdd.4401038}}</ref><ref name = pmid12527898>{{cite journal | vauthors = Alcivar A, Hu S, Tang J, Yang X | title = DEDD and DEDD2 associate with caspase-8/10 and signal cell death | journal = Oncogene | volume = 22 | issue = 2 | pages = 291–7 | date = January 2003 | pmid = 12527898 | doi = 10.1038/sj.onc.1206099 }}</ref><ref name = pmid9774341>{{cite journal | vauthors = Stegh AH, Schickling O, Ehret A, Scaffidi C, Peterhänsel C, Hofmann TG, Grummt I, Krammer PH, Peter ME | title = DEDD, a novel death effector domain-containing protein, targeted to the nucleolus | journal = EMBO J. | volume = 17 | issue = 20 | pages = 5974–86 | date = October 1998 | pmid = 9774341 | pmc = 1170924 | doi = 10.1093/emboj/17.20.5974 }}</ref> | |||
* [[FADD]],<ref name = pmid15659383/><ref name = pmid12887920/><ref name = pmid9228018/><ref name = pmid19060883/><ref name = pmid12911633>{{cite journal | vauthors = Henshall DC, Araki T, Schindler CK, Shinoda S, Lan JQ, Simon RP | title = Expression of death-associated protein kinase and recruitment to the tumor necrosis factor signaling pathway following brief seizures | journal = J. Neurochem. | volume = 86 | issue = 5 | pages = 1260–70 | date = September 2003 | pmid = 12911633 | doi = 10.1046/j.1471-4159.2003.01934.x }}</ref><ref name = pmid8681376>{{cite journal | vauthors = Boldin MP, Goncharov TM, Goltsev YV, Wallach D | title = Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death | journal = Cell | volume = 85 | issue = 6 | pages = 803–15 | date = June 1996 | pmid = 8681376 | doi = 10.1016/s0092-8674(00)81265-9 }}</ref><ref name = pmid12107169>{{cite journal | vauthors = Thomas LR, Stillman DJ, Thorburn A | title = Regulation of Fas-associated death domain interactions by the death effector domain identified by a modified reverse two-hybrid screen | journal = J. Biol. Chem. | volume = 277 | issue = 37 | pages = 34343–8 | date = September 2002 | pmid = 12107169 | doi = 10.1074/jbc.M204169200 }}</ref> | |||
* [[Fas ligand|FasL]],<ref name = pmid15659383/><ref name = pmid12887920/> | |||
* [[Fas receptor|FasR]],<ref name = pmid15659383/><ref name = pmid9208847/><ref name = pmid9325248>{{cite journal | vauthors = MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES | title = Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL | journal = J. Biol. Chem. | volume = 272 | issue = 41 | pages = 25417–20 | date = October 1997 | pmid = 9325248 | doi = 10.1074/jbc.272.41.25417 }}</ref> | |||
* [[IFT57]],<ref name = pmid11788820>{{cite journal | vauthors = Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst CA, Leavitt BR, Metzler M, Hackam AS, Tam J, Vaillancourt JP, Houtzager V, Rasper DM, Roy S, Hayden MR, Nicholson DW | title = Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi | journal = Nat. Cell Biol. | volume = 4 | issue = 2 | pages = 95–105 | date = February 2002 | pmid = 11788820 | doi = 10.1038/ncb735 }}</ref> | |||
* [[NOL3]],<ref name = pmid9560245>{{cite journal | vauthors = Koseki T, Inohara N, Chen S, Núñez G | title = ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 95 | issue = 9 | pages = 5156–60 | date = April 1998 | pmid = 9560245 | pmc = 20230 | doi = 10.1073/pnas.95.9.5156 }}</ref> | |||
* [[PEA15]],<ref name = pmid10493725>{{cite journal | vauthors = Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H | title = Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis | journal = J. Neurosci. | volume = 19 | issue = 19 | pages = 8244–51 | date = October 1999 | pmid = 10493725 | doi = }}</ref><ref name = pmid10442631>{{cite journal | vauthors = Condorelli G, Vigliotta G, Cafieri A, Trencia A, Andalò P, Oriente F, Miele C, Caruso M, Formisano P, Beguinot F | title = PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis | journal = Oncogene | volume = 18 | issue = 31 | pages = 4409–15 | date = August 1999 | pmid = 10442631 | doi = 10.1038/sj.onc.1202831 }}</ref> | |||
* [[RIPK1]],<ref name = pmid19060883>{{cite journal | vauthors = Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Benedict Yen TS, Yen B, Woo T, Malynn BA, Ma A | title = ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development | journal = Nature | volume = 457 | issue = 7231 | pages = 906–9 | date = February 2009 | pmid = 19060883 | pmc = 2642523 | doi = 10.1038/nature07575 }}</ref><ref name = pmid11002417>{{cite journal | vauthors = Chaudhary PM, Eby MT, Jasmin A, Kumar A, Liu L, Hood L | title = Activation of the NF-kappaB pathway by caspase 8 and its homologs | journal = Oncogene | volume = 19 | issue = 39 | pages = 4451–60 | date = September 2000 | pmid = 11002417 | doi = 10.1038/sj.onc.1203812 }}</ref><ref name = pmid18570872>{{cite journal | vauthors = Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA | title = cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination | journal = Mol. Cell | volume = 30 | issue = 6 | pages = 689–700 | date = June 2008 | pmid = 18570872 | doi = 10.1016/j.molcel.2008.05.014 }}</ref> | |||
* [[TNFRSF10B]],<ref name = pmid15659383/><ref name = pmid9325248/> and | |||
* [[TRAF1]].<ref name = pmid12887920/><ref name = pmid11098060>{{cite journal | vauthors = Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC | title = TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis | journal = J. Biol. Chem. | volume = 276 | issue = 11 | pages = 8087–93 | date = March 2001 | pmid = 11098060 | doi = 10.1074/jbc.M009450200 }}</ref> | |||
{{Div col end}} | |||
==Additional photos== | |||
{| | |||
|[[Image:TNF signaling.jpg|thumbnail|200px|Signaling pathway of [[Tumor necrosis factor-alpha|TNF]]-R1. Dashed grey lines represent multiple steps]][[Image:Signal transduction pathways.svg|200px|thumb|Overview of signal transduction pathways involved in [[apoptosis]].]] | |||
|} | |||
== See also == | |||
{{Portal|Molecular and Cellular Biology|border=no}} | |||
* [[Caspase]] | |||
* [[FADD]] | |||
* [[The Proteolysis Map]] | |||
== References == | |||
{{reflist|2}} | |||
== Further reading == | |||
{{refbegin | 2}} | {{refbegin | 2}} | ||
*{{cite journal | * {{cite journal | vauthors = Jia SH, Parodo J, Kapus A, Rotstein OD, Marshall JC | title = Dynamic Regulation of Neutrophil Survival through Tyrosine Phosphorylation or Dephosphorylation of Caspase-8 | journal = Journal of Biological Chemistry | volume = 283 | issue = 9 | pages = 5402–5413 | year = 2008 | pmid = 18086677 | doi = 10.1074/jbc.M706462200 }} | ||
* {{cite journal | vauthors = Cohen GM | title = Caspases: the executioners of apoptosis | journal = Biochem. J. | volume = 326 | issue = Pt 1 | pages = 1–16 | year = 1997 | pmid = 9337844 | pmc = 1218630 | doi = 10.1042/bj3260001}} | |||
* {{cite journal | vauthors = Siegel RM, Chan FK, Chun HJ, Lenardo MJ | title = The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity | journal = Nat. Immunol. | volume = 1 | issue = 6 | pages = 469–74 | year = 2001 | pmid = 11101867 | doi = 10.1038/82712 }} | |||
*{{cite journal | * {{cite journal | vauthors = Ye S, Goldsmith EJ | title = Serpins and other covalent protease inhibitors | journal = Curr. Opin. Struct. Biol. | volume = 11 | issue = 6 | pages = 740–5 | year = 2002 | pmid = 11751056 | doi = 10.1016/S0959-440X(01)00275-5 }} | ||
*{{cite journal | * {{cite journal | vauthors = Gupta S | title = Tumor necrosis factor-alpha-induced apoptosis in T cells from aged humans: a role of TNFR-I and downstream signaling molecules | journal = Exp. Gerontol. | volume = 37 | issue = 2–3 | pages = 293–9 | year = 2002 | pmid = 11772515 | doi = 10.1016/S0531-5565(01)00195-4 }} | ||
*{{cite journal | * {{cite journal | vauthors = Pomerantz RJ | title = Effects of HIV-1 Vpr on neuroinvasion and neuropathogenesis | journal = DNA Cell Biol. | volume = 23 | issue = 4 | pages = 227–38 | year = 2004 | pmid = 15142380 | doi = 10.1089/104454904773819815 }} | ||
*{{cite journal | * {{cite journal | vauthors = Zhao LJ, Zhu H | title = Structure and function of HIV-1 auxiliary regulatory protein Vpr: novel clues to drug design | journal = Curr. Drug Targets Immune Endocr. Metabol. Disord. | volume = 4 | issue = 4 | pages = 265–75 | year = 2005 | pmid = 15578977 | doi = 10.2174/1568008043339668 }} | ||
*{{cite journal | |||
*{{cite journal | |||
}} | |||
{{refend}} | {{refend}} | ||
== | == External links == | ||
* [[ | * The [[MEROPS]] online database for peptidases and their inhibitors: [http://merops.sanger.ac.uk/cgi-bin/merops.cgi?id=C14.009 C14.009] | ||
* [https://www.youtube.com/watch?v=29AMumxsEo0 Apoptosis & Caspase 8]—[[The Proteolysis Map]] (animation) | |||
* {{MeshName|Caspase+8}} | * {{MeshName|Caspase+8}} | ||
* [http://deathbase.org/search_protein_id.php?id=casp8 caspase-8] | |||
{{PDB Gallery|geneid=841}} | |||
{{Fas apoptosis signaling pathway}} | |||
{{Cysteine proteases}} | |||
{{Enzymes}} | |||
[[Category:Peptidase]] | |||
[[Category:EC 3.4.22]] | |||
[[Category:Caspases]] | |||
Latest revision as of 16:03, 16 February 2018
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Caspase-8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase-3. CASP8 orthologs[1] have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds.
Function
The CASP8 gene encodes a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes composed of a prodomain, a large protease subunit, and a small protease subunit. Activation of caspases requires proteolytic processing at conserved internal aspartic residues to generate a heterodimeric enzyme consisting of the large and small subunits. This protein is involved in the programmed cell death induced by Fas and various apoptotic stimuli. The N-terminal FADD-like death effector domain of this protein suggests that it may interact with Fas-interacting protein FADD. This protein was detected in the insoluble fraction of the affected brain region from Huntington disease patients but not in those from normal controls, which implicated the role in neurodegenerative diseases. Many alternatively spliced transcript variants encoding different isoforms have been described, although not all variants have had their full-length sequences determined.[2]
Clinical significance
A very rare genetic disorder of the immune system can also be caused by mutations in this gene. This disease, called CEDS, stands for “Caspase eight deficiency state.” CEDS has features similar to ALPS, another genetic disease of apoptosis, with the addition of an immunodeficient phenotype. Thus, the clinical manifestations include splenomegaly and lymphadenopathy, in addition to recurrent sinopulmonary infections, recurrent mucocutaneous herpesvirus, persistent warts and molluscum contagiosum infections, and hypogammaglobulinemia. There is sometimes lymphocytic infiltrative disease in parenchymal organs, but autoimmunity is minimal and lymphoma has not been observed in the CEDS patients. CEDS is inherited in an autosomal recessive manner.[3]
The clinical phenotype of CEDS patients represented a paradox since caspase-8 was considered to be chiefly a proapoptotic protease, that was mainly involved in signal transduction from Tumor necrosis factor receptor family death receptors such as Fas. The defect in lymphocyte activation and protective immunity suggested that caspase-8 had additional signaling roles in lymphocytes. Further work revealed that caspase-8 was essential for the induction of the transcription factor “nuclear factor κB” (NF-κB) after stimulation through antigen receptors, Fc receptors, or Toll-like receptor 4 in T, B, and natural killer cells.[3]
Biochemically, caspase-8 was found to enter the complex of the inhibitor of NF-κB kinase (IKK) with the upstream Bcl10-MALT1 (mucosa-associated lymphatic tissue) adapter complex which were crucial for the induction of nuclear translocation of NF-κB. Moreover, the biochemical form of caspase-8 differed in the two pathways. For the death pathway, the caspase-8 zymogen is cleaved into subunits that assemble to form the mature, highly active caspase heterotetramer whereas for the activation pathway, the zymogen appears to remain intact perhaps to limit its proteolytic function but enhance its capability as an adapter protein.[3]
Interactions
Caspase-8 has been shown to interact with:
- BCAP31,[4]
- BID,[5][6]
- Bcl-2,[6][7]
- CFLAR,[8][9][10][11][12][13][14]
- Caspase-10,[5][6][8][15]
- Caspase-2,[6][15]
- Caspase-3,[6][15]
- Caspase-6,[6][15][16]
- Caspase-7,[6][15]
- Caspase-9,[6][15]
- DEDD,[17][18][19]
- FADD,[5][8][11][20][21][22][23]
- FasL,[5][8]
- FasR,[5][9][24]
- IFT57,[25]
- NOL3,[26]
- PEA15,[27][28]
- RIPK1,[20][29][30]
- TNFRSF10B,[5][24] and
- TRAF1.[8][31]
Additional photos
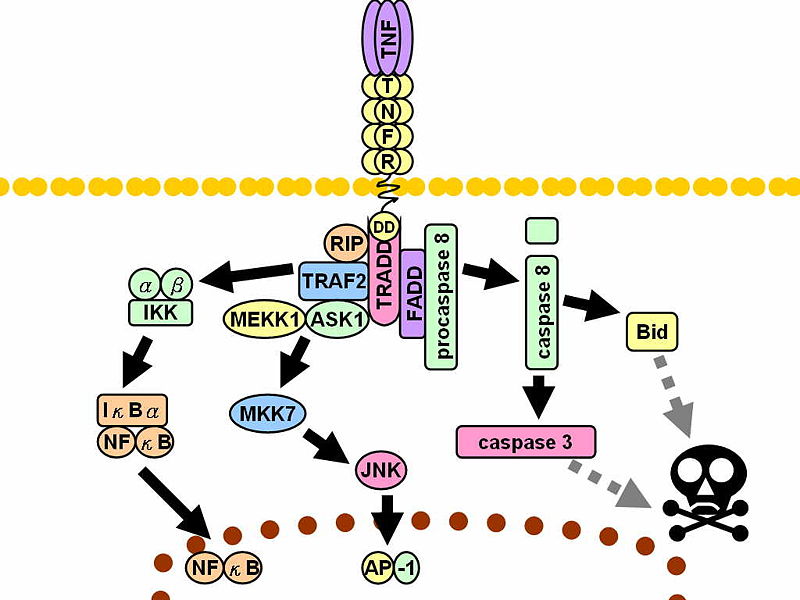 |
See also
References
- ↑ "OrthoMaM phylogenetic marker: CASP8 coding sequence".
- ↑ "Entrez Gene: CASP8 caspase 8, apoptosis-related cysteine peptidase".
- ↑ 3.0 3.1 3.2 Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson TP, Straus SE, Lenardo MJ (2002). "Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency". Nature. 419 (6905): 395–9. doi:10.1038/nature01063. PMID 12353035.
- ↑ Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC (October 1997). "p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum". J. Cell Biol. 139 (2): 327–38. doi:10.1083/jcb.139.2.327. PMC 2139787. PMID 9334338.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Gajate C, Mollinedo F (March 2005). "Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy". J. Biol. Chem. 280 (12): 11641–7. doi:10.1074/jbc.M411781200. PMID 15659383.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (April 2002). "Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria". J. Biol. Chem. 277 (16): 13430–7. doi:10.1074/jbc.M108029200. PMID 11832478.
- ↑ Poulaki V, Mitsiades N, Romero ME, Tsokos M (June 2001). "Fas-mediated apoptosis in neuroblastoma requires mitochondrial activation and is inhibited by FLICE inhibitor protein and Bcl-2". Cancer Res. 61 (12): 4864–72. PMID 11406564.
- ↑ 8.0 8.1 8.2 8.3 8.4 Micheau O, Tschopp J (July 2003). "Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes". Cell. 114 (2): 181–90. doi:10.1016/s0092-8674(03)00521-x. PMID 12887920.
- ↑ 9.0 9.1 Shu HB, Halpin DR, Goeddel DV (June 1997). "Casper is a FADD- and caspase-related inducer of apoptosis". Immunity. 6 (6): 751–63. doi:10.1016/s1074-7613(00)80450-1. PMID 9208847.
- ↑ Goltsev YV, Kovalenko AV, Arnold E, Varfolomeev EE, Brodianskii VM, Wallach D (August 1997). "CASH, a novel caspase homologue with death effector domains". J. Biol. Chem. 272 (32): 19641–4. doi:10.1074/jbc.272.32.19641. PMID 9289491.
- ↑ 11.0 11.1 Srinivasula SM, Ahmad M, Ottilie S, Bullrich F, Banks S, Wang Y, Fernandes-Alnemri T, Croce CM, Litwack G, Tomaselli KJ, Armstrong RC, Alnemri ES (July 1997). "FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis". J. Biol. Chem. 272 (30): 18542–5. doi:10.1074/jbc.272.30.18542. PMID 9228018.
- ↑ Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grütter MG (November 2002). "The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex". J. Biol. Chem. 277 (47): 45162–71. doi:10.1074/jbc.M206882200. PMID 12215447.
- ↑ Han DK, Chaudhary PM, Wright ME, Friedman C, Trask BJ, Riedel RT, Baskin DG, Schwartz SM, Hood L (October 1997). "MRIT, a novel death-effector domain-containing protein, interacts with caspases and BclXL and initiates cell death". Proc. Natl. Acad. Sci. U.S.A. 94 (21): 11333–8. doi:10.1073/pnas.94.21.11333. PMC 23459. PMID 9326610.
- ↑ Roth W, Stenner-Liewen F, Pawlowski K, Godzik A, Reed JC (March 2002). "Identification and characterization of DEDD2, a death effector domain-containing protein". J. Biol. Chem. 277 (9): 7501–8. doi:10.1074/jbc.M110749200. PMID 11741985.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES (December 1996). "Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases". Proc. Natl. Acad. Sci. U.S.A. 93 (25): 14486–91. doi:10.1073/pnas.93.25.14486. PMC 26159. PMID 8962078.
- ↑ Cowling V, Downward J (October 2002). "Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain". Cell Death Differ. 9 (10): 1046–56. doi:10.1038/sj.cdd.4401065. PMID 12232792.
- ↑ Zhan Y, Hegde R, Srinivasula SM, Fernandes-Alnemri T, Alnemri ES (April 2002). "Death effector domain-containing proteins DEDD and FLAME-3 form nuclear complexes with the TFIIIC102 subunit of human transcription factor IIIC". Cell Death Differ. 9 (4): 439–47. doi:10.1038/sj.cdd.4401038. PMID 11965497.
- ↑ Alcivar A, Hu S, Tang J, Yang X (January 2003). "DEDD and DEDD2 associate with caspase-8/10 and signal cell death". Oncogene. 22 (2): 291–7. doi:10.1038/sj.onc.1206099. PMID 12527898.
- ↑ Stegh AH, Schickling O, Ehret A, Scaffidi C, Peterhänsel C, Hofmann TG, Grummt I, Krammer PH, Peter ME (October 1998). "DEDD, a novel death effector domain-containing protein, targeted to the nucleolus". EMBO J. 17 (20): 5974–86. doi:10.1093/emboj/17.20.5974. PMC 1170924. PMID 9774341.
- ↑ 20.0 20.1 Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Benedict Yen TS, Yen B, Woo T, Malynn BA, Ma A (February 2009). "ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development". Nature. 457 (7231): 906–9. doi:10.1038/nature07575. PMC 2642523. PMID 19060883.
- ↑ Henshall DC, Araki T, Schindler CK, Shinoda S, Lan JQ, Simon RP (September 2003). "Expression of death-associated protein kinase and recruitment to the tumor necrosis factor signaling pathway following brief seizures". J. Neurochem. 86 (5): 1260–70. doi:10.1046/j.1471-4159.2003.01934.x. PMID 12911633.
- ↑ Boldin MP, Goncharov TM, Goltsev YV, Wallach D (June 1996). "Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death". Cell. 85 (6): 803–15. doi:10.1016/s0092-8674(00)81265-9. PMID 8681376.
- ↑ Thomas LR, Stillman DJ, Thorburn A (September 2002). "Regulation of Fas-associated death domain interactions by the death effector domain identified by a modified reverse two-hybrid screen". J. Biol. Chem. 277 (37): 34343–8. doi:10.1074/jbc.M204169200. PMID 12107169.
- ↑ 24.0 24.1 MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES (October 1997). "Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL". J. Biol. Chem. 272 (41): 25417–20. doi:10.1074/jbc.272.41.25417. PMID 9325248.
- ↑ Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst CA, Leavitt BR, Metzler M, Hackam AS, Tam J, Vaillancourt JP, Houtzager V, Rasper DM, Roy S, Hayden MR, Nicholson DW (February 2002). "Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi". Nat. Cell Biol. 4 (2): 95–105. doi:10.1038/ncb735. PMID 11788820.
- ↑ Koseki T, Inohara N, Chen S, Núñez G (April 1998). "ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases". Proc. Natl. Acad. Sci. U.S.A. 95 (9): 5156–60. doi:10.1073/pnas.95.9.5156. PMC 20230. PMID 9560245.
- ↑ Kitsberg D, Formstecher E, Fauquet M, Kubes M, Cordier J, Canton B, Pan G, Rolli M, Glowinski J, Chneiweiss H (October 1999). "Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis". J. Neurosci. 19 (19): 8244–51. PMID 10493725.
- ↑ Condorelli G, Vigliotta G, Cafieri A, Trencia A, Andalò P, Oriente F, Miele C, Caruso M, Formisano P, Beguinot F (August 1999). "PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis". Oncogene. 18 (31): 4409–15. doi:10.1038/sj.onc.1202831. PMID 10442631.
- ↑ Chaudhary PM, Eby MT, Jasmin A, Kumar A, Liu L, Hood L (September 2000). "Activation of the NF-kappaB pathway by caspase 8 and its homologs". Oncogene. 19 (39): 4451–60. doi:10.1038/sj.onc.1203812. PMID 11002417.
- ↑ Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA (June 2008). "cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination". Mol. Cell. 30 (6): 689–700. doi:10.1016/j.molcel.2008.05.014. PMID 18570872.
- ↑ Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC (March 2001). "TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis". J. Biol. Chem. 276 (11): 8087–93. doi:10.1074/jbc.M009450200. PMID 11098060.
Further reading
- Jia SH, Parodo J, Kapus A, Rotstein OD, Marshall JC (2008). "Dynamic Regulation of Neutrophil Survival through Tyrosine Phosphorylation or Dephosphorylation of Caspase-8". Journal of Biological Chemistry. 283 (9): 5402–5413. doi:10.1074/jbc.M706462200. PMID 18086677.
- Cohen GM (1997). "Caspases: the executioners of apoptosis". Biochem. J. 326 (Pt 1): 1–16. doi:10.1042/bj3260001. PMC 1218630. PMID 9337844.
- Siegel RM, Chan FK, Chun HJ, Lenardo MJ (2001). "The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity". Nat. Immunol. 1 (6): 469–74. doi:10.1038/82712. PMID 11101867.
- Ye S, Goldsmith EJ (2002). "Serpins and other covalent protease inhibitors". Curr. Opin. Struct. Biol. 11 (6): 740–5. doi:10.1016/S0959-440X(01)00275-5. PMID 11751056.
- Gupta S (2002). "Tumor necrosis factor-alpha-induced apoptosis in T cells from aged humans: a role of TNFR-I and downstream signaling molecules". Exp. Gerontol. 37 (2–3): 293–9. doi:10.1016/S0531-5565(01)00195-4. PMID 11772515.
- Pomerantz RJ (2004). "Effects of HIV-1 Vpr on neuroinvasion and neuropathogenesis". DNA Cell Biol. 23 (4): 227–38. doi:10.1089/104454904773819815. PMID 15142380.
- Zhao LJ, Zhu H (2005). "Structure and function of HIV-1 auxiliary regulatory protein Vpr: novel clues to drug design". Curr. Drug Targets Immune Endocr. Metabol. Disord. 4 (4): 265–75. doi:10.2174/1568008043339668. PMID 15578977.
External links
- The MEROPS online database for peptidases and their inhibitors: C14.009
- Apoptosis & Caspase 8—The Proteolysis Map (animation)
- Caspase+8 at the US National Library of Medicine Medical Subject Headings (MeSH)
- caspase-8