Capecitabine: Difference between revisions
m (Protected "Capecitabine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 23: | Line 23: | ||
| excretion = Renal 95.5%, faecal 2.6% }} | | excretion = Renal 95.5%, faecal 2.6% }} | ||
{{SI}} | {{SI}} | ||
==Overview== | ==Overview== | ||
| Line 72: | Line 72: | ||
{{Chemotherapeutic agents}} | {{Chemotherapeutic agents}} | ||
[[Category:Chemotherapeutic agents]] | [[Category:Chemotherapeutic agents]] | ||
[[Category:Prodrugs]] | [[Category:Prodrugs]] | ||
Revision as of 23:26, 8 August 2012
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extensive |
| Protein binding | < 60% |
| Metabolism | Hepatic, to 5'-DFCR, 5'-DFUR (inactive); neoplastic tissue, 5'-DFUR to active fluorouracil |
| Elimination half-life | 38–45 minutes |
| Excretion | Renal 95.5%, faecal 2.6% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
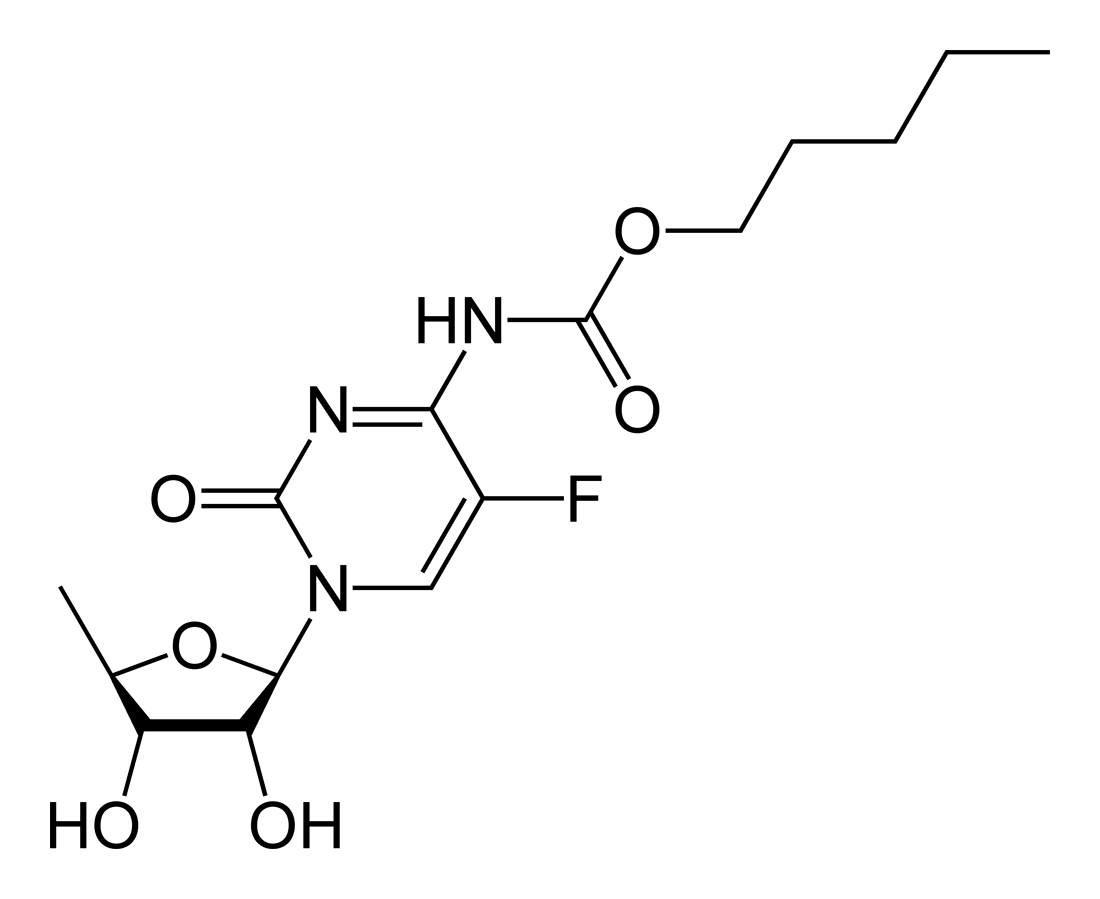
| Formula | C15H22FN3O6 |
| Molar mass | 359.35 g/mol |
|
WikiDoc Resources for Capecitabine |
|
Articles |
|---|
|
Most recent articles on Capecitabine Most cited articles on Capecitabine |
|
Media |
|
Powerpoint slides on Capecitabine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Capecitabine at Clinical Trials.gov Clinical Trials on Capecitabine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Capecitabine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Capecitabine Discussion groups on Capecitabine Patient Handouts on Capecitabine Directions to Hospitals Treating Capecitabine Risk calculators and risk factors for Capecitabine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Capecitabine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Capecitabine (INN) (IPA: Template:IPA) is an orally-administered chemotherapeutic agent used in the treatment of metastatic breast and colorectal cancers. Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows growth of tumor tissue. The activation of capecitabine follows a pathway with three enzymatic steps and two intermediary metabolites, 5'-deoxy-5-fluorocytidine (5'-DFCR) and 5'-deoxy-5-fluorouridine (5'-DFUR), to form 5-fluorouracil. Capecitabine is marketed under the trade name Xeloda (Roche).
Indications
Capecitabine is FDA-approved for:
- Adjuvant in colorectal cancer Stage III Dukes' C - used as first-line monotherapy.
- Metastatic colorectal cancer - used as first-line monotherapy, if appropriate.
- Metastatic breast cancer - used in combination with docetaxel, after failure of anthracycline-based treatment. Also as monotherapy, if the patient has failed paclitaxel-based treatment, and if anthracycline-based treatment has either failed or cannot be continued for other reasons (i.e., the patient has already received the maximum lifetime dose of an anthracycline).
In the UK, capecitabine is approved by the National Institute for Health and Clinical Excellence (NICE) for colon and colorectal cancer, and locally advanced or metastatic breast cancer[1].
Dose
The usual starting dose is 2,500 mg/m2/day in two divided doses, 12 hours apart. One cycle includes two weeks of treatment followed by one week without treatment. Cycles can be repeated every three weeks.
Dose adjustments
- For mild renal dysfunction (creatinine clearance 30-50 mL/min), it is recommended to reduce dose by 25%.
- For severe renal dysfunction (creatinine clearance <30 mL/min), treatment is not recommended.
- There is no recommendation for hepatic dysfunction.
- For elderly patients, lower doses may be required due to higher incidences of serious adverse reactions.
Side effects
Potential major adverse reactions include:
- Cardiovascular: EKG changes, myocardial infarction, angina (these may be more common in patients with pre-existing coronary artery disease)
- Dermatological: Hand-foot syndrome (numbness, tingling, pain, redness, or blistering of the palms of the hands and soles of the feet)
- Gastrointestinal: Diarrhea (sometimes severe), nausea, stomatitis
- Hematological: Neutropenia, anemia, thrombocytopenia
- Hepatic: Hyperbilirubinemia
Drug interactions
- May interact with warfarin and increase bleeding risk.
- May inhibit cytochrome CYP2C9 enzyme, and therefore increase levels of substrates such as phenytoin and other substrates of CYP2C9.
- Much as fluorouracil, the concomitant use of leucovorin may increase both the efficacy and the toxicity of capecitabine.

Formulation
Capecitabine (as brand-name Xeloda®) is available in light peach 150 mg tablets and peach 500 mg tablets.
References
- Lacy, Charles F; Armstrong, Lora L; Goldman, Morton P; Lance, Leonard L (2004). Lexi-Comp's Drug Information Handbook (12th Edition). Lexi-Comp Inc. ISBN 1-59195-083-X
- Fischer, David S; Knobf, M Tish; Durivage, Henry J; Beaulieu, Nancy J (2003). The Cancer Chemotherapy Handbook (6th Edition). Mosby. ISBN 0-323-01890-4
- Thomson Centerwatch: Drugs Approved by the FDA (Xeloda) Retrieved 6/05
External links
- Xeloda.com(patient information, tools, and resources)
- [2] (patient information)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents
- Prodrugs