Amrubicin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
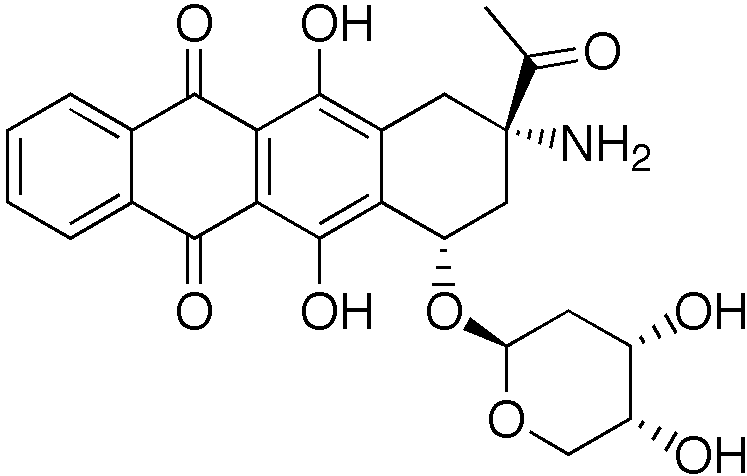
| Formula | C25H25NO9 |
| Molar mass | 483.46 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Amrubicin (INN; previously known as SM-5887) is an anthracycline used in the treatment of lung cancer.[1] It is marketed in Japan since 2002 by Sumitomo Pharmaceuticals under the brand name Calsed.[2]
Amrubicin acts by inhibiting topoisomerase II, and has been compared in clinical trials with topotecan, a Topoisomerase I inhibitor.[3] [4]
It has also been studied for the treatment of bladder carcinoma[5] and gastric cancer.[6]
References
- ↑ Ueoka H, Ohnoshi T, Kimura I (November 1992). "[New anthracycline analogues in the treatment of lung cancer]". Gan To Kagaku Ryoho (in Japanese). 19 (13): 2146–9. PMID 1332624.
- ↑ Sumitomo Pharmaceuticals Co., Ltd. (2003). "CALSED for Injection (English)" (PDF). Retrieved 2008-08-17.[dead link]
- ↑ Celgene Corporation (2008). "Amrubicin(R) Receives FDA Orphan Drug Designation for the Treatment of Small Cell Lung Cancer". Retrieved 2009-07-10.
- ↑ Medical News Today (2007). "Pharmion's Amrubicin Shows Encouraging Results Compared To Standard Of Care In Second Line Treatment Of Small Cell Lung Cancer". Retrieved 2009-07-10.
- ↑ Ohmori H, Tsushima T, Kobashi K (April 1996). "[Experimental studies on intravesical instillation of SM-5887, a novel anthracycline derivative for treatment of bladder carcinoma]". Gan To Kagaku Ryoho (in Japanese). 23 (5): 601–6. PMID 8678519.
- ↑ Tsushima K, Sakata Y, Munakata A; et al. (June 1991). "[A phase II study of SM-5887 for advanced gastric cancer]". Gan To Kagaku Ryoho (in Japanese). 18 (7): 1151–4. PMID 1647150.
Categories:
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Unrecognized language
- All articles with dead external links
- Articles with dead external links from October 2010
- Articles with invalid date parameter in template
- CS1 maint: Explicit use of et al.
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Anthracyclines
- Ketones
- Alcohols
- Quinones