Sorafenib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Alberto Plate [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sorafenib is a kinase inhibitor that is FDA approved for the treatment of unresectable hepatocellular carcinoma, advanced renal cell carcinoma, locally recurrent or metastatic, progressive, differentiated thyroid carcinoma refractory to radioactive iodine treatment. Common adverse reactions include hypertension, acral erythema, alopecia, Peeling of skin, rash, hypoalbuminemia, hypocalcemia, hypophosphatemia, raised TSH level, weight decreased, abdominal pain, decrease in appetite, diarrhea, Increased serum lipase level, Loss of appetite, nausea, serum amylase raised, lymphocytopenia, thrombocytopenia, ALT/SGPT level raised, Infectious diseases, fatigue and pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose for Hepatocellular Carcinoma, Renal Cell Carcinoma, and Differentiated Thyroid Carcinoma
- Dosage: 200 mg q12h, taken 1 hour before or 2 hours after a meal. Treatment should continue until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. Temporary interruption of Sorafenib is recommended in patients undergoing major surgical procedures.
- Temporary interruption or permanent discontinuation of Sorafenib may be required for the following:
- Cardiac ischemia or infarction
- Hemorrhage requiring medical intervention
- Severe or persistent hypertension despite adequate anti-hypertensive therapy
- Gastrointestinal perforation
- QTc prolongation
- Severe drug-induced liver injury
- Temporary interruption or permanent discontinuation of Sorafenib may be required for the following:
Dose modifications for Hepatocellular Carcinoma and Renal Cell Carcinoma
When dose reduction is necessary, the Sorafenib dose may be reduced to 400 mg once daily. If additional dose reduction is required, Sorafenib may be reduced to a single 400 mg dose every other day. If dermatological toxicity presented, the following scheme should be followed:

Dose Modifications for Differentiated Thyroid Carcinoma
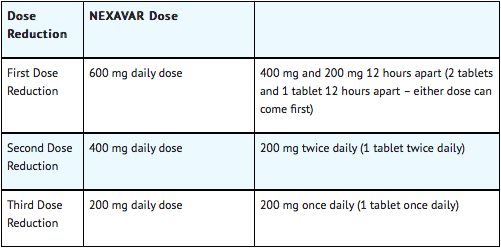
Dose Modification in Skin Toxicity

Following improvement of Grade 2 or 3 dermatologic toxicity to Grade 0–1 after at least 28 days of treatment on a reduced dose of Sorafenib, the dose of Sorafenib may be increased one dose level from the reduced dose. Approximately 50% of patients requiring a dose reduction for dermatologic toxicity are expected to meet these criteria for resumption of the higher dose and roughly 50% of patients resuming the previous dose are expected to tolerate the higher dose (that is, maintain the higher dose level without recurrent Grade 2 or higher dermatologic toxicity)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sorafenib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sorafenib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sorafenib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sorafenib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sorafenib in pediatric patients.
Contraindications
- Sorafenib is contraindicated in patients with known severe hypersensitivity to sorafenib or any other component of Sorafenib.
- Sorafenib in combination with carboplatin and paclitaxel is contraindicated in patients with squamous cell lung cancer.
Warnings
5.1 Risk of Cardiac Ischemia and/or Infarction
In the HCC study, the incidence of cardiac ischemia/infarction was 2.7% in NEXAVAR-treated patients compared with 1.3% in the placebo-treated group, in RCC Study 1, the incidence of cardiac ischemia/infarction was higher in the NEXAVAR-treated group (2.9%) compared with the placebo-treated group (0.4%), and in the DTC study, the incidence of cardiac ischemia/infarction was 1.9% in the NEXAVAR-treated group compared with 0% in the placebo-treated group. Patients with unstable coronary artery disease or recent myocardial infarction were excluded from this study. Temporary or permanent discontinuation of NEXAVAR should be considered in patients who develop cardiac ischemia and/or infarction.
5.2 Risk of Hemorrhage
An increased risk of bleeding may occur following NEXAVAR administration. In the HCC study, an excess of bleeding regardless of causality was not apparent and the rate of bleeding from esophageal varices was 2.4% in NEXAVAR-treated patients and 4% in placebo-treated patients. Bleeding with a fatal outcome from any site was reported in 2.4% of NEXAVAR-treated patients and 4% in placebo-treated patients. In RCC Study 1, bleeding regardless of causality was reported in 15.3% of patients in the NEXAVAR-treated group and 8.2% of patients in the placebo-treated group. The incidence of CTCAE Grade 3 and 4 bleeding was 2% and 0%, respectively, in NEXAVAR-treated patients, and 1.3% and 0.2%, respectively, in placebo-treated patients. There was one fatal hemorrhage in each treatment group in RCC Study 1. In the DTC study, bleeding was reported in 17.4% of NEXAVAR-treated patients and 9.6% of placebo-treated patients; however the incidence of CTCAE Grade 3 bleeding was 1% in NEXAVAR-treated patients and 1.4% in placebo-treated patients. There was no Grade 4 bleeding reported and there was one fatal hemorrhage in a placebo-treated patient. If any bleeding necessitates medical intervention, permanent discontinuation of NEXAVAR should be considered. Due to the potential risk of bleeding, tracheal, bronchial, and esophageal infiltration should be treated with local therapy prior to administering NEXAVAR in patients with DTC.
5.3 Risk of Hypertension
Monitor blood pressure weekly during the first 6 weeks of NEXAVAR. Thereafter, monitor blood pressure and treat hypertension, if required, in accordance with standard medical practice. In the HCC study, hypertension was reported in approximately 9.4% of NEXAVAR-treated patients and 4.3% of patients in the placebo-treated group. In RCC Study 1, hypertension was reported in approximately 16.9% of NEXAVAR-treated patients and 1.8% of patients in the placebo-treated group. In the DTC study, hypertension was reported in 40.6% of NEXAVAR-treated patients and 12.4% of placebo-treated patients. Hypertension was usually mild to moderate, occurred early in the course of treatment, and was managed with standard antihypertensive therapy. In cases of severe or persistent hypertension despite institution of antihypertensive therapy, consider temporary or permanent discontinuation of NEXAVAR. Permanent discontinuation due to hypertension occurred in 1 of 297 NEXAVAR-treated patients in the HCC study, 1 of 451 NEXAVAR-treated patients in RCC Study 1, and 1 of 207 NEXAVAR-treated patients in the DTC study.
5.4 Risk of Dermatologic Toxicities
Hand-foot skin reaction and rash represent the most common adverse reactions attributed to NEXAVAR. Rash and hand-foot skin reaction are usually CTCAE Grade 1 and 2 and generally appear during the first six weeks of treatment with NEXAVAR. Management of dermatologic toxicities may include topical therapies for symptomatic relief, temporary treatment interruption and/or dose modification of NEXAVAR, or in severe or persistent cases, permanent discontinuation of NEXAVAR [see Dosage and Administration (2.2)]. Permanent discontinuation of therapy due to hand-foot skin reaction occurred in 4 (1.3%) of 297 NEXAVAR-treated patients with HCC, 3 (0.7%) of 451 NEXAVAR-treated patients with RCC, and 11 (5.3%) of 207 NEXAVAR-treated patients with DTC.
There have been reports of severe dermatologic toxicities, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). These cases may be life-threatening. Discontinue NEXAVAR if SJS or TEN are suspected.
5.5 Risk of Gastrointestinal Perforation
Gastrointestinal perforation is an uncommon adverse reaction and has been reported in less than 1% of patients taking NEXAVAR. In some cases this was not associated with apparent intra-abdominal tumor. In the event of a gastrointestinal perforation, discontinue NEXAVAR.
5.6 Warfarin
Infrequent bleeding or elevations in the International Normalized Ratio (INR) have been reported in some patients taking warfarin while on NEXAVAR. Monitor patients taking concomitant warfarin regularly for changes in prothrombin time (PT), INR or clinical bleeding episodes.
5.7 Wound Healing Complications
No formal studies of the effect of NEXAVAR on wound healing have been conducted. Temporary interruption of NEXAVAR is recommended in patients undergoing major surgical procedures. There is limited clinical experience regarding the timing of reinitiation of NEXAVAR following major surgical intervention. Therefore, the decision to resume NEXAVAR following a major surgical intervention should be based on clinical judgment of adequate wound healing.
5.8 Increased Mortality Observed with NEXAVAR Administered in Combination with Carboplatin/Paclitaxel and Gemcitabine/Cisplatin in Squamous Cell Lung Cancer
In a subset analysis of two randomized controlled trials in chemo-naive patients with Stage IIIB-IV non-small cell lung cancer, patients with squamous cell carcinoma experienced higher mortality with the addition of NEXAVAR compared to those treated with carboplatin/paclitaxel alone (HR 1.81, 95% CI 1.19–2.74) and gemcitabine/cisplatin alone (HR 1.22, 95% CI 0.82-1.80). The use of NEXAVAR in combination with carboplatin/paclitaxel is contraindicated in patients with squamous cell lung cancer. NEXAVAR in combination with gemcitabine/cisplatin is not recommended in patients with squamous cell lung cancer. The safety and effectiveness of NEXAVAR has not been established in patients with non-small cell lung cancer.
5.9 Risk of QT Interval Prolongation
NEXAVAR can prolong the QT/QTc interval. QT/QTc interval prolongation increases the risk for ventricular arrhythmias.Avoid NEXAVAR in patients with congenital long QT syndrome. Monitor electrolytes and electrocardiograms in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics. Correct electrolyte abnormalities (magnesium, potassium, calcium). Interrupt NEXAVAR if QTc interval is greater than 500 milliseconds or for an increase from baseline of 60 milliseconds or greater [see Clinical Pharmacology (12.2)].
5.10 Drug-Induced Hepatitis
Sorafenib-induced hepatitis is characterized by a hepatocellular pattern of liver damage with significant increases of transaminases which may result in hepatic failure and death. Increases in bilirubin and INR may also occur. The incidence of severe drug-induced liver injury, defined as elevated transaminase levels above 20 times the upper limit of normal or transaminase elevations with significant clinical sequelae (for example, elevated INR, ascites, fatal, or transplantation), was two of 3,357 patients (0.06%) in a global monotherapy database. Monitor liver function tests regularly. In case of significantly increased transaminases without alternative explanation, such as viral hepatitis or progressing underlying malignancy, discontinue NEXAVAR.
5.11 Embryofetal Risk
Based on its mechanism of action and findings in animals, NEXAVAR may cause fetal harm when administered to a pregnant woman. Sorafenib caused embryo-fetal toxicities in animals at maternal exposures that were significantly lower than the human exposures at the recommended dose of 400 mg twice daily. Advise women of childbearing potential to avoid becoming pregnant while on NEXAVAR because of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
5.12 Impairment of Thyroid Stimulating Hormone Suppression in Differentiated Thyroid Carcinoma
NEXAVAR impairs exogenous thyroid suppression. In the DTC study, 99% of patients had a baseline thyroid stimulating hormone (TSH) level less than 0.5 mU/L. Elevation of TSH level above 0.5 mU/L was observed in 41% of NEXAVAR-treated patients as compared with 16% of placebo-treated patients. For patients with impaired TSH suppression while receiving NEXAVAR, the median maximal TSH was 1.6 mU/L and 25% had TSH levels greater than 4.4 mU/L.
Monitor TSH levels monthly and adjust thyroid replacement medication as needed in patients with DTC
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Sorafenib Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Sorafenib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sorafenib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Sorafenib in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sorafenib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sorafenib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sorafenib in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Sorafenib in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Sorafenib in geriatric settings.
Gender
There is no FDA guidance on the use of Sorafenib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sorafenib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sorafenib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sorafenib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sorafenib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sorafenib in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sorafenib Administration in the drug label.
Monitoring
There is limited information regarding Sorafenib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sorafenib and IV administrations.
Overdosage
There is limited information regarding Sorafenib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sorafenib Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sorafenib Mechanism of Action in the drug label.
Structure
There is limited information regarding Sorafenib Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sorafenib Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sorafenib Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sorafenib Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sorafenib Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sorafenib How Supplied in the drug label.
Storage
There is limited information regarding Sorafenib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sorafenib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sorafenib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sorafenib Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sorafenib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sorafenib Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sorafenib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Clinical data | |
|---|---|
| Synonyms | Nexavar Sorafenib tosylate |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 29-49% |
| Protein binding | 99.5% |
| Metabolism | Hepatic oxidation and glucuronidation (CYP3A4-mediated) |
| Elimination half-life | 25–48 hours |
| Excretion | Fecal (77%) and renal (19%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H16ClF3N4O3 |
| Molar mass | 464.825 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Sorafenib |
|
Articles |
|---|
|
Most recent articles on Sorafenib |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sorafenib at Clinical Trials.gov Clinical Trials on Sorafenib at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sorafenib
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sorafenib Discussion groups on Sorafenib Directions to Hospitals Treating Sorafenib Risk calculators and risk factors for Sorafenib
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sorafenib |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Sorafenib (rINN), marketed as Nexavar by Bayer, is a drug approved for the treatment of advanced renal cell carcinoma (primary kidney cancer). It has also received "Fast Track" designation by the FDA[1] for the treatment of advanced hepatocellular carcinoma (primary liver cancer), and has since performed well in Phase III trials[2].
Pharmacology
It is a small molecular inhibitor of Raf kinase, PDGF (platelet-derived growth factor), VEGF receptor 2 & 3 kinases and c Kit the receptor for Stem cell factor. A growing number of drugs target most of these pathways. The originality of Sorafenib lays in its simultaneous targeting of the Raf/Mek/Erk pathway.[3]
Approval
Sorafenib was approved by the U.S. Food and Drug Administration (FDA) on December 20, 2005 and received a E.U. marketing authorisation on July 19, 2006[4].
The European Commission has granted marketing authorization to Nexavar® (sorafenib) tablets for the treatment of patients with hepatocellular carcinoma (HCC), the most common form of liver cancer, on October 30, 2007. [5].
Studies
A New England Journal of Medicine article published in January 2007 showed compared with placebo, treatment with sorafenib prolongs progression-free survival in patients with advanced clear-cell renal-cell carcinoma in whom previous therapy has failed; the median progression-free survival was 5.5 months in the sorafenib group and 2.8 months in the placebo group (hazard ratio for disease progression in the sorafenib group, 0.44; 95% confidence interval [CI], 0.35 to 0.55; P<0.01). The first interim analysis of overall survival in May 2005 showed that sorafenib reduced the risk of death, as compared with placebo (hazard ratio, 0.72; 95% CI, 0.54 to 0.94; P=0.02), although this benefit was not statistically significant according to the O'Brien–Fleming threshold. Partial responses were reported as the best response in 10% of patients receiving sorafenib and in 2% of those receiving placebo (P<0.001).
At ASCO 2007, results from the SHARP trial were presented, which showed efficacy of sorafenib in hepatocellular carcinoma. The primary endpoint was overall survival, which showed a 44% improvement in patients who received sorafenib compared to placebo (hazard ratio 0.69; 95% CI, 0.55 to 0.87; p=0.0001). Both median survival and time to progression showed 3-month improvements.
Regulatory filing is planned.
Side effects
Side effects of sorafenib included skin rash, hand-foot skin reactions, diarrhea, and hypertension.
Footnotes
- ↑ https://lnn605.aus.us.siteprotect.com/medicalnews.php?newsid=45119
- ↑ http://www.healthtech.com/news/top_news/2007/June/Nexavar_Extends_Survival.asp
- ↑ "SorafenibSunitinibdifferences". Retrieved 2007-08-15.
- ↑ European Commission - Enterprise and industry. Nexavar. Retrieved April 24,2007.
- ↑ DGnews [1]. Retrieved November 02, 2007.
External links
- Nexavar.com – Manufacturer's website
- Prescribing Information – includes data from the key studies justifying the use of sorafenib for the treatment of kidney cancer (particularly clear cell renal cell carcinoma, which is associated with the von Hippel-Lindau gene)
- Patient Information from FDA
- Sorafenib in Treating Patients With Soft Tissue Sarcomas
- Clinical trial number NCT00217399 at ClinicalTrials.gov - Sorafenib and Anastrozole in Treating Postmenopausal Women With Metastatic Breast Cancer
- [http:/mpablog.typepad.com/david_foster] Two year experience blog on nexavar, sutent, side effects, etc.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Cancer treatments
- Orphan drugs