Zopiclone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Zopiclone is a central nervous system agent , nonbarbiturate hypnotic that is FDA approved for the treatment of insomnia. Common adverse reactions include unpleasant taste, headache, somnolence, respiratory infection, dizziness, dry mouth, rash, anxiety, hallucinations, and viral infections..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Zopiclone are indicated for the treatment of insomnia. In controlled outpatient and sleep laboratory studies, Zopiclone administered at bedtime decreased sleep latency and improved sleep maintenance.
- The clinical trialsperformed in support of efficacy were up to 6 months in duration. The final formal assessments of sleep latency and maintenance were performed at 4 weeks in the 6-week study (adults only), at the end of both 2-week studies (elderly only) and at the end of the 6-month study (adults only).
Dosing Information
- Use the lowest effective dose for the patient.
- Dosage in Adults:
- The recommended starting dose is 1 mg. Dosing can be raised to 2 mg or 3 mg if clinically indicated. In some patients, the higher morning blood levels of Zopiclone following use of the 2 mg or 3 mg dose increase the risk of next day impairment of driving and other activities that require full alertness. The total dose of Zopiclone should not exceed 3 mg, once daily immediately before bedtime.
- Geriatric or Debilitated Patients:
- The total dose of Zopiclone should not exceed 2 mg in elderly or debilitated patients.
- Patients with Severe Hepatic Impairment, or Taking Potent CYP3A4 Inhibitors
- In patients with severe hepatic impairment, or in patients co-administered Zopiclone with potent CYP3A4inhibitors, the total dose of Zopiclone should not exceed 2 mg.
- Use with CNS Depressants:
- Dosage adjustments may be necessary when Zopiclone is combined with other CNS Depressant drugs because of the potentially additive effects.
- Administration with Food:
- Taking Zopiclone with or immediately after a heavy, high-fat meal results in slower absorption and would be expected to reduce the effect of Zopiclone on [[sleep latency]]
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Zopiclone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Zopiclone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Zopiclone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Zopiclone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Zopiclone in pediatric patients.
Contraindications
- Zopiclone is contraindicated in patients with known hypersensitivity to Zopiclone. Hypersensitivity reactions include anaphylaxis and angioedema.
Warnings
CNS Depressant Effects and Next-Day Impairment==
- Zopiclone is a central nervous system depressant and can impair daytime function in some patients at the higher doses (2 mg or 3 mg), even when used as prescribed. Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of symptoms (or even with subjective improvement), and impairment may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of Zopiclone may develop, patients using 3 mg Zopiclone should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness the day after use.
- Additive effects occur with concomitant use of other CNS Depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol), including daytime use. Downward dose adjustment of Zopiclone and concomitant CNS Depressants should be considered.
- The use of Zopiclone with other sedative-hypnotics at bedtime or the middle of the night is not recommended.
- The risk of next-day psychomotor impairment is increased if Zopiclone is taken with less than a full night of sleep remaining (7- to 8 hours); if higher than the recommended dose is taken; if co- administered with other CNS Depressants; or co-administered with other drugs that increase the blood levels of Zopiclone.
Need to Evaluate for Co-Morbid Diagnoses
- Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric disorders or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including Zopiclone. Because some of the important adverse effects of Zopiclone appear to be dose-related, it is important to use the lowest possible effective dose, especially in the elderly.
Severe Anaphylactic and Anaphylactoid Reactions
- Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including Zopiclone. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with Zopiclone should not be rechallenged with the drug.
Abnormal Thinking and Behavioral Changes
- A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS Depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization. Amnesia and other neuropsychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of sedative/hypnotics.
- Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with Zopiclone alone at therapeutic doses, the use of alcohol and other CNS Depressants with Zopiclone appears to increase the risk of such behaviors, as does the use of Zopiclone at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of Zopiclone should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
- It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above are drug-induced, spontaneous in origin, or a result of an underlying psychiatric disorders or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Withdrawal Effects
- Following rapid dose decrease or abrupt discontinuation of the use of sedative/hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs.
Timing of Drug Administration
- Zopiclone should be taken immediately before bedtime. Taking a sedative/hypnotic while still up and about may result in short-term memory impairment, hallucinations, impaired coordination, dizziness, and lightheadedness.
Special Populations
- Use in Elderly and/or Debilitated Patients:
- Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. The dose should not exceed 2 mg in elderly or debilitated patients.
- Use in Patients with concomitant illness:
- Clinical experience with Zopiclone in patients with concomitant illness is limited. Zopiclone should be used with caution in patients with diseases or conditions that could affect metabolism or hemodynamic responses.
- A study in healthy volunteers did not reveal respiratory-depressant effects at doses 2.5-fold higher (7 mg) than the recommended dose of Zopiclone. adverse effects is advised, however, if Zopiclone is prescribed to patients with compromised respiratory function.
- The dose of Zopiclone should not exceed 2 mg in patients with severe hepatic impairment, because systemic exposure is doubled in such subjects. No dose adjustment appears necessary for subjects with mild or moderate hepatic impairment. No dose adjustment appears necessary in subjects with any degree of renal impairment, since less than 10% of Zopiclone is excreted unchanged in the urine.
- The dose of Zopiclone should be reduced in patients who are administered potent inhibitors of CYP3A4, such as ketoconazole, while taking Zopiclone. Downward dose adjustment is also recommended when Zopiclone is administered with agents having known CNS-depressant effects.
- Use in Patients with Depression:
- sedative/hypnotic drugs should be administered with caution to patients exhibiting signs and symptoms of depression. Suicidal tendencies may be present in such patients, and protective measures may be required. Intentional overdose is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.
Adverse Reactions
Clinical Trials Experience
- Because clinical trialsare conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trialsof another drug and may not reflect the rates observed in clinical practice
- The premarketing development program for Zopiclone included Zopiclone exposures in patients and/or normal subjects from two different groups of studies: approximately 400 normal subjects in clinical pharmacology/pharmacokinetic studies, and approximately 1550 patients in placebo-controlled clinical effectiveness studies, corresponding to approximately 263 patient-exposure years. The conditions and duration of treatment with Zopiclone varied greatly and included (in overlapping categories) open-label and double-blind phases of studies, inpatients and outpatients, and short-term and longer-term exposure. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.
- The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, adverse reaction of the type listed. A reaction was considered treatment-emergent if it occurred for the first time or worsened while the patient was receiving therapy following baseline evaluation.
clinical trials Experience
- Adverse Reactions Resulting in Discontinuation of Treatment:
- In placebo-controlled, parallel-group clinical trialsin the elderly, 3.8% of 208 patients who received placebo, 2.3% of 215 patients who received 2 mg Zopiclone, and 1.4% of 72 patients who received 1 mg Zopiclone discontinued treatment due to an adverse reaction. In the 6‑week parallel-group study in adults, no patients in the 3 mg arm discontinued because of an adverse reaction. In the long-term 6-month study in adult insomnia patients, 7.2% of 195 patients who received placebo and 12.8% of 593 patients who received 3 mg Zopiclone discontinued due to an adverse reaction. No reaction that resulted in discontinuation occurred at a rate of greater than 2%.
- Adverse Reactions Observed at an Incidence of ≥2% in Controlled Trials:
- Table 1 shows the incidence of adverse reactions from a Phase 3 placebo-controlled study of Zopiclone at doses of 2 or 3 mg in non-elderly adults. Treatment duration in this trial was 44 days. The table includes only reactions that occurred in 2% or more of patients treated with Zopiclone 2 mg or 3 mg in which the incidence in patients treated with Zopiclone was greater than the incidence in placebo-treated patients.



- Adverse reactions from Table 1 that suggest a dose-response relationship in adults include viral infection, dry mouth, dizziness, hallucinations, infection, rash, and unpleasant taste, with this relationship clearest for unpleasant taste.
- Table 2 shows the incidence of adverse reactions from combined Phase 3 placebo-controlled studies of Zopiclone at doses of 1 or 2 mg in elderly adults (ages 65 to 86). Treatment duration in these trials was 14 days. The table includes only reactions that occurred in 2% or more of patients treated with Zopiclone 1 mg or 2 mg in which the incidence in patients treated with Zopiclone was greater than the incidence in placebo-treated patients.
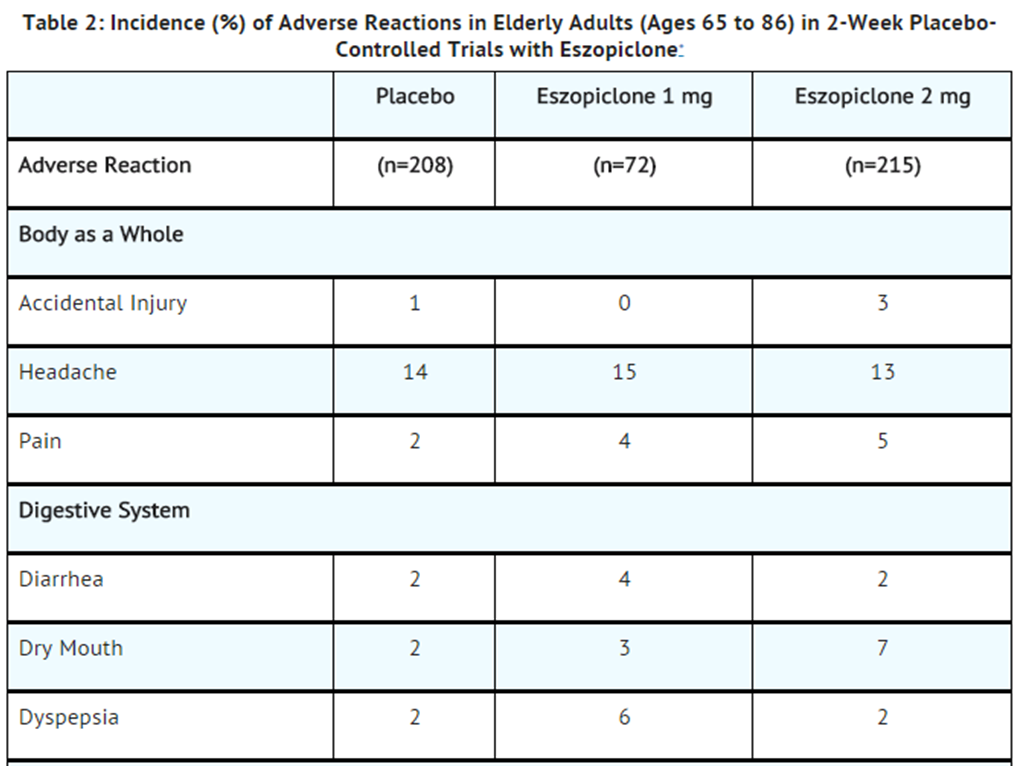

- Adverse reactions from Table 2 that suggest a dose-response relationship in elderly adults include pain, dry mouth, and unpleasant taste, with this relationship again clearest for unpleasant taste.
- These figures cannot be used to predict the incidence of adverse reactions in the course of usual medical practice because patient characteristics and other factors may differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contributions of drug and non-drug factors to the adverse reaction incidence rate in the population studied.
- Other Reactions Observed During the Premarketing Evaluation of Zopiclone
- Following is a list of modified COSTART terms that reflect adverse reactions as defined in the introduction to the Adverse Reactions section and reported by approximately 1550 subjects treated with Zopiclone at doses in the range of 1 to 3.5 mg/day during Phase 2 and 3 clinical trialsthroughout the United States and Canada. All reported reactions are included except those already listed in Tables 1 and 2 or elsewhere in labeling, minor reactions common in the general population, and reactions unlikely to be drug-related. Although the reactions reported occurred during treatment with Zopiclone, they were not necessarily caused by it.
- Reactions are further categorized by body system and listed in order of decreasing frequency according to the following definitions: frequent adverse reactions are those that occurred on one or more occasions in at least 1/100 patients; infrequent adverse reactions are those that occurred in fewer than 1/100 patients but in at least 1/1,000 patients; rare adverse reactions are those that occurred in fewer than 1/1,000 patients. Gender-specific reactions are categorized based on their incidence for the appropriate gender.
Body as a Whole:
- Frequent:
- Infrequent:
Cardiovascular System:
- Frequent:
- Infrequent:
- Rare:
Digestive System:
- Infrequent:
- Aorexia,cholelithiasis, increased appetite, melena, mouth ulceration, thirst, ulcerative stomatitis;
- Rare:
Hemic and Lymphatic System:
- Infrequent:
Metabolic and Nutritional:
- Frequent:
- Infrequent:
- Rare:
Musculoskeletal System:
- Infrequent:
- Arthritis, bursitis, joint disorder (mainly swelling, stiffness, and pain), leg cramps, myasthenia, twitching
- Rare:
Nervous System:
- Infrequent:
- Rare:
Respiratory System:
- Infrequent:
Skin and Appendages:
- Infrequent:
- Rare:
- Erythema multiforme, furunculosis, herpes zoster, hirsutism, maculopapular rash, vesiculobullous rash
Special Senses:
- Infrequent:
- Conjunctivitis, dry eyes, ear pain, otitis externa, otitis media, tinnitus, vestibular disorder
- Rare:
Urogenital System:
- Infrequent:
- Rare:
Postmarketing Experience
Post-Marketing Experience
- In addition to the adverse reactions observed during clinical trials, dysosmia, an olfactory dysfunction that is characterized by distortion of the sense of smell, has been reported during post-marketing surveillance with Zopiclone. Because this event is reported spontaneously from a population of unknown size, it is not possible to estimate the frequency of this event.
Drug Interactions
CNS Active Drugs
- Ethanol:
- An additive effect on psychomotor performance was seen with coadministration of Zopiclone and ethanol.
- Olanzapine:
- Coadministration of Zopiclone and olanzapine produced a decrease in DSST scores. The interaction was pharmacodynamic; there was no alteration in the pharmacokinetics of either drug.
Drugs that Inhibit or Induce CYP3A4
- Drugs That Inhibit CYP3A4 (Ketoconazole):
- CYP3A4 is a major metabolic pathway for elimination of Zopiclone. The exposure of Zopiclone was increased by coadministration of ketoconazole, a potent inhibitor of CYP3A4. Other strong inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, nefazodone, troleandomycin, ritonavir, nelfinavir) would be expected to behave similarly. Dose reduction of Zopiclone is needed for patient co-administered Zopiclone with potent CYP3A4 inhibitors.
- Drugs that Induce CYP3A4 (Rifampicin):
- Racemic zopiclone exposure was decreased 80% by concomitant use of rifampicin, a potent inducer of CYP3A4. A similar effect would be expected with Zopiclone. Combination use with CYP3A4 inducer may decrease exposure and effects of Zopiclone.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- There are no adequate and well-controlled studies in pregnant women. Zopiclone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Oral administration of Zopiclone to pregnant rats (62.5, 125, or 250 mg/kg/day) and rabbits (4, 8, or 16 mg/kg/day) throughout organogenesis showed no evidence of teratogenicity up to the highest doses tested. In rats, reduced fetal weight and increased incidences of skeletal variations and/or delayed ossification were observed at the mid and high doses. The no-observed-effect dose for adverse effects on embryofetal development is 200 times the maximum recommended human dose (MRHD) of 3 mg/day on a mg/m2 basis. No effects on embryofetal development were observed in rabbits; the highest dose tested is approximately 100 times the MRHD on a mg/m2 basis.
- Oral administration of Zopiclone (60, 120, or 180 mg/kg/day) to pregnant rats throughout the pregnancy and lactation resulted in increased post-implantation loss, decreased postnatal pup weights and survival, and increased pup startle response at all doses. The lowest dose tested is approximately 200 times the MRHD on a mg/m2 basis. Zopiclone had no effects on other developmental measures or reproductive function in the offspring.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Zopiclone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Zopiclone during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Zopiclone with respect to nursing mothers.
Pediatric Use
- Safety and effectiveness have not been established in pediatric patients.
- The labeling for Sunovion Pharmaceutical Inc.’s Zopiclone tablets includes additional information from a clinical study in which efficacy was not demonstrated in pediatric patients. However, due to Sunovion Pharmaceuticals, Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
- In studies in which Zopiclone (2 to 300 mg/kg/day) was orally administered to young rats from weaning through sexual maturity, neurobehavioral impairment (altered auditory startle response) and reproductive toxicity (adverse effects on male reproductive organ weights and histopathology) were observed at doses ≥ 5 mg/kg/day. Delayed sexual maturation was noted in males and females at ≥ 10 mg/kg/day. The no-effect dose (2 mg/kg) was associated with plasma exposures (AUC) for Zopiclone and metabolite (S)-desmethylzopiclone [(S)-DMZ] approximately 2 times plasma exposures in humans at the maximum recommended dose (MRHD) in adults (3 mg/day).
- When Zopiclone (doses from 1 to 50 mg/kg/day) was orally administered to young dogs from weaning through sexual maturity, neurotoxicity (convulsions) was observed at doses ≥ 5 mg/kg/day. Hepatotoxicity (elevated liver enzymes and hepatocellular vacuolation and degeneration) and reproductive toxicity (adverse effects on male reproductive organ weights and histopathology) were noted at dose ≥ 10 mg/kg/day. The no-effect dose (1 mg/kg) was associated with plasma exposures (AUC) to Zopiclone and (S)-DMZ approximately 3 and 2 times, respectively, plasma exposures in humans at the MRHD in adults.
Geriatic Use
- A total of 287 subjects in double-blind, parallel-group, placebo-controlled clinical trialswho received Zopiclone were 65 to 86 years of age. The overall pattern of adverse events for elderly subjects (median age = 71 years) in 2-week studies with nighttime dosing of 2 mg Zopiclone was not different from that seen in younger adults. Zopiclone 2 mg exhibited significant reduction in sleep latency and improvement in sleep maintenance in the elderly population. Compared with non-elderly adults, subjects 65 years and older had longer elimination and higher total exposure to Zopiclone. Therefore, dose reduction is recommended in the elderly patients.
Gender
There is no FDA guidance on the use of Zopiclone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Zopiclone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Zopiclone in patients with renal impairment.
Hepatic Impairment
- No dose adjustment is necessary for patients with mild-to-moderate hepatic impairment. Exposure was increased in severely impaired patients compared with the healthy volunteers. The dose of Zopiclone should not exceed 2 mg in patients with severe Hepatic Impairment. Zopiclone should be used with caution in patients with hepatic impairment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Zopiclone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Zopiclone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Zopiclone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Zopiclone in the drug label.
Overdosage
- In clinical trialswith Zopiclone, one case of overdose with up to 36 mg of Zopiclone was reported in which the subject fully recovered. Since commercial marketing began, spontaneous cases of Zopiclone overdoses up to 270 mg (90 times the maximum recommended dose of Zopiclone) have been reported, in which patients have recovered. Fatalities related to Zopiclone overdoses were reported only in combination with other CNS drugs or alcohol.
Signs and Symptoms
- Signs and symptoms of overdose effects of CNS Depressants can be expected to present as exaggerations of the pharmacological effects noted in preclinical testing. Impairment of consciousness ranging from somnolence to coma has been described. Rare individual instances of fatal outcomes following overdose with racemic zopiclone have been reported in European postmarketing reports, most often associated with overdose with other CNS-depressant agents.
Recommended Treatment
- General symptomatic and supportive measures should be used along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. Flumazenil may be useful. As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate signs should be monitored and general supportive measures employed. Hypotension and CNS depression should be monitored and treated by appropriate medical intervention. The value of dialysis in the treatment of overdosage has not been determined.
- As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage.
Chronic Overdose
- There is limited information regarding Chronic Overdose of Zopiclonein the drug label.
Pharmacology
| |
| |
ZopicloneZopiclone
| |
| Systematic (IUPAC) name | |
| (RS)-6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 388.808 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 52-59% bound to plasma protein |
| Metabolism | Various cytochrome P450 liver enzymes |
| Half life | ~6 hours ~9 hours for over 65 |
| Excretion | Urine |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral tablets, 3.75mg (UK), 5 or 7.5 mg |
Mechanism of Action
- The precise mechanism of action of Zopiclone as a hypnotic is unknown, but its effect is believed to result from its interaction with GABA-receptor complexes at binding domains located close to or allosterically coupled to benzodiazepine receptors. Zopiclone is a nonbenzodiazepine hypnotic that is a pyrrolopyrazine derivative of the cyclopyrrolone class with a chemical structure unrelated to pyrazolopyrimidines, imidazopyridines, benzodiazepines, barbiturates, or other drugs with known hypnotic properties.
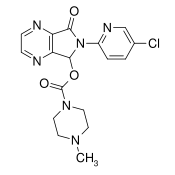
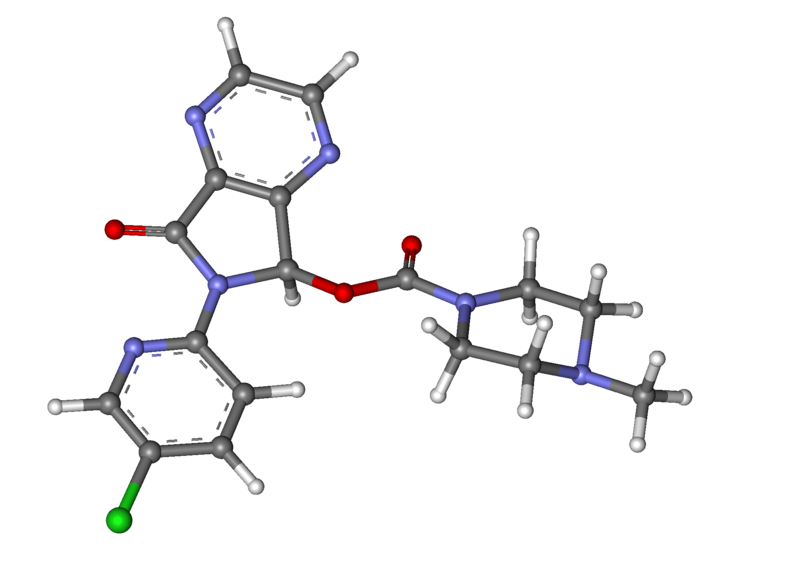
Structure
- Zopiclone are a nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of Zopiclone is (+)-(5S)-6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazin-5-yl 4-methylpiperazine-1-carboxylate. Its molecular weight is 388.81, and its empirical formula is C17H17ClN6O3. Zopiclone has a single chiral center with an (S)-configuration. It has the following chemical structure:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Zopiclone in the drug label.
Pharmacokinetics
- The pharmacokinetics of Zopiclone have been investigated in healthy subjects (adult and elderly) and in patients with hepatic disease or renal disease. In healthy subjects, the pharmacokinetic profile was examined after single doses of up to 7.5 mg and after once-daily administration of 1, 3, and 6 mg for 7 days. Zopiclone is rapidly absorbed, with a time to peak concentration (tmax) of approximately 1 hour and a terminal-phase elimination half-life (t1/2) of approximately 6 hours. In healthy adults, Zopiclone does not accumulate with once-daily administration, and its exposure is dose-proportional over the range of 1 to 6 mg.
Absorption and Distribution
- Zopiclone is rapidly absorbed following oral administration. Peak plasma concentrations are achieved within approximately 1 hour after oral administration. Zopiclone is weakly bound to plasma protein (52 to 59%). The large free fraction suggests that Zopiclone disposition should not be affected by drug-drug interactions caused by protein binding. The blood-to-plasma ratio for Zopiclone is less than one, indicating no selective uptake by red blood cells.
Metabolism:
- Following oral administration, Zopiclone is extensively metabolized by oxidation and demethylation. The primary plasma metabolites are (S)-zopiclone-N-oxide and (S)-N-desmethyl zopiclone; the latter compound binds to GABA receptors with substantially lower potency than Zopiclone, and the former compound shows no significant binding to this receptor. In vitro studies have shown that CYP3A4 and CYP2E1 enzymes are involved in the metabolism of Zopiclone. Zopiclone did not show any inhibitory potential on CYP450 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 in cryopreserved human hepatocytes.
Elimination:
- After oral administration, Zopiclone is eliminated with a mean t1/2 of approximately 6 hours. Up to 75% of an oral dose of racemic zopiclone is excreted in the urine, primarily as metabolites. A similar excretion profile would be expected for Zopiclone, the S-isomer of racemic zopiclone. Less than 10% of the orally administered Zopiclone dose is excreted in the urine as parent drug.
Effect of Food:
- In healthy adults, administration of a 3 mg dose of Zopiclone after a high-fat meal resulted in no change in AUC, a reduction in mean Cmax of 21%, and delayed tmax by approximately 1 hour. The half-life remained unchanged, approximately 6 hours. The effects of Zopiclone on sleep onset may be reduced if it is taken with or immediately after a high-fat/heavy meal.
Specific Populations:
- Age
- Compared with non-elderly adults, subjects 65 years and older had an increase of 41% in total exposure (AUC) and a slightly prolonged elimination of Zopiclone (t1/2 approximately 9 hours). Cmax was unchanged. Therefore, in elderly patients the dose should not exceed 2 mg.
- Gender
- The pharmacokinetics of Zopiclone in men and women are similar.
- Race
- In an analysis of data on all subjects participating in Phase 1 studies of Zopiclone, the pharmacokinetics for all races studied appeared similar.
- Hepatic Impairment
- Pharmacokinetics of a 2 mg Zopiclone dose were assessed in 16 healthy volunteers and in 8 subjects with mild, moderate, and severe liver disease. Exposure was increased 2-fold in severely impaired patients compared with the healthy volunteers. Cmax and tmax were unchanged. No dose adjustment is necessary for patients with mild-to-moderate hepatic impairment. Dose reduction is recommended for patients with severe hepatic impairment. Zopiclone should be used with caution in patients with hepatic impairment.
- Renal Impairment
- The pharmacokinetics of Zopiclone were studied in 24 patients with mild, moderate, or severe renal impairment. AUC and Cmax were similar in the patients compared with demographically matched healthy control subjects. No dose adjustment is necessary in patients with renal impairment, since less than 10% of the orally administered Zopiclone dose is excreted in the urine as parent drug.
Drug Interactions
- Zopiclone is metabolized by CYP3A4 and CYP2E1 via demethylation and oxidation. There were no pharmacokinetic or pharmacodynamic interactions between Zopiclone and paroxetine. When Zopiclone was coadministered with olanzapine, no pharmacokinetic interaction was detected in levels of Zopiclone or olanzapine, but a pharmacodynamic interaction was seen on a measure of psychomotor function. Zopiclone and lorazepam decreased each other’s Cmax by 22%. Coadministration of Zopiclone 3 mg to subjects receiving ketoconazole, a potent inhibitor of CYP3A4, 400 mg daily for 5 days, resulted in a 2.2-fold increase in exposure to Zopiclone. Cmax and t1/2 were increased 1.4-fold and 1.3-fold, respectively. Zopiclone would not be expected to alter the clearance of drugs metabolized by common CYP450 enzymes.
- Paroxetine
- Coadministration of single dose of Zopiclone and paroxetine produced no pharmacokinetic or pharmacodynamic interaction. The lack of a drug interaction following single-dose administration does not predict the complete absence of a pharmacodynamic effect following chronic administration.
- Coadministration of single doses of Zopiclone and lorazepam did not have clinically relevant effects on the pharmacodynamics or pharmacokinetics of either drug. The lack of a drug interaction following single-dose administration does not predict the complete absence of a pharmacodynamic effect following chronic administration.
- Drugs with a Narrow Therapeutic Index:
- Digoxin
- A single dose of Zopiclone 3 mg did not affect the pharmacokinetics of digoxin measured at steady state following dosing of 0.5 mg twice daily for one day and 0.25 mg daily for the next 6 days.
- Digoxin
- Warfarin
- Zopiclone 3 mg administered daily for 5 days did not affect the pharmacokinetics of (R)- or (S)-warfarin, nor were there any changes in the pharmacodynamic profile (prothrombin time) following a single 25 mg oral dose of warfarin.
- Warfarin
- Drugs Highly Bound to Plasma Protein:
- Zopiclone is not highly bound to plasma proteins (52 to 59% bound); therefore, the disposition of Zopiclone is not expected to be sensitive to alterations in protein binding. Administration of Zopiclone 3 mg to a patient taking another drug that is highly protein-bound would not be expected to cause an alteration in the free concentration of either drug.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility:
- Carcinogenesis:
- In a carcinogenicity study in rats, oral administration of Zopiclone for 97 (males) or 104 (females) weeks resulted in no increases in tumors; plasma levels (AUC) of Zopiclone at the highest dose tested (16 mg/kg/day) are approximately 80 (females) and 20 (males) times those in humans at the maximum recommended human dose (MRHD) of 3 mg/day. However, in a 2-year carcinogenicity study in rats, oral administration of racemic zopiclone (1, 10, or 100 mg/kg/day) resulted in increases in mammary gland adenocarcinomas (females) and thyroid gland follicular cell adenomas and carcinomas (males) at the highest dose tested. Plasma levels of Zopiclone at this dose are approximately 150 (females) and 70 (males) times those in humans at the MRHD of Zopiclone. The mechanism for the increase in mammary adenocarcinomas is unknown. The increase in thyroid tumors is thought to be due to increased levels of TSH secondary to increased metabolism of circulating thyroid hormones, a mechanism not considered relevant to humans.
- In a 2-year carcinogenicity study in mice, oral administration of racemic zopiclone (1, 10, or 100 mg/kg/day) produced increases in pulmonary carcinomas and carcinomas plus adenomas (females) and skin fibromas and sarcomas (males) at the highest dose tested. The skin tumors were due to skin lesions induced by aggressive behavior, a mechanism not relevant to humans. A carcinogenicity study of Zopiclone was conducted in mice at oral doses up to 100 mg/kg/day. Although this study did not reach a maximum tolerated dose, and was thus inadequate for overall assessment of carcinogenic potential, no increases in either pulmonary or skin tumors were seen at doses producing plasma levels of Zopiclone approximately 90 times those in humans at the MRHD of Zopiclone (and 12 times the exposure in the racemate study).
- Zopiclone did not increase tumors in a p53 transgenic mouse bioassay at oral doses up to 300 mg/kg/day.
- Mutagenesis:
- Zopiclone was clastogenic in in vitro (mouse lymphoma and chromosomal aberration) assays in mammalian cells. Zopiclone was negative in the in vitro bacterial gene mutation (Ames) assay and in an in vivo micronucleus assay.
- (S)-N-desmethyl zopiclone, a metabolite of Zopiclone, was positive in in vitro chromosomal aberration assays in mammalian cells. (S)-N-desmethyl zopiclone was negative in the in vitro bacterial gene mutation (Ames) assay and in an in vivo chromosomal aberration and micronucleus assay.
- Impairment of Fertility:
- Oral administration of Zopiclone to rats prior to and during mating, and continuing in females to day 7 of gestation (doses up to 45 mg/kg/day to males and females or up to 180 mg/kg/day to females only) resulted in decreased fertility, with no pregnancy at the highest dose tested when both males and females were treated. In females, there was an increase in abnormal estrus cycles at the highest dose tested. In males, decreases in sperm number and motility and increases in morphologically abnormal sperm were observed at the mid and high doses. The no-effect dose for adverse effects on fertility (5 mg/kg/day) is 16 times the MRHD on a mg/m2 basis.
Clinical Studies
- The effect of Zopiclone on reducing sleep latency and improving sleep maintenance was established in studies with 2100 subjects (ages 18 to 86) with chronic and transient insomnia in six placebo-controlled trials of up to 6 months’ duration. Two of these trials were in elderly patients (n=523). Overall, at the recommended adult dose (2 to 3 mg) and elderly dose (1 to 2 mg), Zopiclone significantly decreased sleep latency and improved measures of sleep maintenance(objectively measured as wake time after sleep onset WASO and subjectively measured as total sleep time).
Transient [insomnia
- Healthy adults were evaluated in a model of transient insomnia (n=436) in a sleep laboratory in a double-blind, parallel-group, single-night trial comparing two doses of Zopiclone and placebo. Zopiclone 3 mg was superior to placebo on measures of sleep latency and sleep maintenance, including polysomnographic (PSG) parameters of latency to persistent sleep (LPS) and WASO.
Chronic insomnia (Adults and Elderly)
- The effectiveness of Zopiclone was established in five controlled studies in chronic insomnia. Three controlled studies were in adult subjects, and two controlled studies were in elderly subjects with chronic insomnia.
- Adults
- In the first study, adults with chronic insomnia (n=308) were evaluated in a double-blind, parallel-group trial of 6 weeks’ duration comparing Zopiclone 2 mg and 3 mg with placebo. Objective endpoints were measured for 4 weeks. Both 2 mg and 3 mg were superior to placebo on LPS at 4 weeks. The 3 mg dose was superior to placebo on WASO.
- In the second study, adults with chronic insomnia (n=788) were evaluated using subjective measures in a double-blind, parallel-group trial comparing the safety and efficacy of Zopiclone 3 mg with placebo administered nightly for 6 months. Zopiclone was superior to placebo on subjective measures of sleep latency, total sleep time, and WASO.
- In addition, a 6-period cross-over PSG study evaluating Zopiclone doses of 1 to 3 mg, each given over a 2-day period, demonstrated effectiveness of all doses on LPS, and of 3 mg on WASO. In this trial, the response was dose-related.
- Elderly
- Elderly subjects (ages 65 to 86) with chronic insomnia were evaluated in two double-blind, parallel-group trials of 2 weeks duration. One study (n=231) compared the effects of Zopiclone with placebo on subjective outcome measures, and the other (n=292) on objective and subjective outcome measures. The first study compared 1 mg and 2 mg of Zopiclone with placebo, while the second study compared 2 mg of Zopiclone with placebo. All doses were superior to placebo on measures of sleep latency. In both studies, 2 mg of Zopiclone was superior to placebo on measures of sleep maintenance.
Studies Pertinent to Safety Concerns for sedative hypnotic Drugs
- Next Day Residual Effects
- In a double-blind study of 91 healthy adults age 25- to 40 years, the effects of Zopiclone 3 mg on psychomotor function were assessed between 7.5 and 11.5 hours the morning after dosing. Measures included tests of psychomotor coordination that are correlated with ability to maintain a motor vehicle in the driving lane, tests of working memory, and subjective perception of sedation and coordination. Compared with placebo, Zopiclone 3 mg was associated with next- morning psychomotor and memory impairment that was most severe at 7.5 hours, but still present and potentially clinically meaningful at 11.5 hours. Subjective perception of sedation and coordination from Zopiclone 3 mg was not consistently different from placebo, even though subjects were objectively impaired.
- In a 6-month double-blind, placebo-controlled trial of nightly administered Zopiclone 3 mg, memory impairment was reported by 1.3% (8/593) of subjects treated with Zopiclone 3 mg compared to 0% (0/195) of subjects treated with placebo. In a 6-week adult study of nightly administered Zopiclone confusion was reported by 3% of patients treated with Zopiclone 3 mg, compared to 0% of subjects treated with placebo. In the same study, memory impairment was reported by 1% of patents treated with either 2 mg or 3 mg Zopiclone, compared to 0% treated with placebo.
- In a 2-week study of 264 elderly insomniacs, 1.5% of patients treated with Zopiclone 2 mg reported memory impairment compared to 0% treated with placebo. In another 2-week study of 231 elderly insomniacs, 2.5% of patients treated with Zopiclone 2 mg reported confusion compared to 0% treated with placebo.
- A study of normal subjects exposed to single fixed doses of Zopiclone from 1 to 7.5 mg using the DSST to assess sedation and psychomotor function at fixed times after dosing (hourly up to 16 hours) found the expected sedation and reduction in psychomotor function. This was maximal at 1 hour and present up to 4 hours, but was no longer present by 5 hours.
- In another study, patients with insomnia were given 2 or 3 mg doses of Zopiclone nightly, with DSST assessed on the mornings following days 1, 15, and 29 of treatment. While both the placebo and Zopiclone 3 mg groups showed an improvement in DSST scores relative to baseline the following morning (presumably due to a learning effect), the improvement in the placebo group was greater and reached statistical significance on night 1, although not on nights 15 and 29. For the Zopiclone 2 mg group, DSST change scores were not significantly different from placebo at any time point.
- During nightly use for an extended period, pharmacodynamic tolerance or adaptation has been observed with other hypnotics. If a drug has a short elimination half-life, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This is believed to be responsible for two clinical findings reported to occur after several weeks of nightly use of other rapidly eliminated hypnotics: increased wakefulness during the last quarter of the night and the appearance of increased signs of daytime anxiety.
- In a 6-month double-blind, placebo-controlled study of nightly administration of Zopiclone 3 mg, rates of anxiety reported as an adverse event were 2.1% in the placebo arm and 3.7% in the Zopiclone arm. In a 6-week adult study of nightly administration, anxiety was reported as an adverse event in 0%, 2.9%, and 1% of the placebo, 2 mg, and 3 mg treatment arms, respectively. In this study, single-blind placebo was administered on nights 45 and 46, the first and second days of withdrawal from study drug. New adverse events were recorded during the withdrawal period, beginning with day 45, up to 14 days after discontinuation. During this withdrawal period, 105 subjects previously taking nightly Zopiclone 3 mg for 44 nights spontaneously reported anxiety (1%), abnormal dreams (1.9%), hyperesthesia (1%), and neurosis (1%), while none of 99 subjects previously taking placebo reported any of these adverse events during the withdrawal period.
- Rebound insomnia, defined as a dose-dependent temporary worsening in sleep parameters (latency, sleep efficiency, and number of awakenings) compared with baseline following discontinuation of treatment, is observed with short- and intermediate-acting hypnotics. Rebound insomnia following discontinuation of Zopiclone relative to placebo and baseline was examined objectively in a 6-week adult study on the first 2 nights of discontinuation (nights 45 and 46) following 44 nights of active treatment with 2 mg or 3 mg. In the Zopiclone 2 mg group, compared with baseline, there was a significant increase in WASO and a decrease in sleep efficiency, both occurring only on the first night after discontinuation of treatment. No changes from baseline were noted in the Zopiclone 3 mg group on the first night after discontinuation, and there was a significant improvement in LPS and sleep efficiency compared with baseline following the second night of discontinuation. Comparisons of changes from baseline between Zopiclone and placebo were also performed. On the first night after discontinuation of Zopiclone 2 mg, LPS and WASO were significantly increased and sleep efficiency was reduced; there were no significant differences on the second night. On the first night following discontinuation of Zopiclone 3 mg, sleep efficiency was significantly reduced. No other differences from placebo were noted in any other sleep parameter on either the first or second night following discontinuation. For both doses, the discontinuation-emergent effect was mild, had the characteristics of the return of the symptoms of chronic insomnia, and appeared to resolve by the second night after Zopiclone discontinuation.
How Supplied
- Zopiclone tablets are round, white, film-coated tablets and are supplied as follows:
- The 1 mg tablets are debossed with product identification “54 746” on one side and plain on the other side.
- NDC 0054-0290-13 1 mg, bottle of 30
- NDC 0054-0290-25 1 mg, bottle of 100
- The 2 mg tablets are debossed with product identification “54 029” on one side and plain on the other side.
- NDC 0054-0291-25 2 mg, bottle of 100
- The 3 mg tablets are debossed with product identification “54 396” on one side and plain on the other side.
- NDC 0054-0292-25 3 mg, bottle of 100
Storage
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F)
Images
Drug Images
{{#ask: Page Name::Zopiclone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Zopiclone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients and their families about the benefits and risks of treatment with Zopiclone. Inform patients of the availability of a Medication Guide and instruct them to read the Medication Guide prior to initiating treatment with Zopiclone and with each prescription refill. Review the Zopiclone Medication Guide with every patient prior to initiation of treatment. Instruct patients or caregivers that Zopiclone should be taken only as prescribed.
- CNS depressant effects and next-day impairment: Tell patients that Zopiclone can cause next-day impairment even when used as prescribed, and that this risk is increased if dosing instructions are not carefully followed. Caution patients taking the 3 mg dose against driving and other activities requiring complete mental alertness the day after use. Inform patients that impairment can be present despite feeling fully awake.
- Severe anaphylactic and anaphylactoid reactions: Inform patients that severe anaphylactic and anaphylactoid reactions have occurred with Zopiclone. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if any of them occur.
- “sleep-Driving” and other complex behaviors: Instruct patients and their families that sedative hypnotics can cause abnormal thinking and behavior change, including "sleep driving" and other complex behaviors while not being fully awake (preparing and eating food, making phone calls, or having sex). Tell patients to call you immediately if they develop any of these symptoms.
- Alcohol and Other Drugs: Ask patients about alcohol consumption, medicines they are taking, and drugs they may be taking without a prescription. Advise patients not to use Zopiclone if they drank alcohol that evening or before bed.
- Tolerance, Abuse, and Dependence: Tell patients not to increase the dose of Zopiclone on their own, and to inform you if they believe the drug "does not work".
- Administration Instructions: Patients should be counseled to take Zopiclone right before they get into bed and only when they are able to stay in bed a full night (7–8 hours) before being active again. Zopiclone tablets should not be taken with or immediately after a meal. Advise patients NOT to take Zopiclone if they drank alcohol that evening.
- Roxane Laboratories, Inc.
- Columbus, Ohio 43216
- 10005923/02 Revised May 2014
- ©RLI, 2014
Precautions with Alcohol
- Additive effects occur with concomitant use of other CNS Depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol), including daytime use. Downward dose adjustment of Zopiclone and concomitant CNS Depressants should be considered
- Ask patients about alcohol consumption, medicines they are taking, and drugs they may be taking without a prescription. Advise patients not to use Zopiclone if they drank alcohol that evening or before bed.
Abnormal Thinking and Behavioral Changes
- A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS Depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization. Amnesia and other neuropsychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of sedative/hypnotics.
- Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with Zopiclone alone at therapeutic doses, the use of alcohol and other CNS Depressants with Zopiclone appears to increase the risk of such behaviors, as does the use of Zopiclone at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of Zopiclone should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
- It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above are drug-induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Dependence
- The clinical trial experience with Zopiclone revealed no evidence of a serious withdrawal syndrome. Nevertheless, the following adverse events included in DSM-IV criteria for uncomplicated sedative/hypnotic withdrawal were reported during clinical trialsfollowing placebo substitution occurring within 48 hours following the last Zopiclone treatment: anxiety, abnormal dreams, nausea, and upset stomach. These reported adverse events occurred at an incidence of 2% or less. Use of benzodiazepines and similar agents may lead to physical and psychological dependence. The risk of abuse and dependence increases with the dose and duration of treatment and concomitant use of other psychoactive drugs. The risk is also greater for patients who have a history of alcohol or drug abuse or history of psychiatric disorders. These patients should be under careful surveillance when receiving Zopiclone or any other hypnotic.
OVERDOSAGE
- In clinical trialswith Zopiclone, one case of overdose with up to 36 mg of Zopiclone was reported in which the subject fully recovered. Since commercial marketing began, spontaneous cases of Zopiclone overdoses up to 270 mg (90 times the maximum recommended dose of Zopiclone) have been reported, in which patients have recovered. Fatalities related to Zopiclone overdoses were reported only in combination with other CNS drugs or alcohol.
Brand Names
There is limited information regarding Zopiclone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Zopiclone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Zopiclone
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Zopiclone |Label Name=Zopiclone drug lable 01.png
}}
{{#subobject:
|Label Page=Zopiclone |Label Name=Zopiclone drug lable 02.png
}}
{{#subobject:
|Label Page=Zopiclone |Label Name=Zopiclone drug lable 03.png
}}
