Oxime
Overview

An oxime is one in a class of chemical compounds with the general formula R1R2CNOH, where R1 is an organic side chain and R2 is either hydrogen, forming an aldoxime, or another organic group, forming a ketoxime.
Oximes can be formed by the action of hydroxylamine on aldehydes or ketones.
The term oxime dates to the 19th century, a condensation of the words oxygen and imide.
Oximes exist as two stereoisomers: a syn isomer and an anti isomer. Aldoximes, except for aromatic aldoximes, exist only as a syn isomer, while ketoximes can be separated almost completely and obtained as a syn isomer and an anti isomer.
Chemistry of oximes
Organic synthesis
Oximes can be synthesized by condensation of an aldehyde or a ketone with hydroxylamine. The condensation of aldehydes with hydroxylamine gives aldoxime, and ketoxime is produced from ketones and hydroxylamine. Generally, oximes exist as colorless crystals and do not easily dissolve in water. Therefore, oximes are used for the identification of ketone or aldehyde.
Oximes can also be obtained from reaction of nitrites such as isoamyl nitrite with compounds containing an acidic hydrogen atom. Examples are the reaction of ethyl acetoacetate and sodium nitrite in acetic acid,[1][2] the reaction of methyl ethyl ketone with ethyl nitrite in hydrochloric acid.[3] and a similar reaction with propiophenone,[4] the reaction of phenacyl chloride,[5] the reaction of malononitrile with sodium nitrite in acetic acid[6]
A conceptually related reaction is the Japp-Klingemann reaction.
Organic reactions
The hydrolysis of oximes proceeds easily by heating in the presence of various inorganic acids, and the oximes decompose into the corresponding ketones or aldehydes, and hydroxylamines. The reduction of oximes by sodium amalgam or hydrogenation produces amines. The reduction of aldoximes gives both primary amines and secondary amines.
Generally oximes can be changed to the corresponding amide derivatives by treatment with various acids. This reaction is called Beckmann rearrangement. In this reaction, a hydroxyl group is exchanged with the group that is in the anti position of the hydroxyl group. The amide derivatives that are obtained by Beckmann rearrangement can be transformed into a carboxylic acid and an amine by hydrolysis. Beckmann rearrangement is used for the industrial synthesis of caprolactam, which is the material used to make nylon-6.
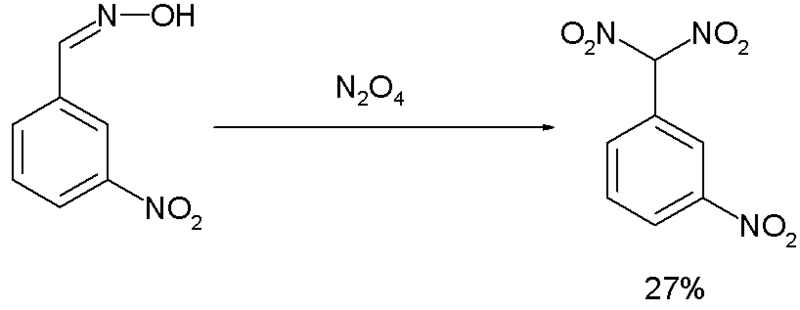
The Ponzio reaction (1906) [7] concerning the conversion of m-nitrobenzaldoxime to m-Nitrophenyldinitromethane with Dinitrogen tetroxide, was the result of research into TNT-like high explosives [8]:
Uses of oximes
Oxime compounds are used as antidotes for nerve agents. A nerve agent inactivates acetylcholinesterase molecules by phosphonylation of the molecule. Oxime compounds can reactivate acetylcholinesterate by attaching to the phosphorus atom and forming an oxime-phosphonate which then splits away from the acetylcholinesterase molecule. The most effective oxime nerve-agent antidotes are pralidoxime, obidoxime, methoxime, HI-6, Hlo-7, 2-PAM, and TMB-4.[9] The effectiveness of the oxime treatment depends on the particular nerve agent used.[10] The oxime of perillaldehyde is used as an artificial sweetener in Japan, as it is 2000 times sweeter than sucrose. Salicylaldoxime is a chelator.
References
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Template:OrgSynth
- ↑ Ponzio, J. prakf chcm , 73, 494 (1906),
- ↑ Aromatic-Aliphatic Nitro Compounds. III. The Ponzio Reaction; 2,4,6-Trinitrobenzyl Nitrate Louis F. Fieser and William von E. Doering J. Am. Chem. Soc.; 1946; 68(11) pp 2252 - 2253; doi:10.1021/ja01215a040
- ↑ Template:Cite
- ↑ Kassa, J. (2002). "Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents". Journal of Toxicology — Clinical Toxicology. 40: 803. doi:10.1081/CLT-120015840.
cs:Oxim de:Oxime he:אוקסים nl:Oxime sv:Oxim Template:Jb1 Template:WH Template:WikiDoc Sources