Venezuelan equine encephalitis: Difference between revisions
No edit summary |
m (Changes made per Mahshid's request) |
||
| (31 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
{{CMG}} {{AE}} {{AG}} | {{CMG}} {{AE}} {{AG}} | ||
{{SK}} VEE; VEEV; Venezuelan encephalitis; Venezuelan equine encephalomyelitis | {{SK}} VEE; VEEV; Venezuelan equine encephalitis virus; Venezuelan encephalitis; Venezuelan equine encephalomyelitis; Venezuelan equine fever | ||
==Overview== | ==Overview== | ||
Venezuelan equine encephalitis is a mild to moderate, though sometimes [[fatal]], infection of the [[central nervous system]]. Venezuelan equine encephalitis belongs to the Group IV positive-sense ssRNA virus within the ''[[Togaviridae]]'' family of viruses, and the genus ''[[Alphavirus]]''. Venezuelan equine encephalitis virus is usually transmitted via [[mosquito]]s to the human host, primarily ''Culex melanoconion'' or ''[[Aedes]]''. Venezuelan equine encephalitis virus must be differentiated from other diseases that cause [[fever]], [[headache]], [[seizures]], and [[altered mental status]]. Prognosis for Venezuelan equine encephalitis is generally good; less than 1% of patients infected with the Venezuelan equine encephalitis virus present with symptoms. Symptomatic patients often recover within 2-3 weeks of infection. The [[case fatality|case-fatality]] rate of Venezuelan equine encephalitis is approximately 0.7. If possible, a detailed and thorough history from the patient is necessary. If the patient is female, a pregnancy test should be administered to monitor for potential [[miscarriage]]. Venezuelan equine encephalitis is usually asymptomatic. The diagnostic method of choice for Venezuelan equine encephalitis is laboratory testing. There is no treatment for Venezuelan equine encephalitis; the mainstay of therapy is supportive care. There is a vaccination approved for limited use for Venezuelan equine encephalitis, though its effectiveness is often questioned. | |||
==Historical Perspective== | ==Historical Perspective== | ||
| Line 11: | Line 11: | ||
==Classification== | ==Classification== | ||
Venezuelan equine encephalitis may be classified according to location of the disease into 2 subtypes: systemic or encephalitic. Venezuelan equine encephalitis may also be classified according to [[invasive|neuroinvasiveness]] of the disease into 2 subtypes: neuroinvasive and non-neuroinvasive. Venezuelan equine encephalitis belongs to the Group IV positive-sense ssRNA virus within the [[Togaviridae]] family of viruses, and the genus [[Alphavirus]]. Venezuelan equine encephalitis is closely related to [[eastern equine encephalitis]] virus and [[western equine encephalitis]] virus. Venezuelan equine encephalitis is known as an [[arbovirus]], or an arthropod-borne virus. | Venezuelan equine encephalitis may be classified according to location of the disease into 2 subtypes: systemic or encephalitic. Venezuelan equine encephalitis may also be classified according to [[invasive|neuroinvasiveness]] of the disease into 2 subtypes: neuroinvasive and non-neuroinvasive.<ref name=CDCCritArboInvasive> Arboviral diseases, neuroinvasive and non-neuroinvasive 2015 Case Definition. National Notifiable Diseases Surveillance System (NNDSS). Centers for Disease Control (2015). https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ Accessed on March 31, 2016. </ref> Venezuelan equine encephalitis belongs to the Group IV positive-sense ssRNA virus within the ''[[Togaviridae]]'' family of viruses, and the genus ''[[Alphavirus]]''. Venezuelan equine encephalitis is closely related to [[eastern equine encephalitis]] virus and [[western equine encephalitis]] virus. Venezuelan equine encephalitis is known as an [[arbovirus]], or an arthropod-borne virus. | ||
==Pathophysiology== | ==Pathophysiology== | ||
Venezuelan equine encephalitis virus is usually transmitted via [[mosquito]]s to the human host. Venezuelan equine encephalitis virus contains [[positive-sense ssRNA virus|positive-sense]] viral [[RNA]]; this RNA has its genome directly utilized as if it were mRNA, producing a single protein which is modified by host and viral proteins to form the various proteins needed for [[replication]]. The following table is a summary of the Venezuelan equine encephalitis virus:<ref name=ViralZoneAlpha> Alphavirus. SIB Swiss Institute of Bioinformatics. http://viralzone.expasy.org/viralzone/all_by_species/625.html Accessed on March 15, 2016 </ref> | |||
{| class="wikitable" | |||
! style="text-align: center; font-weight: bold;" | Characteristic | |||
! style="text-align: center; font-weight: bold;" | Data | |||
|- | |||
| Nucleic acid | |||
| [[RNA]] | |||
|- | |||
| Sense | |||
| [[positive-sense ssRNA virus|ssRNA(+)]] | |||
|- | |||
| Virion | |||
| [[enveloped virus|Enveloped]] | |||
|- | |||
| [[Capsid]] | |||
| Spherical | |||
|- | |||
| Symmetry | |||
| Yes; T=4 [[icosahedral]] | |||
|- | |||
| Capsid [[monomers]] | |||
| 240 | |||
|- | |||
| Monomer length (diameter) | |||
| 70 nm | |||
|- | |||
| Additional envelope information | |||
| 80 spikes; each spike is a [[trimer]] of E1/E2 proteins | |||
|- | |||
| Genome shape | |||
| Linear | |||
|- | |||
| Genome length | |||
| 11-12 kb | |||
|- | |||
| [[Nucleotide]] cap | |||
| Yes | |||
|- | |||
| [[Polyadenylation|Polyadenylated]] tail | |||
| Yes | |||
|- | |||
| [[Incubation period]] | |||
| 1-6 day(s) | |||
|} | |||
[[Image: Alphavirus06.jpeg|thumbnail|This negatively-stained 1975 [[transmission electron micrograph]] ([[TEM]]) revealed the presence of a number of Venezuelan equine encephalitis (VEE) virus virions in this tissue specimen, which had been fixed using [[phosphotungstic acid]] (PTA).<ref name=PHIL> {{Cite web | title = Public Health Image Library (PHIL) | url = http://phil.cdc.gov/phil/home.asp}}</ref>]] | |||
Venezuelan equine encephalitis is contracted by the [[bite]] of an infected [[mosquito]], primarily ''Culex melanoconion'' or ''[[Aedes]]''. Venezuelan equine encephalitis virus circulates between a mosquito vector, usually ''Culex melanoconion'', and forest [[rodent]]s in Central and South America. Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans, such as some ''[[Aedes]]'' and other ''Culex'' species. The [[incubation period]] is 1-6 day(s).<ref name=VEECanPH2> VENEZUELAN EQUINE ENCEPHALITIS VIRUS: PATHOGEN SAFETY DATA SHEET - INFECTIOUS SUBSTANCES. Public Health Agency of Canada. (2011) http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/ven-encephalit-eng.php Accessed on March 31, 2016. </ref> In contrast to many other [[arbovirus|arboviral]] infections, infected humans possess sufficient [[viremia]] to infect uninfected mosquitos.<ref name=Mandell1> M.D. JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Expert Consult Premium Edition. Saunders; 2014.</ref> Additionally, while a link has never been proven, there is speculation that transmission between humans is possible, as 40% of cases demonstrate infection in the [[pharynx]].<ref name="pmid956360">{{cite journal| author=Bowen GS, Calisher CH| title=Virological and serological studies of Venezuelan equine encephalomyelitis in humans. | journal=J Clin Microbiol | year= 1976 | volume= 4 | issue= 1 | pages= 22-7 | pmid=956360 | doi= | pmc=PMC274383 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=956360 }} </ref> | |||
Venezuelan equine encephalitis virus is transmitted in the following pattern:<ref name=ViralZoneAlpha> Alphavirus. SIB Swiss Institute of Bioinformatics. http://viralzone.expasy.org/viralzone/all_by_species/625.html Accessed on March 15, 2016 </ref> | |||
#Attachment of the viral E [[glycoprotein]] to host receptors mediates [[clathrin]] [[endocytosis]] of virus into the host cell. | |||
#Fusion of [[biological membrane|virus membrane]] with the host [[cell membrane]]. RNA genome is released into the [[cytoplasm]]. | |||
#The [[positive-sense ssRNA virus]] is [[translate]]d into a [[polyprotein]], which is cleaved into non-structural proteins necessary for RNA synthesis ([[replication]] and [[transcription]]). | |||
#[[Replication]] takes place in [[cytoplasm]]ic viral factories at the surface of [[endosome]]s. A [[dsRNA]] [[genome]] is synthesized from the genomic ssRNA(+). | |||
#The [[dsRNA]] [[genome]] is [[transcribed]] thereby providing viral [[mRNA]]s (new ssRNA(+) genomes). | |||
#Expression of the subgenomic RNA (sgRNA) gives rise to the structural proteins. | |||
#Virus assembly occurs at the [[endoplasmic reticulum]]. | |||
#[[Virion]]s bud at the [[endoplasmic reticulum]], are transported to the [[Golgi apparatus]], and then exit the cell via the [[secretory pathway]]. | |||
On microscopic histopathological analysis, the enveloped, spherical, and [[icosahedral]] virion shape are characteristic findings of Venezuelan equine encephalitis. | |||
==Causes== | ==Causes== | ||
Venezuelan equine encephalitis may be caused by Venezuelan equine encephalitis virus. | |||
==Differentiating Venezuelan equine encephalitis from Other Diseases== | |||
Venezuelan equine encephalitis virus must be differentiated from other diseases that cause [[fever]], [[headache]], [[seizures]], and [[altered mental status]], such as:<ref name=Mandell1> M.D. JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Expert Consult Premium Edition. Saunders; 2014.</ref><ref name="pmid14978145">{{cite journal| author=Kennedy PG| title=Viral encephalitis: causes, differential diagnosis, and management. | journal=J Neurol Neurosurg Psychiatry | year= 2004 | volume= 75 Suppl 1 | issue= | pages= i10-5 | pmid=14978145 | doi= | pmc=PMC1765650 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14978145 }} </ref><ref name=NYDeptofHealth> Arboviral Infections (arthropod-borne encephalitis, eastern equine encephalitis, St. Louis encephalitis, California encephalitis, Powassan encephalitis, West Nile encephalitis). New York State Department of Health (2006). https://www.health.ny.gov/diseases/communicable/arboviral/fact_sheet.htm Accessed on February 23, 2016 </ref><ref name="pmid21932127">{{cite journal| author=Eckstein C, Saidha S, Levy M| title=A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. | journal=J Neurol | year= 2012 | volume= 259 | issue= 5 | pages= 801-16 | pmid=21932127 | doi=10.1007/s00415-011-6240-5 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21932127 }} </ref><ref name="pmid11260760">{{cite journal| author=De Kruijk JR, Twijnstra A, Leffers P| title=Diagnostic criteria and differential diagnosis of mild traumatic brain injury. | journal=Brain Inj | year= 2001 | volume= 15 | issue= 2 | pages= 99-106 | pmid=11260760 | doi=10.1080/026990501458335 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11260760 }} </ref> | |||
{| style="border: 0px; font-size: 90%; margin: 3px;" align=center | |||
|+ | |||
! style="background: #4479BA; width: 50px;" | {{fontcolor|#FFF|Disease}} | |||
! style="background: #4479BA; width: 100px;" | {{fontcolor|#FFF|Similarities}} | |||
! style="background: #4479BA; width: 150px;" | {{fontcolor|#FFF|Differentials}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Meningitis]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Classic triad of [[fever]], [[nuchal rigidity]], and [[altered mental status]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |[[Photophobia]], [[phonophobia]], [[rash]] associated with [[meningococcemia]], concomitant [[sinusitis]] or [[otitis]], swelling of the [[fontanelle]] in infants (0-6 months) | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" |'''[[Brain abscess]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Fever]], [[headache]], [[hemiparesis]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Varies depending on the location of the abscess; clinically, [[visual disturbance]] including [[papilledema]], decreased [[sensation]]; on imaging, a [[lesion]] demonstrates both ring enhancement and central restricted diffusion | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Demyelinating disease]]s''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Ataxia]], [[lethargy]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |[[Multiple sclerosis]]: clinically, [[nystagmus]], [[internuclear ophthalmoplegia]], [[Lhermitte's sign]]; on imaging, well-demarcated ovoid lesions with possible T1 hypointensities (“black holes”) | |||
[[Acute disseminated encephalomyelitis]]: clinically, [[somnolence]], [[myoclonic]] movements, and [[hemiparesis]]; on imaging, diffuse or multi-lesion enhancement, with indistinct lesion borders | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Substance abuse]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Tremor]], [[headache]], [[altered mental status]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Varies depending on type of substance: prior history, drug-seeking behavior, attention-seeking behavior, [[paranoia]], sudden [[panic]], [[anxiety]], [[hallucination]]s | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Electrolyte disturbance]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Fatigue]], [[headache]], [[nausea]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Varies depending on deficient ions; clinically, [[edema]], [[constipation]], [[hallucination]]s; on [[EKG]], abnormalities in [[T wave]], [[P wave]], [[QRS complex]]; possible presentations include [[arrhythmia]], [[dehydration]], [[renal failure]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Stroke]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Ataxia]], [[aphasia]], [[dizziness]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" |Varies depending on classification of stroke; presents with positional [[vertigo]], high [[blood pressure]], [[extremities|extremity]] weakness | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Intracranial hemorrhage]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Headache]], [[coma]], [[dizziness]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Lobar [[hemorrhage]], [[numbness]], [[tingling]], [[hypertension]], [[hemorrhagic diathesis]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC;" | '''[[Trauma]]''' | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Headache]], [[altered mental status]] | |||
| style="padding: 5px 5px; background: #F5F5F5;" | [[Amnesia]], [[loss of consciousness]], [[dizziness]], [[concussion]], [[contusion]] | |||
|- | |||
|} | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
The [[case fatality|case-fatality]] rate of Venezuelan equine encephalitis is approximately 0.7.<ref name="pmid8709783">{{cite journal| author=Weaver SC, Salas R, Rico-Hesse R, Ludwig GV, Oberste MS, Boshell J et al.| title=Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. | journal=Lancet | year= 1996 | volume= 348 | issue= 9025 | pages= 436-40 | pmid=8709783 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8709783 }} </ref> | |||
===Age=== | ===Age=== | ||
Patients of all age groups may develop Venezuelan equine encephalitis.<ref name="pmid9086137">{{cite journal| author=Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A et al.| title=Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. | journal=J Infect Dis | year= 1997 | volume= 175 | issue= 4 | pages= 828-32 | pmid=9086137 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9086137 }} </ref> | |||
===Gender=== | ===Gender=== | ||
Venezuelan equine encephalitis affects men and women equally.<ref name="pmid9086137">{{cite journal| author=Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A et al.| title=Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. | journal=J Infect Dis | year= 1997 | volume= 175 | issue= 4 | pages= 828-32 | pmid=9086137 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9086137 }} </ref> | |||
===Race=== | ===Race=== | ||
There is no racial predilection for Venezuelan equine encephalitis. | |||
===Seasonal=== | |||
Venezuelan equine encephalitis is most commonly observed in the summer months or after periods of heavy rainfall. | |||
===Geographic Distribution=== | |||
The majority of Venezuelan equine encephalitis cases are reported in South America, specifically Columbia and Venezuela. | |||
==Risk Factors== | ==Risk Factors== | ||
Common [[risk factor]]s in the development of Venezuelan equine encephalitis are: | |||
*Summer season | |||
*Outdoor recreational activities | |||
*Residing or visiting Central and South America | |||
*Contact with: | |||
**[[Mosquito]]s | |||
**Birds | |||
**Horses | |||
**[[Rodent]]s | |||
**Infected persons | |||
==Natural History, Complications, and Prognosis== | |||
===Natural History=== | |||
If left untreated, patients with Venezuelan equine encephalitis may progress to [[febrile]] [[prodrome]] followed by [[meningismus]], [[weakness]], [[tremor]]s, and [[altered mental status]]. | |||
===Complications=== | |||
Complications of Venezuelan equine encephalitis include: | |||
*[[Seizure]]s | |||
*Loss of basic [[motor skill]]s | |||
*Loss of [[coordination]] | |||
*[[Meningitis]] | |||
*[[Dysarthria]] | |||
*[[Affective disorders]] | |||
*[[Miscarriage]] in pregnant women | |||
===Prognosis=== | |||
Prognosis for Venezuelan equine encephalitis is generally good; less than 1% of patients infected with the Venezuelan equine encephalitis virus present with symptoms. Symptomatic patients often recover within 2-3 weeks of infection. | |||
== | ==Diagnosis== | ||
===Diagnostic criteria=== | |||
Neuroinvasive vs non-neuroinvasive Venezuelan equine encephalitis can be differentiated based on both clinical and laboratory findings. These include:<ref name=CDCCritArboInvasive> Arboviral diseases, neuroinvasive and non-neuroinvasive 2015 Case Definition. National Notifiable Diseases Surveillance System (NNDSS). Centers for Disease Control (2015). https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ Accessed on March 31, 2016. </ref> | |||
{| class="wikitable" | |||
! style="text-align: center; font-weight: bold;" | Venezuelan Equine Encephalitis Subtype | |||
! style="text-align: center; font-weight: bold;" | Clinical Presentation | |||
= | ! style="text-align: center; font-weight: bold;" | Laboratory Findings | ||
|- | |||
| style="font-style: italic;" | Neuroinvasive | |||
: | | | ||
: | :{{unicode|☑}} [[Meningitis]], [[encephalitis]], acute flaccid [[paralysis]], or other acute signs of central or peripheral neurologic dysfunction, as documented by a [[physician]] '''AND''' | ||
: | :{{unicode|☑}} Absence of a more likely clinical explanation | ||
: | | | ||
:{{unicode|☑}} Isolation of [[virus]] from, or demonstration of specific viral [[antigen]] or [[nucleic acid in]], [[tissue]], [[blood]], [[cerebrospinal fluid]] (CSF) '''OR''' | |||
=== Symptoms === | :{{unicode|☑}} Four-fold or greater change in virus-specific quantitative antibody [[titer]]s in paired sera '''OR''' | ||
:{{unicode|☑}} Virus-specific [[IgM]] antibodies in [[serum]] with confirmatory virus-specific neutralizing antibodies in the same or a later specimen '''OR''' | |||
* | :{{unicode|☑}} Virus-specific [[IgM]] antibodies in [[cerebrospinal fluid]], with or without a reported [[pleocytosis]], and a negative result for other IgM antibodies in cerebrospinal fluid for arboviruses endemic to the region where exposure occurred | ||
|- | |||
| style="font-style: italic;" | Non-neuroinvasive | |||
| | |||
:{{unicode|☑}} [[Fever]] and [[chills]] as reported by the [[patient]] or a [[health care provider]] '''AND''' | |||
:{{unicode|☑}} Absence of [[invasive|neuroinvasive]] disease '''AND''' | |||
:{{unicode|☑}} Absence of a more likely clinical explanation | |||
| | |||
=== Physical Examination === | :{{unicode|☑}} Isolation of [[virus]] from, or demonstration of specific viral [[antigen]] or [[nucleic acid]] in, [[tissue]], [[blood]], or other body fluid, excluding [[cerebrospinal fluid]] '''OR''' | ||
* | :{{unicode|☑}} Four-fold or greater change in virus-specific quantitative antibody [[titer]]s in paired sera '''OR''' | ||
* | :{{unicode|☑}} Virus-specific [[IgM]] antibodies in [[serum]] with confirmatory virus-specific neutralizing antibodies in the same or a later specimen | ||
|} | |||
===History and Symptoms=== | |||
If possible, a detailed and thorough history from the patient is necessary. If the patient is female, a pregnancy test should be administered to monitor for potential [[miscarriage]]. Venezuelan equine encephalitis is usually asymptomatic. Less common symptoms of Venezuelan equine encephalitis include:<ref name=Mandell1> M.D. JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Expert Consult Premium Edition. Saunders; 2014.</ref><ref name= NINDS> Meningitis and Encephalitis Fact Sheet. National Institute of Neurological Disorders and Stroke. National Institutes of Health (2015). http://www.ninds.nih.gov/disorders/encephalitis_meningitis/detail_encephalitis_meningitis.htm Accessed on February 9, 2015 </ref> | |||
*[[Fever]] | |||
*[[Chills]] | |||
*[[Headache]] | |||
=== Laboratory Findings === | *[[Fatigue]] | ||
*[[myalgia|Muscle pain]] | |||
*[[Arthralgia|Joint pain]] | |||
*[[pharyngeal|Throat pain]] | |||
*[[Dizziness]] | |||
*[[photophobia|Light-sensitivity]] | |||
*[[Altered mental status]] | |||
===Physical Examination=== | |||
Physical examination for Venezuelan equine encephalitis may be remarkable for:<ref name="pmid20551475">{{cite journal| author=Steele KE, Twenhafel NA| title=REVIEW PAPER: pathology of animal models of alphavirus encephalitis. | journal=Vet Pathol | year= 2010 | volume= 47 | issue= 5 | pages= 790-805 | pmid=20551475 | doi=10.1177/0300985810372508 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20551475 }} </ref> | |||
*[[Fever]] | |||
*[[Ataxia]] | |||
*[[Seizure]]s | |||
*[[Obtundation]] | |||
*[[Myalgia]] | |||
*[[myelitis|Acute flaccid myelitis]] | |||
*[[Lethargy]] | |||
*[[Meningism]] | |||
*[[Photophobia]] | |||
*[[Somnolence]] | |||
*[[Coma]] | |||
*[[Motor neuron]] dysfunction | |||
*[[Tremor]] | |||
*[[Pharyngeal]] pain | |||
*[[Cervical]] [[lymphadenopathy]] | |||
===Laboratory Findings=== | |||
The diagnostic method of choice for Venezuelan equine encephalitis is laboratory testing. The positive presence of [[IgM]] [[antibody|antibodies]] is diagnostic of Venezuelan equine encephalitis. Other laboratory findings consistent with the diagnosis of Venezuelan equine encephalitis include:<ref name=IDSAEnceph> The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America. http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/PDF_Library/Encephalitis.pdf Accessed on February 16, 2016.</ref> | |||
*[[Serologic]] [[cross-reactivity]] | |||
*Persistence of [[IgG]] and neutralizing [[antibody|antibodies]] | |||
*Confirmation of arboviral-specific neutralizing antibodies in [[enzyme linked immunosorbent assay (ELISA)]] or [[polymerase chain reaction|polymerase chain reaction (PCR)]] | |||
*In [[cerebrospinal fluid]]: | |||
**[[Pleocytosis]] | |||
**Increased protein levels | |||
**Normal to slightly elevated [[glucose]] levels | |||
===Imaging Findings=== | ===Imaging Findings=== | ||
[[MRI]] is the imaging modality of choice for Venezuelan equine encephalitis. Findings of Venezuelan equine encephalitis on MRI include T2 hyperintensity and restricted diffusion in the [[basal ganglia]] and [[thalamus]].<ref name=EncephGeneralMRI> Flavivirus encephalitis. Radiopaedia.org (2016). http://radiopaedia.org/articles/flavivirus-encephalitis Accessed on March 29, 2016. </ref> [[CT scan]] appears normal. [[EEG]] typically demonstrates diffuse slowing; some cases present with focal [[temporal]] slowing, resembling [[herpes simplex encephalitis]]. | |||
==Treatment== | |||
===Medical Therapy=== | |||
There is no treatment for Venezuelan equine encephalitis; the mainstay of therapy is supportive care. Because supportive care is the only treatment for Venezuelan equine encephalitis, physicians often do not request the tests required to specifically identify the Venezuelan equine encephalitis virus. | |||
===Surgery=== | |||
Surgical intervention is not recommended for the management of Venezuelan equine encephalitis. | |||
===Prevention=== | |||
== Treatment == | TC-83 is is a live, [[attenuated]] [[vaccine]] recommended for at risk military and [[laboratory]] personnel to prevent Venezuelan equine encephalitis. Mild to moderate [[adverse drug reaction|adverse reactions]] have been noted in up to 25% of patients.<ref name="pmid19837294">{{cite journal| author=Paessler S, Weaver SC| title=Vaccines for Venezuelan equine encephalitis. | journal=Vaccine | year= 2009 | volume= 27 Suppl 4 | issue= | pages= D80-5 | pmid=19837294 | doi=10.1016/j.vaccine.2009.07.095 | pmc=PMC2764542 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19837294 }} </ref> In some cases, the TC-83 strain does not provide sufficient [[immunization]] and may be bolstered with a C-84 vaccine. The vaccine has not been proven as fully effective against [[aerosol]] exposure.<ref name=MicroBioVEEV> Venezuelan equine encephalitis virus. MicrobeWiki (2010) https://microbewiki.kenyon.edu/index.php/Venezuelan_equine_encephalitis_virus Accessed on April 5, 2016. </ref> Currently only the C-84 vaccine is licensed for use in horses in the United States, although countries, such as Mexico and Colombia, still produce the live vaccine for horses. | ||
=== Medical Therapy === | |||
Other primary prevention strategies for Venezuelan equine encephalitis include:<ref name=EEENYPubHealth> Eastern Equine Encephalitis (EEE). New York State Department of Public Health (2012). https://www.health.ny.gov/diseases/communicable/eastern_equine_encephalitis/fact_sheet.htm Accessed on March 15, 2016.</ref> | |||
*Removal of [[standing water]] | |||
*Screens on doors and windows | |||
*When outdoors, wearing: | |||
**Insect repellent containing [[DEET]] | |||
**Long sleeves, pants; tucking in pants into high socks | |||
=== | |||
*[ | |||
* | |||
* | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category: | [[Category:Viruses]] | ||
[[Category:Neurology]] | |||
{{WikiDoc Help Menu}} | |||
Latest revision as of 19:07, 18 September 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Anthony Gallo, B.S. [2]
Synonyms and keywords: VEE; VEEV; Venezuelan equine encephalitis virus; Venezuelan encephalitis; Venezuelan equine encephalomyelitis; Venezuelan equine fever
Overview
Venezuelan equine encephalitis is a mild to moderate, though sometimes fatal, infection of the central nervous system. Venezuelan equine encephalitis belongs to the Group IV positive-sense ssRNA virus within the Togaviridae family of viruses, and the genus Alphavirus. Venezuelan equine encephalitis virus is usually transmitted via mosquitos to the human host, primarily Culex melanoconion or Aedes. Venezuelan equine encephalitis virus must be differentiated from other diseases that cause fever, headache, seizures, and altered mental status. Prognosis for Venezuelan equine encephalitis is generally good; less than 1% of patients infected with the Venezuelan equine encephalitis virus present with symptoms. Symptomatic patients often recover within 2-3 weeks of infection. The case-fatality rate of Venezuelan equine encephalitis is approximately 0.7. If possible, a detailed and thorough history from the patient is necessary. If the patient is female, a pregnancy test should be administered to monitor for potential miscarriage. Venezuelan equine encephalitis is usually asymptomatic. The diagnostic method of choice for Venezuelan equine encephalitis is laboratory testing. There is no treatment for Venezuelan equine encephalitis; the mainstay of therapy is supportive care. There is a vaccination approved for limited use for Venezuelan equine encephalitis, though its effectiveness is often questioned.
Historical Perspective
Venezuelan equine encephalitis was first discovered in 1938 after the virus was isolated from the brains of dead horses following an outbreak in the Venezuelan countryside.[1] There have been several outbreaks of Venezuelan equine encephalitis. In 1995, the last major outbreak occurred in Venezuela and Columbia and resulted in approximately 75,000 cases, of which 3,000 had severe neurological complications and 300 progressed to mortality.[2] The last reported case of Venezuelan equine encephalitis in the United States occurred in southern Texas in 1972.[3]
Classification
Venezuelan equine encephalitis may be classified according to location of the disease into 2 subtypes: systemic or encephalitic. Venezuelan equine encephalitis may also be classified according to neuroinvasiveness of the disease into 2 subtypes: neuroinvasive and non-neuroinvasive.[4] Venezuelan equine encephalitis belongs to the Group IV positive-sense ssRNA virus within the Togaviridae family of viruses, and the genus Alphavirus. Venezuelan equine encephalitis is closely related to eastern equine encephalitis virus and western equine encephalitis virus. Venezuelan equine encephalitis is known as an arbovirus, or an arthropod-borne virus.
Pathophysiology
Venezuelan equine encephalitis virus is usually transmitted via mosquitos to the human host. Venezuelan equine encephalitis virus contains positive-sense viral RNA; this RNA has its genome directly utilized as if it were mRNA, producing a single protein which is modified by host and viral proteins to form the various proteins needed for replication. The following table is a summary of the Venezuelan equine encephalitis virus:[5]
| Characteristic | Data |
|---|---|
| Nucleic acid | RNA |
| Sense | ssRNA(+) |
| Virion | Enveloped |
| Capsid | Spherical |
| Symmetry | Yes; T=4 icosahedral |
| Capsid monomers | 240 |
| Monomer length (diameter) | 70 nm |
| Additional envelope information | 80 spikes; each spike is a trimer of E1/E2 proteins |
| Genome shape | Linear |
| Genome length | 11-12 kb |
| Nucleotide cap | Yes |
| Polyadenylated tail | Yes |
| Incubation period | 1-6 day(s) |
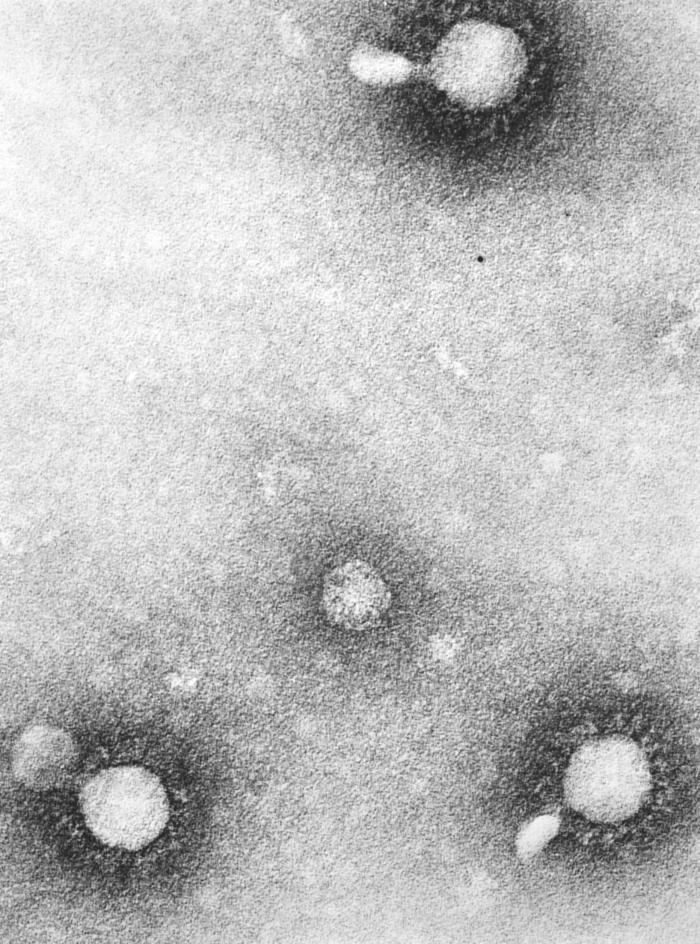
Venezuelan equine encephalitis is contracted by the bite of an infected mosquito, primarily Culex melanoconion or Aedes. Venezuelan equine encephalitis virus circulates between a mosquito vector, usually Culex melanoconion, and forest rodents in Central and South America. Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans, such as some Aedes and other Culex species. The incubation period is 1-6 day(s).[7] In contrast to many other arboviral infections, infected humans possess sufficient viremia to infect uninfected mosquitos.[8] Additionally, while a link has never been proven, there is speculation that transmission between humans is possible, as 40% of cases demonstrate infection in the pharynx.[9]
Venezuelan equine encephalitis virus is transmitted in the following pattern:[5]
- Attachment of the viral E glycoprotein to host receptors mediates clathrin endocytosis of virus into the host cell.
- Fusion of virus membrane with the host cell membrane. RNA genome is released into the cytoplasm.
- The positive-sense ssRNA virus is translated into a polyprotein, which is cleaved into non-structural proteins necessary for RNA synthesis (replication and transcription).
- Replication takes place in cytoplasmic viral factories at the surface of endosomes. A dsRNA genome is synthesized from the genomic ssRNA(+).
- The dsRNA genome is transcribed thereby providing viral mRNAs (new ssRNA(+) genomes).
- Expression of the subgenomic RNA (sgRNA) gives rise to the structural proteins.
- Virus assembly occurs at the endoplasmic reticulum.
- Virions bud at the endoplasmic reticulum, are transported to the Golgi apparatus, and then exit the cell via the secretory pathway.
On microscopic histopathological analysis, the enveloped, spherical, and icosahedral virion shape are characteristic findings of Venezuelan equine encephalitis.
Causes
Venezuelan equine encephalitis may be caused by Venezuelan equine encephalitis virus.
Differentiating Venezuelan equine encephalitis from Other Diseases
Venezuelan equine encephalitis virus must be differentiated from other diseases that cause fever, headache, seizures, and altered mental status, such as:[8][10][11][12][13]
| Disease | Similarities | Differentials |
|---|---|---|
| Meningitis | Classic triad of fever, nuchal rigidity, and altered mental status | Photophobia, phonophobia, rash associated with meningococcemia, concomitant sinusitis or otitis, swelling of the fontanelle in infants (0-6 months) |
| Brain abscess | Fever, headache, hemiparesis | Varies depending on the location of the abscess; clinically, visual disturbance including papilledema, decreased sensation; on imaging, a lesion demonstrates both ring enhancement and central restricted diffusion |
| Demyelinating diseases | Ataxia, lethargy | Multiple sclerosis: clinically, nystagmus, internuclear ophthalmoplegia, Lhermitte's sign; on imaging, well-demarcated ovoid lesions with possible T1 hypointensities (“black holes”)
Acute disseminated encephalomyelitis: clinically, somnolence, myoclonic movements, and hemiparesis; on imaging, diffuse or multi-lesion enhancement, with indistinct lesion borders |
| Substance abuse | Tremor, headache, altered mental status | Varies depending on type of substance: prior history, drug-seeking behavior, attention-seeking behavior, paranoia, sudden panic, anxiety, hallucinations |
| Electrolyte disturbance | Fatigue, headache, nausea | Varies depending on deficient ions; clinically, edema, constipation, hallucinations; on EKG, abnormalities in T wave, P wave, QRS complex; possible presentations include arrhythmia, dehydration, renal failure |
| Stroke | Ataxia, aphasia, dizziness | Varies depending on classification of stroke; presents with positional vertigo, high blood pressure, extremity weakness |
| Intracranial hemorrhage | Headache, coma, dizziness | Lobar hemorrhage, numbness, tingling, hypertension, hemorrhagic diathesis |
| Trauma | Headache, altered mental status | Amnesia, loss of consciousness, dizziness, concussion, contusion |
Epidemiology and Demographics
The case-fatality rate of Venezuelan equine encephalitis is approximately 0.7.[14]
Age
Patients of all age groups may develop Venezuelan equine encephalitis.[2]
Gender
Venezuelan equine encephalitis affects men and women equally.[2]
Race
There is no racial predilection for Venezuelan equine encephalitis.
Seasonal
Venezuelan equine encephalitis is most commonly observed in the summer months or after periods of heavy rainfall.
Geographic Distribution
The majority of Venezuelan equine encephalitis cases are reported in South America, specifically Columbia and Venezuela.
Risk Factors
Common risk factors in the development of Venezuelan equine encephalitis are:
- Summer season
- Outdoor recreational activities
- Residing or visiting Central and South America
- Contact with:
Natural History, Complications, and Prognosis
Natural History
If left untreated, patients with Venezuelan equine encephalitis may progress to febrile prodrome followed by meningismus, weakness, tremors, and altered mental status.
Complications
Complications of Venezuelan equine encephalitis include:
- Seizures
- Loss of basic motor skills
- Loss of coordination
- Meningitis
- Dysarthria
- Affective disorders
- Miscarriage in pregnant women
Prognosis
Prognosis for Venezuelan equine encephalitis is generally good; less than 1% of patients infected with the Venezuelan equine encephalitis virus present with symptoms. Symptomatic patients often recover within 2-3 weeks of infection.
Diagnosis
Diagnostic criteria
Neuroinvasive vs non-neuroinvasive Venezuelan equine encephalitis can be differentiated based on both clinical and laboratory findings. These include:[4]
| Venezuelan Equine Encephalitis Subtype | Clinical Presentation | Laboratory Findings |
|---|---|---|
| Neuroinvasive |
|
|
| Non-neuroinvasive |
|
|
History and Symptoms
If possible, a detailed and thorough history from the patient is necessary. If the patient is female, a pregnancy test should be administered to monitor for potential miscarriage. Venezuelan equine encephalitis is usually asymptomatic. Less common symptoms of Venezuelan equine encephalitis include:[8][15]
- Fever
- Chills
- Headache
- Fatigue
- Muscle pain
- Joint pain
- Throat pain
- Dizziness
- Light-sensitivity
- Altered mental status
Physical Examination
Physical examination for Venezuelan equine encephalitis may be remarkable for:[16]
- Fever
- Ataxia
- Seizures
- Obtundation
- Myalgia
- Acute flaccid myelitis
- Lethargy
- Meningism
- Photophobia
- Somnolence
- Coma
- Motor neuron dysfunction
- Tremor
- Pharyngeal pain
- Cervical lymphadenopathy
Laboratory Findings
The diagnostic method of choice for Venezuelan equine encephalitis is laboratory testing. The positive presence of IgM antibodies is diagnostic of Venezuelan equine encephalitis. Other laboratory findings consistent with the diagnosis of Venezuelan equine encephalitis include:[17]
- Serologic cross-reactivity
- Persistence of IgG and neutralizing antibodies
- Confirmation of arboviral-specific neutralizing antibodies in enzyme linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR)
- In cerebrospinal fluid:
- Pleocytosis
- Increased protein levels
- Normal to slightly elevated glucose levels
Imaging Findings
MRI is the imaging modality of choice for Venezuelan equine encephalitis. Findings of Venezuelan equine encephalitis on MRI include T2 hyperintensity and restricted diffusion in the basal ganglia and thalamus.[18] CT scan appears normal. EEG typically demonstrates diffuse slowing; some cases present with focal temporal slowing, resembling herpes simplex encephalitis.
Treatment
Medical Therapy
There is no treatment for Venezuelan equine encephalitis; the mainstay of therapy is supportive care. Because supportive care is the only treatment for Venezuelan equine encephalitis, physicians often do not request the tests required to specifically identify the Venezuelan equine encephalitis virus.
Surgery
Surgical intervention is not recommended for the management of Venezuelan equine encephalitis.
Prevention
TC-83 is is a live, attenuated vaccine recommended for at risk military and laboratory personnel to prevent Venezuelan equine encephalitis. Mild to moderate adverse reactions have been noted in up to 25% of patients.[19] In some cases, the TC-83 strain does not provide sufficient immunization and may be bolstered with a C-84 vaccine. The vaccine has not been proven as fully effective against aerosol exposure.[20] Currently only the C-84 vaccine is licensed for use in horses in the United States, although countries, such as Mexico and Colombia, still produce the live vaccine for horses.
Other primary prevention strategies for Venezuelan equine encephalitis include:[21]
- Removal of standing water
- Screens on doors and windows
- When outdoors, wearing:
- Insect repellent containing DEET
- Long sleeves, pants; tucking in pants into high socks
References
- ↑ Beck CE, Wyckoff RW (1938). "VENEZUELAN EQUINE ENCEPHALOMYELITIS". Science. 88 (2292): 530. doi:10.1126/science.88.2292.530. PMID 17840536.
- ↑ 2.0 2.1 2.2 Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A; et al. (1997). "Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995". J Infect Dis. 175 (4): 828–32. PMID 9086137.
- ↑ Venezuelan Equine Encephalomyelitis - Fact Sheet. Canadian Food Inspection Agency (2012). http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reportable/venezuelan-equine-encephalomyelitis/fact-sheet/eng/1329841926239/1329842048136 Accessed on March 31, 2016.
- ↑ 4.0 4.1 Arboviral diseases, neuroinvasive and non-neuroinvasive 2015 Case Definition. National Notifiable Diseases Surveillance System (NNDSS). Centers for Disease Control (2015). https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ Accessed on March 31, 2016.
- ↑ 5.0 5.1 Alphavirus. SIB Swiss Institute of Bioinformatics. http://viralzone.expasy.org/viralzone/all_by_species/625.html Accessed on March 15, 2016
- ↑ "Public Health Image Library (PHIL)".
- ↑ VENEZUELAN EQUINE ENCEPHALITIS VIRUS: PATHOGEN SAFETY DATA SHEET - INFECTIOUS SUBSTANCES. Public Health Agency of Canada. (2011) http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/ven-encephalit-eng.php Accessed on March 31, 2016.
- ↑ 8.0 8.1 8.2 M.D. JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Expert Consult Premium Edition. Saunders; 2014.
- ↑ Bowen GS, Calisher CH (1976). "Virological and serological studies of Venezuelan equine encephalomyelitis in humans". J Clin Microbiol. 4 (1): 22–7. PMC 274383. PMID 956360.
- ↑ Kennedy PG (2004). "Viral encephalitis: causes, differential diagnosis, and management". J Neurol Neurosurg Psychiatry. 75 Suppl 1: i10–5. PMC 1765650. PMID 14978145.
- ↑ Arboviral Infections (arthropod-borne encephalitis, eastern equine encephalitis, St. Louis encephalitis, California encephalitis, Powassan encephalitis, West Nile encephalitis). New York State Department of Health (2006). https://www.health.ny.gov/diseases/communicable/arboviral/fact_sheet.htm Accessed on February 23, 2016
- ↑ Eckstein C, Saidha S, Levy M (2012). "A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis". J Neurol. 259 (5): 801–16. doi:10.1007/s00415-011-6240-5. PMID 21932127.
- ↑ De Kruijk JR, Twijnstra A, Leffers P (2001). "Diagnostic criteria and differential diagnosis of mild traumatic brain injury". Brain Inj. 15 (2): 99–106. doi:10.1080/026990501458335. PMID 11260760.
- ↑ Weaver SC, Salas R, Rico-Hesse R, Ludwig GV, Oberste MS, Boshell J; et al. (1996). "Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group". Lancet. 348 (9025): 436–40. PMID 8709783.
- ↑ Meningitis and Encephalitis Fact Sheet. National Institute of Neurological Disorders and Stroke. National Institutes of Health (2015). http://www.ninds.nih.gov/disorders/encephalitis_meningitis/detail_encephalitis_meningitis.htm Accessed on February 9, 2015
- ↑ Steele KE, Twenhafel NA (2010). "REVIEW PAPER: pathology of animal models of alphavirus encephalitis". Vet Pathol. 47 (5): 790–805. doi:10.1177/0300985810372508. PMID 20551475.
- ↑ The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America. http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/PDF_Library/Encephalitis.pdf Accessed on February 16, 2016.
- ↑ Flavivirus encephalitis. Radiopaedia.org (2016). http://radiopaedia.org/articles/flavivirus-encephalitis Accessed on March 29, 2016.
- ↑ Paessler S, Weaver SC (2009). "Vaccines for Venezuelan equine encephalitis". Vaccine. 27 Suppl 4: D80–5. doi:10.1016/j.vaccine.2009.07.095. PMC 2764542. PMID 19837294.
- ↑ Venezuelan equine encephalitis virus. MicrobeWiki (2010) https://microbewiki.kenyon.edu/index.php/Venezuelan_equine_encephalitis_virus Accessed on April 5, 2016.
- ↑ Eastern Equine Encephalitis (EEE). New York State Department of Public Health (2012). https://www.health.ny.gov/diseases/communicable/eastern_equine_encephalitis/fact_sheet.htm Accessed on March 15, 2016.