Thrombotic thrombocytopenic purpura pathophysiology: Difference between revisions
| (25 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Thrombotic thrombocytopenic purpura}} | {{Thrombotic thrombocytopenic purpura}} | ||
{{CMG}} | {{CMG}} {{AE}} {{S.G.}} | ||
==Overview== | ==Overview== | ||
The exact [[pathogenesis]] of thrombotic thrombocytopenic purpura (TTP) is not fully understood. It is thought that TTP is caused by the [[deficiency]] of a [[plasma]] [[Metalloproteinase|metalloprotease]], [[ADAMTS13]]. | |||
== Pathophysiology == | == Pathophysiology == | ||
=== | * The exact [[pathogenesis]] of TTP is not completely understood.<ref name="pmid20058209">{{cite journal |vauthors=Tsai HM |title=Pathophysiology of thrombotic thrombocytopenic purpura |journal=Int. J. Hematol. |volume=91 |issue=1 |pages=1–19 |date=January 2010 |pmid=20058209 |pmc=3159000 |doi=10.1007/s12185-009-0476-1 |url=}}</ref> | ||
* It is understood that TTP is caused by either [[deficiency]] of a [[plasma]] [[Metalloproteinase|metalloprotease]], [[ADAMTS13]]( ('''A''' '''D'''isintegrin '''A'''nd [[Metalloproteinase|'''M'''etalloprotease]] with a '''T'''hrombo'''S'''pondin type 1 motif, member '''13'''). | |||
* [[ADAMTS13]] is member of human family the ADAMTS.<ref name="pmid15554875">{{cite journal |vauthors=Porter S, Clark IM, Kevorkian L, Edwards DR |title=The ADAMTS metalloproteinases |journal=Biochem. J. |volume=386 |issue=Pt 1 |pages=15–27 |date=February 2005 |pmid=15554875 |pmc=1134762 |doi=10.1042/BJ20040424 |url=}}</ref> | |||
* [[ADAMTS13]] is a [[plasma]] reprolysin-like [[Metalloproteinase|metalloprotease]] divides [[Von Willebrand factor|von Willebrand factor (VWF)]].<ref name="pmid23809107">{{cite journal |vauthors=Zheng XL |title=Structure-function and regulation of ADAMTS-13 protease |journal=J. Thromb. Haemost. |volume=11 Suppl 1 |issue= |pages=11–23 |date=June 2013 |pmid=23809107 |pmc=3713533 |doi=10.1111/jth.12221 |url=}}</ref> | |||
* The [[von Willebrand factor]] ([[Von Willebrand factor|VWF]]) is produced by the [[endothelial]] [[Cell (biology)|cells]] as an [[Ultra high molecular weight polyethylene|ultra-high-molecular-weight multimers]]. Normally, [[VWF]] is sliced by a [[plasma]] [[metalloproteinase]] called [[ADAMTS13]] into smaller multimers. When the [[Activity (chemistry)|activity]] or the [[Amount of substance|amount]] of the [[protease]] is not enough, the [[Ultra high molecular weight polyethylene|ultra-high-molecular-weight multimers]] of [[Von Willebrand factor|VWF]] commence [[platelet]] [[aggregation]] and [[Thrombosis|thrombosi]]<nowiki/>s in small [[vessels]].<ref name="pmid20058209" /> | |||
* [[Autoantibodies]] against the [[von Willebrand factor]] ([[VWF]]) cleaving [[Metalloproteinase|metalloprotease]] [[ADAMTS13|ADAMTS-13]]. Severe [[deficiency]] of [[plasma]] [[ADAMTS13|ADAMTS-13]] activity with or without detectable [[inhibitory]] [[Autoantibody|autoantibodies]] against [[ADAMTS13|ADAMTS-13]] supports the [[diagnosis]] of [[TTP]]. | |||
* [[Deficiency]] of [[ADAMTS13]] is caused by [[gene]] [[Mutation|mutations]] or [[Acquired disorder|acquired]]. [[Autoantibody|Autoantibodies]] is main to the [[pathophysiology]] of TTP. Ultra-large [[VWF]] [[Multimeric protein|multimers]] are breaking down by [[ADAMTS13]] [[enzyme]] . | |||
* In [[ADAMTS13]] [[deficiency]], large [[Von willebrand factor a domain containing 7|von willebrand factor]] ([[VWF]]) [[Multimeric protein|multimers]] collect leading to [[platelet]] [[aggregation]], [[hemolysis]] and microthrombi formation. [[Organ (anatomy)|Organs]] are damaged by microthrombi that it cause [[ischemia]] is leading to damage to end [[organs]]. | |||
* The most common [[organs]] being damaged are [[central nervous system]] ([[CNS]]) and [[Kidney|kidneys]]. | |||
* [[Thrombocytopenia]] results from [[platelet]] [[Consumption coagulopathy|consumption]] during [[thrombus]] formation. | |||
* [[Anemia]] results from [[hemolytic]] destruction of [[red blood cells]] as they pass through small [[Blood vessel|vessels]] that are partially occluded by [[thrombi]]. | |||
== Genetics == | |||
[[Gene|Genes]] involved in the [[pathogenesis]] of TTP include:<ref name="pmid28678087">{{cite journal |vauthors=Conboy E, Partain PI, Warad D, Kluge ML, Arndt C, Chen D, Rodriguez V |title=A Severe Case of Congenital Thrombotic Thrombocytopenia Purpura Resulting From Compound Heterozygosity Involving a Novel ADAMTS13 Pathogenic Variant |journal=J. Pediatr. Hematol. Oncol. |volume=40 |issue=1 |pages=60–62 |date=January 2018 |pmid=28678087 |doi=10.1097/MPH.0000000000000895 |url=}}</ref> | |||
*[[Mutation|Mutations]] in the [[ADAMTS13]] [[gene]]. | |||
*The development of TTP is the result of [[inherited]] [[ADAMTS13]] [[deficiency]] but mild [[phenotype]] with increased [[von Willebrand factor]] level. Upshaw–Schulman [[syndrome]] is [[Heredity|hereditary]] of TTP. | |||
*Among some patients with severe, [[Heredity|hereditary]] [[ADAMTS13]] [[deficiency]] do not have signs or [[Symptom|symptoms]] of TTP until their adulthoods .<ref name="pmid21781265">{{cite journal |vauthors=Fujimura Y, Matsumoto M, Isonishi A, Yagi H, Kokame K, Soejima K, Murata M, Miyata T |title=Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan |journal=J. Thromb. Haemost. |volume=9 Suppl 1 |issue= |pages=283–301 |date=July 2011 |pmid=21781265 |doi=10.1111/j.1538-7836.2011.04341.x |url=}}</ref> | |||
==Microscopic Pathology== | |||
On microscopic [[Histopathological|histopathologica]]<nowiki/>l analysis findings of [[Hemolytic-uremic syndrome|TTP]] include: | |||
*Granular (muddy brown) casts | |||
*Characteristic fibrin thrombi in glomerular and interstitial capillaries | |||
*Slough into [[tubular]] [[lumen]] | |||
<nowiki/>[[File:Acute thrombotic microangiopathy - pas - high mag.jpg|300px|thumb|none| High magnification microscopy of HUS Source:By Nephron [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], from Wikimedia Commons]] | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Latest revision as of 12:14, 14 March 2019
|
Thrombotic thrombocytopenic purpura Microchapters |
|
Differentiating Thrombotic thrombocytopenic purpura from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Thrombotic thrombocytopenic purpura pathophysiology On the Web |
|
American Roentgen Ray Society Images of Thrombotic thrombocytopenic purpura pathophysiology |
|
Thrombotic thrombocytopenic purpura pathophysiology in the news |
|
Blogs on Thrombotic thrombocytopenic purpura pathophysiology |
|
Directions to Hospitals Treating Thrombotic thrombocytopenic purpura |
|
Risk calculators and risk factors for Thrombotic thrombocytopenic purpura pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Sogand Goudarzi, MD [2]
Overview
The exact pathogenesis of thrombotic thrombocytopenic purpura (TTP) is not fully understood. It is thought that TTP is caused by the deficiency of a plasma metalloprotease, ADAMTS13.
Pathophysiology
- The exact pathogenesis of TTP is not completely understood.[1]
- It is understood that TTP is caused by either deficiency of a plasma metalloprotease, ADAMTS13( (A Disintegrin And Metalloprotease with a ThromboSpondin type 1 motif, member 13).
- ADAMTS13 is member of human family the ADAMTS.[2]
- ADAMTS13 is a plasma reprolysin-like metalloprotease divides von Willebrand factor (VWF).[3]
- The von Willebrand factor (VWF) is produced by the endothelial cells as an ultra-high-molecular-weight multimers. Normally, VWF is sliced by a plasma metalloproteinase called ADAMTS13 into smaller multimers. When the activity or the amount of the protease is not enough, the ultra-high-molecular-weight multimers of VWF commence platelet aggregation and thrombosis in small vessels.[1]
- Autoantibodies against the von Willebrand factor (VWF) cleaving metalloprotease ADAMTS-13. Severe deficiency of plasma ADAMTS-13 activity with or without detectable inhibitory autoantibodies against ADAMTS-13 supports the diagnosis of TTP.
- Deficiency of ADAMTS13 is caused by gene mutations or acquired. Autoantibodies is main to the pathophysiology of TTP. Ultra-large VWF multimers are breaking down by ADAMTS13 enzyme .
- In ADAMTS13 deficiency, large von willebrand factor (VWF) multimers collect leading to platelet aggregation, hemolysis and microthrombi formation. Organs are damaged by microthrombi that it cause ischemia is leading to damage to end organs.
- The most common organs being damaged are central nervous system (CNS) and kidneys.
- Thrombocytopenia results from platelet consumption during thrombus formation.
- Anemia results from hemolytic destruction of red blood cells as they pass through small vessels that are partially occluded by thrombi.
Genetics
Genes involved in the pathogenesis of TTP include:[4]
- The development of TTP is the result of inherited ADAMTS13 deficiency but mild phenotype with increased von Willebrand factor level. Upshaw–Schulman syndrome is hereditary of TTP.
- Among some patients with severe, hereditary ADAMTS13 deficiency do not have signs or symptoms of TTP until their adulthoods .[5]
Microscopic Pathology
On microscopic histopathological analysis findings of TTP include:
- Granular (muddy brown) casts
- Characteristic fibrin thrombi in glomerular and interstitial capillaries
- Slough into tubular lumen
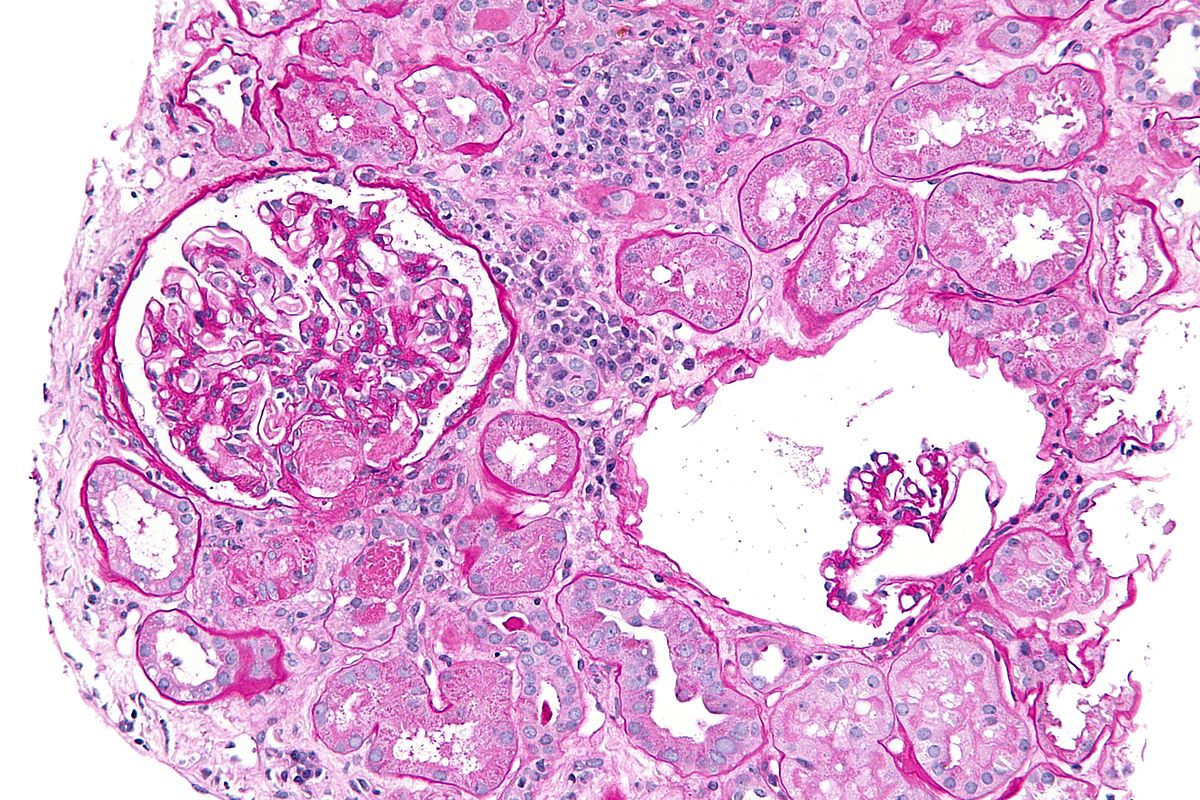
References
- ↑ 1.0 1.1 Tsai HM (January 2010). "Pathophysiology of thrombotic thrombocytopenic purpura". Int. J. Hematol. 91 (1): 1–19. doi:10.1007/s12185-009-0476-1. PMC 3159000. PMID 20058209.
- ↑ Porter S, Clark IM, Kevorkian L, Edwards DR (February 2005). "The ADAMTS metalloproteinases". Biochem. J. 386 (Pt 1): 15–27. doi:10.1042/BJ20040424. PMC 1134762. PMID 15554875.
- ↑ Zheng XL (June 2013). "Structure-function and regulation of ADAMTS-13 protease". J. Thromb. Haemost. 11 Suppl 1: 11–23. doi:10.1111/jth.12221. PMC 3713533. PMID 23809107.
- ↑ Conboy E, Partain PI, Warad D, Kluge ML, Arndt C, Chen D, Rodriguez V (January 2018). "A Severe Case of Congenital Thrombotic Thrombocytopenia Purpura Resulting From Compound Heterozygosity Involving a Novel ADAMTS13 Pathogenic Variant". J. Pediatr. Hematol. Oncol. 40 (1): 60–62. doi:10.1097/MPH.0000000000000895. PMID 28678087.
- ↑ Fujimura Y, Matsumoto M, Isonishi A, Yagi H, Kokame K, Soejima K, Murata M, Miyata T (July 2011). "Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan". J. Thromb. Haemost. 9 Suppl 1: 283–301. doi:10.1111/j.1538-7836.2011.04341.x. PMID 21781265.