Reperfusion injury overview
Editors-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editors-In-Chief: Shivam Singla, M.D
Overview
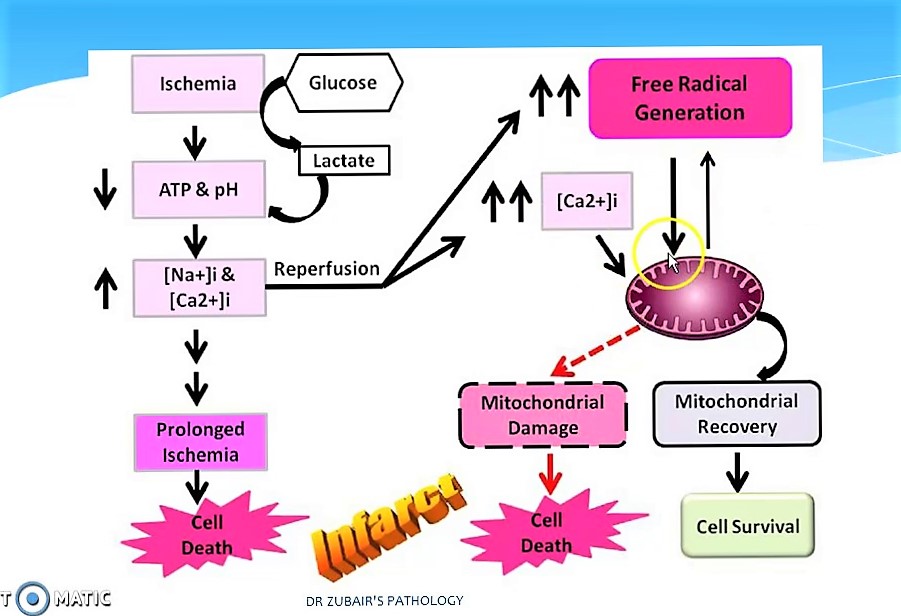
The introduction and wide application of reperfusion strategies in patients with STEMI has significantly reduced the mortality rate over last decade. Despite this, the rate of 30-day mortality remains high and approximately 25% of surviving patients develop heart failure. One of the mechanisms responsible for these adverse outcomes is Reperfusion injury. Reperfusion injury refers to myocardial cell death secondary to restoration of blood flow to the ischemic myocardium. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function.
Ischemia-reperfusion injury creates the base line for tissue damage and cellular apoptosis. The tissue damage follows a natural progression of cellular and metabolic events initiated by an ischemic episode. Ischemia induces various intracellular or extracellular changes leading ton increased calcium intracellularly and ATP depletion that will end up in the cell death if the ongoing process does not stopped. Reperfusion is considered as a stopper for this and leads to flushing of tissues with toxic metabolites , primarily reactive oxygen species . This leads to Increased mitochondrial pore permeability <math>\longrightarrow</math>complement activation & cytochrome release <math>\longrightarrow</math>Inflammation and edema <math>\longrightarrow</math>Neutrophil platelet adhesion and thrombosis leading to progressive tissue death.
Pathophysiology
Pathophysiological Mechanism is as follows:
- The pathophysiologic mechanisms underlying reperfusion injury includes various steps starting from infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochondrial damage and finally leading to activation of coagulation.
- Reperfusion damage occurs after myocardial reperfusion after a period of decreased oxygen supply. The damage from reperfusion injury is partially due to the affected tissue's inflammatory response. White blood cells transported to the region by fresh blood release a host of inflammatory factors such as interleukins and free radicals in response to tissue injury. Blood flow restored reintroduces oxygen inside cells that damages cellular proteins, DNA, and plasma membrane. Damage to the membrane of the cell will in effect cause further free radicals to be released. These reactive species can also act indirectly to turn on apoptosis through redox signaling. Also, leukocytes may build up in small capillaries, block them and cause more ischemia
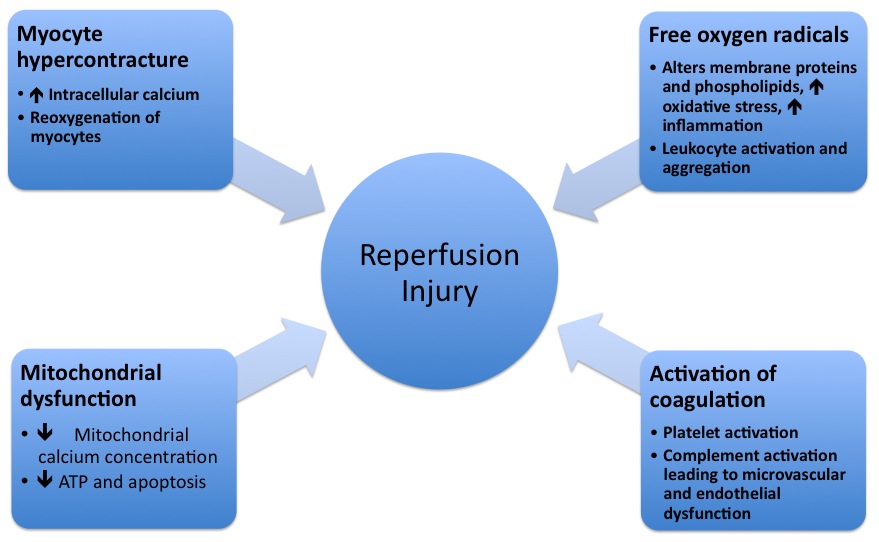
- Mitochondrial dysfunction plays a significant role in reperfusion injury. Although the mitochondrial membrane is normally impermeable to ions and metabolites, ischemia changes permeability by elevating concentrations of intro-mitochondrial calcium, reducing concentrations of adenine nucleotides, and inducing oxidative stress. It gives primacy to the mitochondrial transfer pore permeability (MPTP), which opens when reperfusion occurs. This contributes to increased osmotic load in the mitochondrial body causing swelling and breakup, releasing proteins that induce apoptosis from mitochondria. Mitochondrial activity is impaired, and ATP is hydrolyzed, allowing degrading enzymes to activate. Finally, excessive activation of the Poly ADP ribose polymerase-1 (PARP-1) impairs the work of other organelles and speeds up the development of reactive oxygen species.
- Hypoxanthine is produced as breakdown product of the ATP metabolism in prolonged ischemia (60 minutes or more). As a consequence of higher oxygen availability the enzyme xanthine dehydrogenase is converted to xanthine oxidase. This oxidation contributes to the conversion of molecular oxygen into highly reactive superoxide and hydroxyl radicals. Xanthine oxidase also produces uric acid, which can act both as a pro-oxidant and as a reactive species scavenger such as per-oxinitrite. To produce the potent reactive species per-oxinitrite, too much nitric oxide formed during reperfusion reacts with superoxide. These radicals and reactive oxygen species attack lipids , proteins, and glycosaminoglycan from the cell membrane, causing further damage. Specific biological processes may also be initiated by redox signaling.
Risk Factors
Risk factors for reperfusion injury include
- Hypertension with left ventricular hypertrophy,
- Congestive heart failure,
- Increased age,
- Diabetes, and
- Hyperlipidemia
Natural History, Complications and Prognosis
Reperfusion injury may be responsible for about 50% of the total infarct size after an acute myocardial infarction as well as myocardial stunning, congestive heart failure and reperfusion arrhythmias such as ventricular arrhythmias.[1]
Medical Therapy
Various proposed medical managements studied are:
- Therapeutic hypothermia
It has been shown in rats that neurons sometimes die completely 24 hours after the blood flow returns. Some claim that this delayed reaction is the result of the multiple inflammatory immune responses that occur during reperfusion.[2] Such inflammatory reactions cause intracranial pressure, a pressure that leads to cell damage and cell death in some cases. Hypothermia has been shown to help reduce intracranial pressure and thus decrease the adverse effects of inflammatory immune responses during reperfusion. Besides that, reperfusion also increases free radical development. Hypothermia has also been shown to decrease the patient's development of deadly free radicals during reperfusion.[3]
- Hydrogen sulfide treatment
There are several preliminary studies in mice that seem to show that treatment with hydrogen sulfide ( H2S) could have a protective effect against reperfusion injury.[4]
- Cyclosporine
In addition to its well-known immunosuppressive capabilities, the one-time administration of cyclosporine at the time of percutaneous coronary intervention (PCI) has been found to deliver a 40 percent reduction in infarct size in a small group proof of concept study of human patients with reperfusion injury published in The New England Journal of Medicine in 2008.
Cyclosporine has been confirmed in studies to inhibit the actions of cyclophilin D, a protein which is induced by excessive intracellular calcium flow to interact with other pore components and help open the MPT pore. Inhibiting cyclophilin D has been shown to prevent the opening of the MPT pore and protect the mitochondria and cellular energy production from excessive calcium inflows.
Reperfusion leads to biochemical imbalances within the cell that lead to cell death and increased infarct size. More specifically, calcium overload and excessive production of reactive oxygen species in the first few minutes after reperfusion set off a cascade of biochemical changes that result in the opening of the so-called mitochondrial permeability transition pore (MPT pore) in the mitochondrial membrane of cardiac cells.
The opening of the MPT pore leads to the inrush of water into the mitochondria, resulting in mitochondrial dysfunction and collapse. Upon collapse, the calcium is then released to overwhelm the next mitochondria in a cascading series of events that cause mitochondrial energy production supporting the cell to be reduced or stopped completely. The cessation of energy production results in cellular death. Protecting mitochondria is a viable cardio protective strategy.
Cyclosporine is currently in a phase II/III (adaptive) clinical study in Europe to determine its ability to ameliorate neuronal cellular damage in traumatic brain injury.
- TRO40303
TRO40303 is a new cardio protective compound that was shown to inhibit the MPT pore and reduce infarct size after ischemia-reperfusion. It was developed by Trophos company and currently is in Phase I clinical trial.
- Stem cell therapy
Recent investigations suggest a possible beneficial effect of mesenchymal stem cells on heart and kidney reperfusion injury.
- Superoxide dismutase
Superoxide dismutase is an effective anti-oxidant enzyme which converts superoxide anions to water and hydrogen peroxide. Recent researches have shown significant therapeutic effects on pre-clinical models of reperfusion injury after ischemic stroke.
- Metformin
A series of 2009 studies published in the Journal of Cardiovascular Pharmacology suggest that Metformin may prevent cardiac reperfusion injury by inhibition of Mitochondrial Complex I and the opening of MPT pore and in rats.
- Cannabinoids
A study published in 2012 show that the synthetic analogue of the phytocannabinoid Tetrahydrocannabivarin (THCV), Δ8-Tetrahydrocannabivarin (Δ8-THCV) and its metabolite 11-OH-Δ8-THCV, prevent hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors and thereby decrease tissue injury and inflammation with a protective effect against liver damage. Pretreatment with a CB2 receptor antagonist attenuated the protective effects of Δ8-THCV, while a CB1 antagonist tended to enhance it.
An earlier study published in 2011 found, that Cannabidiol (CBD) also protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response of oxidative and nitrative stress, and thereby cell death and tissue injury, but independent from classical CB1 and CB2 receptors.
Reperfusion protection in obligate hibernators[edit]
Obligatory hibernators such as the ground squirrels show resistance to ischemia/reperfusion (I/R) injury in liver, heart, and small intestine during the hibernation season when there is a switch from carbohydrate metabolism to lipid metabolism for cellular energy supply. This metabolic switch limits anaerobic metabolism and the formation of lactate, a herald of poor prognosis and multi-organ failure (MOF) after I/R injury. In addition, the increase in lipid metabolism generates ketone bodies and activates peroxisome proliferating-activated receptors (PPARs), both of which have been shown to be protective against I/R injury.
While many pharmacotherapies are successful in limiting reperfusion injury in animal studies or ex-vivo, the majority have failed to improve clinical outcomes in randomized clinical trials in patients. Strategies may have failed as a result of targeting the wrong mechanism, because an inadequate dose was studied, because patients with insufficient potential for benefit were studied, and because the drug was administered too late (after reperfusion had already occurred).
References
- ↑ Yellon DM, Hausenloy DJ (2007). "Myocardial reperfusion injury". N. Engl. J. Med. 357 (11): 1121–35. doi:10.1056/NEJMra071667. PMID 17855673. Unknown parameter
|month=ignored (help) - ↑ "Reperfusion injury - Wikipedia".
- ↑ BIGELOW WC (September 1959). "Methods for inducing hypothermia and rewarming". Ann. N. Y. Acad. Sci. 80: 522–32. doi:10.1111/j.1749-6632.1959.tb49229.x. PMID 13800633.
- ↑ "The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial Infarction and Heart Failure".
|
Reperfusion injury Microchapters |
|
Treatment |
|---|
|
Reperfusion injury overview On the Web |
|
American Roentgen Ray Society Images of Reperfusion injury overview |
|
Risk calculators and risk factors for Reperfusion injury overview |