Penbutolol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 99: | Line 99: | ||
In a perinatal and postnatal study in rats, the pup body weight and pup survival rate were reduced at the highest dose level of 160 mg/kg/day (200 times the MRHD). | In a perinatal and postnatal study in rats, the pup body weight and pup survival rate were reduced at the highest dose level of 160 mg/kg/day (200 times the MRHD). | ||
|useInNursing=It is not known whether penbutatol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when penbutatol is administered to a nursing woman. | |useInNursing=It is not known whether penbutatol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when penbutatol is administered to a nursing woman. | ||
|useInPed=Safety and effectiveness of penbutatol in pediatric patients have not been established. | |useInPed=Safety and effectiveness of penbutatol in pediatric patients have not been established. | ||
|useInGeri=Clinical studies of penbutatol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |useInGeri=Clinical studies of penbutatol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | ||
| Line 116: | Line 115: | ||
(Description regarding monitoring, from ''Warnings'' section) | (Description regarding monitoring, from ''Warnings'' section) | ||
| | |overdose=There is no actual experience with penbutatol overdose. The signs and symptoms that would be expected with overdosage of beeta-adrenergic receptor antagonists are symptomatic [[bradycardia]], [[hypotension]], bronchospasm, and acute cardiac failure. In addition to discontinuation of penbutatol, gastric emptying, and close observation of the patient, the following measures might be considered as appropriate: | ||
* Excessive [[bradycardia]]: Administer atropine sulfate to induce vagal blockade. If [[bradycardia]] persists, intravenous [[isoproterenol]] hydrochloride may be administered cautiously; larger than usual doses may be needed. In refractory cases, the use of a transvenous cardiac pacemaker may be necessary. | |||
* [[Hypotension]]: Sympathomimetic drug therapy, such as [[dopamine]], [[dobutamine]], or [[levarterenol]], may be considered if [[hypotension]] persists despite correction of [[bradycardia]]. In refractory cases, administration of glucagon hydrochloride has been reported to be useful. | |||
* [[Bronchospasm]]: A beta-2-agonist or [[Isoproterenol]] hydrochloride may be administered. Additional therapy with [[aminophylline]] may be considered. | |||
* | * [[Acute Cardiac Failure]]: Institute conventional therapy immediately. Intravenous administration of [[dobutamine]] and glucagon hydrochloride has been reported to be useful. | ||
* | * [[Heart Block]] (Second or Third Degree): [[Isoproterenol]] hydrochloride or a transvenous cardiac pacemaker may be used. | ||
* | |||
* | |||
( | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = | | verifiedrevid = | ||
Revision as of 18:47, 11 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Penbutolol is a beta-adrenergic blocker that is FDA approved for the treatment of hypertension. Common adverse reactions include nausea, dizziness, headache, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The usual starting and maintenance dose of penbutolol, used alone or in combination with other antihypertensive agents, such as thiazide-type diuretics, is 20 mg given once daily.
Doses of 40 mg and 80 mg have been well-tolerated but have not been shown to give a greater antihypertensive effect. The full effect of a 20- or 40-mg dose is seen by the end of 2 weeks. A dose of 10 mg also lowers blood pressure, but the full effect is not seen for 4 to 6 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penbutolol in adult patients.
Non–Guideline-Supported Use
Angina Pectoris
- Dosing Information
- 40 mg/day.[1]
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Penbutolol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penbutolol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penbutolol in pediatric patients.
Contraindications
- Cardiogenic shock
- Sinus bradycardia
- Second degree heart block
- Third degree heart block
- Bronchial asthma
- Hypersensitivity to penbutolol
Warnings
Cardiac Failure
Sympathetic stimulation may be essential for supporting circulatory function in patients with heart failure, and its inhibition by ß-adrenergic receptor blockade may precipitate more severe failure. Although ß-blockers should be avoided in overt congestive heart failure, penbutolol can, if necessary, be used with caution in patients with a history of cardiac failure who are well compensated, on treatment with vasodilators, digitalis and/or diuretics. Both digitalis and penbutolol slow AV conduction. Beta-adrenergic receptor antagonists do not inhibit the inotropic action of digitalis on heart muscle. If cardiac failure persists, treatment with penbutolol should be discontinued.
Patients Without History of Cardiac Failure
Continued depression of the myocardium with ß-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first evidence of heart failure, patients receiving penbutolol should be given appropriate treatment, and the response should be closely observed. If cardiac failure continues despite adequate intervention with appropriate drugs, penbutolol should be withdrawn (gradually, if possible).
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Hypersensitivity to catecholamines has been observed in patients who were withdrawn from therapy with ß-blocking agents; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing penbutolol, particularly in patients with ischemic heart disease, the dosage should be reduced gradually over a period of 1 to 2 weeks and the patient should be monitored carefully. If angina becomes more pronounced or acute coronary insufficiency develops, administration of penbutolol should be reinstated promptly, at least on a temporary basis, and appropriate measures should be taken for the management of unstable angina. Patients should be warned against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may not be recognized, it may not be prudent to discontinue penbutolol abruptly, even in patients who are being treated only for hypertension.
Nonallergic Bronchospasm (eg, chronic bronchitis,emphysema)
Penbutolol is contraindicated in bronchial asthma. In general, patients with bronchospastic diseases should not receive ß-blockers. Penbutolol should be administered with caution because it may block bronchodilation produced by endogenous catecholamine stimulation of ß-2 receptors.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes Mellitus and Hypoglycemia
Beta-adrenergic receptor blockade may prevent the appearance of signs and symptoms of acute hypoglycemia, such as tachycardia and blood pressure changes. This is especially important in patients with labile diabetes. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of hypoglycemic drugs. Beta-adrenergic blockade may also impair the homeostatic response to hypoglycemia; in that event, the spontaneous recovery from hypoglycemia may be delayed during treatment with ß-adrenergic receptor antagonists.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs (eg, tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of ß-adrenergic receptor blockers that might precipitate a thyroid storm.
Adverse Reactions
Clinical Trials Experience
Penbutolol is usually well tolerated in properly selected patients. Most adverse effects observed during clinical trials have been mild and reversible.
Table 1 lists the adverse reactions reported from 4 controlled studies conducted in the United States involving once-a-day administration of penbutolol (at doses ranging from 10 to 120 mg) as monotherapy or in combination with hydrochlorothiazide. Penbutolol doses above 40 mg/day are not, however, recommended. The table includes only those events where the prevalence rate in the penbutolol group was at least 1.5%, or where the reaction is of particular interest.
Over a dose range from 10 to 40 mg, once a day, fatigue, nausea, and sexual impotence occurred at a greater frequency as the dose was increased.
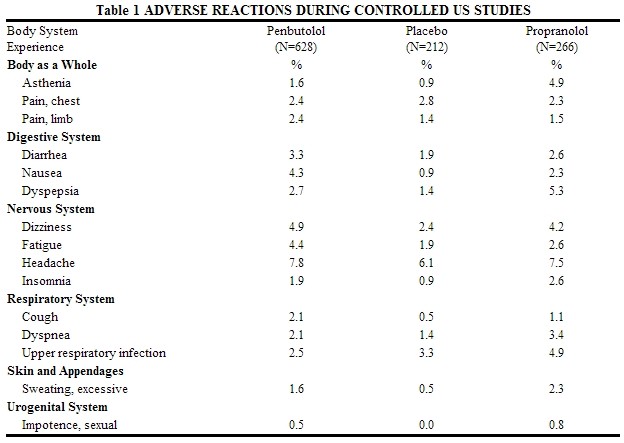
In a double-blind clinical trial comparing In a double-blind clinical trial comparing penbutolol (40 mg and greater once a day) and propranolol (40 mg or more twice a day), heart rates of less than 60 beats/min. were recorded at least once in 25% of the patients in the group receiving penbutolol and in 37% of the patients in the propranolol group. Corresponding figures for heart rates of less than 50 beats/min. were 1.2% and 6% respectively. No symptoms associated with bradycardia were reported.
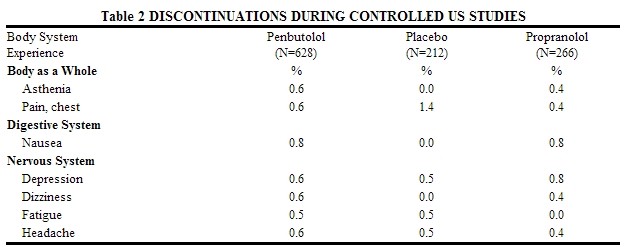
Discontinuations of penbutolol because of adverse reactions have ranged between 2.4% and 6.9% of patients in double-blind, parallel, controlled clinical trials, as compared to 1.8% to 4.1% in the corresponding control groups that were given placebo. The frequency and severity of adverse reactions have not increased during long-term administration of penbutolol. The prevalence of adverse reactions reported from 4 controlled clinical trials (referred to in the table above) as reasons for discontinuation of therapy by>0.5% of the penbutolol group is listed in the table below.
Potential Adverse Effects
In addition, certain adverse effects not listed above have been reported with other ß-blocking agents and should also be considered as potential adverse effects of penbutolol.
- Central Nervous System: Reversible mental depression progressing tocatatonia (an acute syndrome characterized by disorientation for time and place), short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance (neuropsychometrics).
- Cardiovascular: Intensification of AV block.
- Allergic: Erythematous rash, fever combined with aching and sore throat, laryngospasm, and respiratory distress.
- Hematologic: Agranulocytosis, nonthrombocytopenic and thrombocytopenic purpura.
- Gastrointestinal: Mesenteric arterial thrombosis and ischemic colitis.
- Miscellaneous: Reversible alopecia and Peyronie’s disease. The oculomucocutaneous syndrome associated with the ß-blocker practolol has not been reported with penbutolol during investigational use and extensive foreign clinical experience.
Postmarketing Experience
There is limited information regarding Penbutolol Postmarketing Experience in the drug label.
Drug Interactions
- Penbutalol has been used in combination with hydrochlorothiazide in at least 100 patients without unexpected adverse reactions.
- In one study, the combination of penbutolol and alcohol increased the number of errors in the eye-hand psychomotor function test.
- Penbutolol increases the volume of distribution of lidocaine in normal subjects. This could result in a requirement for higher loading doses of lidocaine.
- Cimetidine has no effect on the clearance of penbutolol. The major metabolite of penbutolol is a glucuronide, and it has been shown that cimetidine does not inhibit glucuronidation.
- Synergistic hypotensive effects, bradycardia, and arrhythmias have been reported in some patients receiving ß-adrenergic blocking agents when an oral calcium antagonist was added to the treatment regimen.
- Generally, penbutatol should not be used in patients receiving catecholamine-depleting drugs.
- Digoxin: Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
- Anesthesia: Care should be taken when using anesthetic agents that depress the myocardium, such as ether, cyclopropane, and trichloroethylene, and it is prudent to use the lowest possible dose of penbutolol. Penbutolol, like other beta-blockers, is a competitive inhibitor of beta-receptor agonists, and its effect on the heart can be reversed by cautious administration of such agents (eg, dobutamine or isoproterenol). Manifestations of excessive vagal tone (eg, profound bradycardia, hypotension) may be corrected with atropine 1 to 3 mg IV in divided doses.
Use in Specific Populations
Pregnancy
Teratogenic Effects
Teratology studies in rats and rabbits revealed no teratogenic effects related to treatment with penbutolol at oral doses up to 200 mg/kg/day (250 times the MRHD). In rabbits, a slight increase in the intrauterine fetal mortality and a reduced 24-hour offspring survival rate were observed in the groups treated with 125 mg/kg/day (156 times the MRHD) but not in the groups treated with 0.2 and 5 mg (0.25 to 6 times the MRHD).
There are no adequate and well-controlled studies in pregnant women. Penbutatol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
In a perinatal and postnatal study in rats, the pup body weight and pup survival rate were reduced at the highest dose level of 160 mg/kg/day (200 times the MRHD).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Penbutolol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Penbutolol during labor and delivery.
Nursing Mothers
It is not known whether penbutatol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when penbutatol is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of penbutatol in pediatric patients have not been established.
Geriatic Use
Clinical studies of penbutatol did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Penbutolol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Penbutolol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Penbutolol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Penbutolol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Penbutolol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Penbutolol in patients who are immunocompromised.
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Penbutolol and IV administrations.
Overdosage
There is no actual experience with penbutatol overdose. The signs and symptoms that would be expected with overdosage of beeta-adrenergic receptor antagonists are symptomatic bradycardia, hypotension, bronchospasm, and acute cardiac failure. In addition to discontinuation of penbutatol, gastric emptying, and close observation of the patient, the following measures might be considered as appropriate:
- Excessive bradycardia: Administer atropine sulfate to induce vagal blockade. If bradycardia persists, intravenous isoproterenol hydrochloride may be administered cautiously; larger than usual doses may be needed. In refractory cases, the use of a transvenous cardiac pacemaker may be necessary.
- Hypotension: Sympathomimetic drug therapy, such as dopamine, dobutamine, or levarterenol, may be considered if hypotension persists despite correction of bradycardia. In refractory cases, administration of glucagon hydrochloride has been reported to be useful.
- Bronchospasm: A beta-2-agonist or Isoproterenol hydrochloride may be administered. Additional therapy with aminophylline may be considered.
- Acute Cardiac Failure: Institute conventional therapy immediately. Intravenous administration of dobutamine and glucagon hydrochloride has been reported to be useful.
- Heart Block (Second or Third Degree): Isoproterenol hydrochloride or a transvenous cardiac pacemaker may be used.
Pharmacology
Penbutolol
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Penbutolol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Penbutolol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Penbutolol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Penbutolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Penbutolol Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bowles MJ, Khurmi NS, Bala Subramanian V, Raftery EB (1984). "Efficacy of once daily penbutolol in chronic stable angina. An objective comparison with long-acting propranolol". Int J Cardiol. 5 (2): 131–42. PMID 6365803.