Myxoma pathophysiology: Difference between revisions
No edit summary |
|||
| (24 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Myxoma}} | {{Myxoma}} | ||
{{CMG}} {{AE}} {{MV}}{{CZ}}{{AAM}} | {{CMG}} {{AE}} {{S.G.}} {{MV}}{{CZ}}{{AAM}} | ||
==Overview== | ==Overview== | ||
Cardiac myxoma is a benign intracavitary endocardial mass that represents the most common primary tumor of the heart. | Cardiac myxoma is a [[benign]] intracavitary [[endocardial]] [[mass]] that represents the most common [[primary tumor]] of the [[heart]]. Myxoma cells are characterized by [[undifferentiated]] [[Mesenchymal cell|mesenchymal cells]], which potentially [[differentiate]] into many [[tissues]] such as [[blood vessels]], [[glandular]] structures, and [[bones]]. The primary distribution of cardiac myxoma is the [[left atrium]] (75%) of the [[heart]]; regularly, they tend to be located in the [[fossa ovalis]] and [[endocardium]] of the [[atrial septum]]. | ||
==Pathogenesis== | ==Pathogenesis== | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
* Cardiac myxoma arises from remnants of subendocardial vasoformative reserve cells, which are primitive [[mesenchymal]] cells that are normally involved in the supportive structure of the [[endocardium]].<ref name="pmid10064365">{{cite journal |vauthors=Roscher AA, Kato NS, Quan H, Padmanabhan M |title=Intra-atrial myxomas, clinical-pathologic correlation based on two case studies including historical review |journal=J Cardiovasc Surg (Torino) |volume=37 |issue=6 Suppl 1 |pages=131–7 |year=1996 |pmid=10064365 |doi= |url=}}</ref><ref name="pmid11737312">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Román JJ |title=Prichard's structures of the fossa ovalis are not histogenetically related to cardiac myxoma |journal=Histopathology |volume=39 |issue=5 |pages=529–35 |year=2001 |pmid=11737312 |doi= |url=}}</ref> | * Cardiac myxoma arises from remnants of [[subendocardial]] vasoformative reserve [[Cell (biology)|cells]], which are primitive [[mesenchymal]] [[Cell (biology)|cells]] that are normally involved in the supportive structure of the [[endocardium]].<ref name="pmid433739">{{cite journal |vauthors=Bulkley BH, Hutchins GM |title=Atrial myxomas: a fifty year review |journal=Am. Heart J. |volume=97 |issue=5 |pages=639–43 |year=1979 |pmid=433739 |doi= |url=}}</ref><ref name="pmid109036972">{{cite journal |vauthors=Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR |title=Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation |journal=Radiographics |volume=20 |issue=4 |pages=1073–103; quiz 1110–1, 1112 |year=2000 |pmid=10903697 |doi=10.1148/radiographics.20.4.g00jl081073 |url=}}</ref><ref name="pmid10064365">{{cite journal |vauthors=Roscher AA, Kato NS, Quan H, Padmanabhan M |title=Intra-atrial myxomas, clinical-pathologic correlation based on two case studies including historical review |journal=J Cardiovasc Surg (Torino) |volume=37 |issue=6 Suppl 1 |pages=131–7 |year=1996 |pmid=10064365 |doi= |url=}}</ref><ref name="pmid11737312">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Román JJ |title=Prichard's structures of the fossa ovalis are not histogenetically related to cardiac myxoma |journal=Histopathology |volume=39 |issue=5 |pages=529–35 |year=2001 |pmid=11737312 |doi= |url=}}</ref><ref name="pmid10903697">{{cite journal |vauthors=Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR |title=Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation |journal=Radiographics |volume=20 |issue=4 |pages=1073–103; quiz 1110–1, 1112 |year=2000 |pmid=10903697 |doi=10.1148/radiographics.20.4.g00jl081073 |url=}}</ref> | ||
* The exact pathogenesis of cardiac myxoma is not fully understood.<ref name="pmid10903697">{{cite journal |vauthors=Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR |title=Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation |journal=Radiographics |volume=20 |issue=4 |pages=1073–103; quiz 1110–1, 1112 |year=2000 |pmid=10903697|doi=10.1148/radiographics.20.4.g00jl081073 |url=}}</ref> | * The exact [[pathogenesis]] of cardiac myxoma is not fully understood.<ref name="pmid10903697">{{cite journal |vauthors=Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR |title=Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation |journal=Radiographics |volume=20 |issue=4 |pages=1073–103; quiz 1110–1, 1112 |year=2000 |pmid=10903697|doi=10.1148/radiographics.20.4.g00jl081073 |url=}}</ref> | ||
* It is thought that cardiac myxoma is produced by the neoplastic theory, dysembryoplastic theory, histopathogenesis of glandular cells in myxoma or the thrombotic theory.<ref name="pmid16508920">{{cite journal |vauthors=Orlandi A, Ciucci A, Ferlosio A, Genta R, Spagnoli LG, Gabbiani G |title=Cardiac myxoma cells exhibit embryonic endocardial stem cell features |journal=J. Pathol. |volume=209 |issue=2 |pages=231–9 |year=2006 |pmid=16508920 |doi=10.1002/path.1959 |url=}}</ref><ref name="pmid13129418">{{cite journal |vauthors=Amano J, Kono T, Wada Y, Zhang T, Koide N, Fujimori M, Ito K |title=Cardiac myxoma: its origin and tumor characteristics |journal=Ann Thorac Cardiovasc Surg |volume=9 |issue=4 |pages=215–21 |year=2003 |pmid=13129418 |doi= |url=}}</ref> | * It is thought that cardiac myxoma is produced by the [[neoplastic]] theory, dysembryoplastic theory, histopathogenesis of [[glandular]] [[Cell (biology)|cells]] in myxoma or the [[thrombotic]] theory.<ref name="pmid16508920">{{cite journal |vauthors=Orlandi A, Ciucci A, Ferlosio A, Genta R, Spagnoli LG, Gabbiani G |title=Cardiac myxoma cells exhibit embryonic endocardial stem cell features |journal=J. Pathol. |volume=209 |issue=2 |pages=231–9 |year=2006 |pmid=16508920 |doi=10.1002/path.1959 |url=}}</ref><ref name="pmid13129418">{{cite journal |vauthors=Amano J, Kono T, Wada Y, Zhang T, Koide N, Fujimori M, Ito K |title=Cardiac myxoma: its origin and tumor characteristics |journal=Ann Thorac Cardiovasc Surg |volume=9 |issue=4 |pages=215–21 |year=2003 |pmid=13129418 |doi= |url=}}</ref> | ||
* The site of tumor attachment, normally the foramen ovale, is considered to be consistent with an origin from multipotent mesenchymal cells or from embryonic rests.<ref name="pmid7477198">{{cite journal |vauthors=Reynen K |title=Cardiac myxomas |journal=N. Engl. J. Med. |volume=333 |issue=24 |pages=1610–7 |year=1995 |pmid=7477198 |doi=10.1056/NEJM199512143332407 |url=}}</ref> | * The site of [[Tumor cell|tumor]] attachment, normally the [[foramen ovale]], is considered to be consistent with an origin from [[multipotent]] [[Mesenchymal cell|mesenchymal cells]] or from [[embryonic]] rests.<ref name="pmid7477198">{{cite journal |vauthors=Reynen K |title=Cardiac myxomas |journal=N. Engl. J. Med. |volume=333 |issue=24 |pages=1610–7 |year=1995 |pmid=7477198 |doi=10.1056/NEJM199512143332407 |url=}}</ref> | ||
==Genetics== | ==Genetics== | ||
* Sporadic cardiac myxomas and familial forms are related with several chromosome and gene alterations which involve cardiac development.<ref name="pmid26416542" /> | * Sporadic cardiac myxomas and familial forms are related with several [[chromosome]] and [[gene]] alterations which involve [[Heart|cardiac]] development.<ref name="pmid26416542" /> | ||
* Inherited myxomas are usually present in [[Carney complex]].<ref name="pmid26416542" /> | * Inherited myxomas are usually present in [[Carney complex]].<ref name="pmid26416542" /> | ||
* The development of [[Carney complex]] is a result of [[PRKAR1A]] gene inactivation mutation that is associated with [[chromosome]] 17q24.2-q24.3.<ref name="pmid26416542" /> | * The development of [[Carney complex]] is a result of [[PRKAR1A]] [[gene]] inactivation [[mutation]] that is associated with [[chromosome]] 17q24.2-q24.3.<ref name="pmid26416542" /> | ||
* The gene 17q24.2-q24.3 plays an important role in cardiac development and myxomagenesis. The expression of [[PRKAR1A]] causes myxomatous changes in the endocardium.<ref name="pmid26416542">{{cite journal |vauthors=Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y |title=A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family |journal=Can J Cardiol |volume=31 |issue=11 |pages=1393–401 |year=2015 |pmid=26416542 |doi=10.1016/j.cjca.2015.05.018 |url=}}</ref> | * The [[gene]] 17q24.2-q24.3 plays an important role in [[Heart|cardiac]] development and myxomagenesis. The expression of [[PRKAR1A]] causes myxomatous changes in the [[endocardium]].<ref name="pmid26416542">{{cite journal |vauthors=Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y |title=A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family |journal=Can J Cardiol |volume=31 |issue=11 |pages=1393–401 |year=2015 |pmid=26416542 |doi=10.1016/j.cjca.2015.05.018 |url=}}</ref> | ||
* The encoded protein of [[PRKAR1A]] is a type 1A regulatory subunit of protein kinase A. | * The encoded protein of [[PRKAR1A]] is a type 1A regulatory subunit of [[protein kinase]] A.Inactivating [[Germline mutation|germline mutations]] of this [[gene]] are found in 70% of people with Carney complex.<ref name="pmid26130139">{{cite journal |vauthors=Correa R, Salpea P, Stratakis CA |title=Carney complex: an update |journal=Eur. J. Endocrinol. |volume=173 |issue=4 |pages=M85–97 |date=October 2015 |pmid=26130139 |pmc=4553126 |doi=10.1530/EJE-15-0209 |url=}}</ref> | ||
* Less commonly, the molecular pathogenesis of Carney complex is a variety of [[genetic]] changes at chromosome 2p16.<ref name="StratakisKirschner2001">{{cite journal|last1=Stratakis|first1=Constantine A.|last2=Kirschner|first2=Lawrence S.|last3=Carney|first3=J. Aidan|title=Clinical and Molecular Features of the Carney Complex: Diagnostic Criteria and Recommendations for Patient Evaluation|journal=The Journal of Clinical Endocrinology & Metabolism|volume=86|issue=9|year=2001|pages=4041–4046|issn=0021-972X|doi=10.1210/jcem.86.9.7903}}</ref> | |||
* Less commonly, the molecular pathogenesis of Carney complex is a variety of genetic changes at chromosome 2p16.<ref name=": | * Both types of Carney complex are [[autosomal dominant]]. | ||
* Both types of Carney complex are [[autosomal dominant]]. | * Despite dissimilar [[genetics]], there appears to be no [[phenotype|phenotypic]] difference between [[PRKAR1A]] and [[chromosome]] 2p16 [[Mutation|mutations]].<ref name="StratakisKirschner20012">{{cite journal|last1=Stratakis|first1=Constantine A.|last2=Kirschner|first2=Lawrence S.|last3=Carney|first3=J. Aidan|title=Clinical and Molecular Features of the Carney Complex: Diagnostic Criteria and Recommendations for Patient Evaluation|journal=The Journal of Clinical Endocrinology & Metabolism|volume=86|issue=9|year=2001|pages=4041–4046|issn=0021-972X|doi=10.1210/jcem.86.9.7903}}</ref> | ||
* Despite dissimilar genetics, there appears to be no [[phenotype|phenotypic]] difference between PRKAR1A and chromosome 2p16 mutations.<ref name=" | |||
==Associated Conditions== | ==Associated Conditions== | ||
* The [[Carney complex]] is characterized by myxomatous neoplasms (cardiac, endocrine, cutaneous, and neural), and a host of pigmented lesions of the skin and mucosae, including the rarely occurring epitheloid blue [[nevus]].<ref>Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. ''Medicine'' (Baltimore). 1985;64(4):270-83.</ref><ref>Iglesias C, Torrelo A, Colmenero I, Mediero IG, Zambrano A, Requenca L. Isolated multiple congential epithelioid blue naevus. ''British Journal of Dermatology'' 2005;152:391-393.</ref><ref>Gaissmaier et al. (letter and response) Carney Complex. ''Circulation'' 1999;100 (25); e150 http://circ.ahajournals.org/cgi/reprint/100/25/e150</ref> | * The [[Carney complex]] is characterized by myxomatous neoplasms ([[cardiac]], [[endocrine]], [[cutaneous]], and [[neural]]), and a host of [[pigmented lesions]] of the [[skin]] and [[Mucous membrane|mucosae]], including the rarely occurring epitheloid blue [[nevus]].<ref>Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. ''Medicine'' (Baltimore). 1985;64(4):270-83.</ref><ref>Iglesias C, Torrelo A, Colmenero I, Mediero IG, Zambrano A, Requenca L. Isolated multiple congential epithelioid blue naevus. ''British Journal of Dermatology'' 2005;152:391-393.</ref><ref>Gaissmaier et al. (letter and response) Carney Complex. ''Circulation'' 1999;100 (25); e150 http://circ.ahajournals.org/cgi/reprint/100/25/e150</ref> | ||
* Approximately 7% of all cardiac myxomas are associated with Carney complex.<ref name="Reynen1995">{{Cite journal | last1 = Reynen | first1 = K. | title = Cardiac Myxomas | journal = New England Journal of Medicine | volume = 333 | issue = 24 | pages = 1610–1617 | year = 1995 | pmid = 7477198 | doi = 10.1056/NEJM199512143332407}}</ref> | * Approximately 7% of all cardiac myxomas are associated with [[Carney complex]].<ref name="Reynen1995">{{Cite journal | last1 = Reynen | first1 = K. | title = Cardiac Myxomas | journal = New England Journal of Medicine | volume = 333 | issue = 24 | pages = 1610–1617 | year = 1995 | pmid = 7477198 | doi = 10.1056/NEJM199512143332407}}</ref> | ||
==Gross Pathology== | ==Gross Pathology== | ||
* On gross pathology, external appearance, consistency size, and weight are extremely variable findings of cardiac myxoma | * On gross [[pathology]], external appearance, consistency size, and [[weight]] are extremely variable findings of cardiac myxoma.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
*[[Tumor]] consistency depends on the quantity and distribution of [[fibrous tissue]] and [[calcification]] (it can be smooth, lobulated, friable or gelatinous). | |||
* Myxomas are usually described as having a gelatinous, irregular surface. | * Myxomas are usually described as having a gelatinous, irregular surface. | ||
*Myxomas that have an irregular consistency are more likely to form surface [[thrombi]] and embolize.<ref name="pmid25900256">{{cite journal| author=He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B et al.| title=Risk factors for embolism in cardiac myxoma: a retrospective analysis. | journal=Med Sci Monit | year= 2015 | volume= 21 | issue= | pages= 1146-54 | pmid=25900256 | doi=10.12659/MSM.893855 | pmc=4418206 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25900256 }} </ref> | *Myxomas that have an irregular consistency are more likely to form surface [[thrombi]] and [[Embolization|embolize]].<ref name="pmid25900256">{{cite journal| author=He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B et al.| title=Risk factors for embolism in cardiac myxoma: a retrospective analysis. | journal=Med Sci Monit | year= 2015 | volume= 21 | issue= | pages= 1146-54 | pmid=25900256 | doi=10.12659/MSM.893855 | pmc=4418206 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25900256 }} </ref> | ||
* Morphologically, these lesions tend to be attached to the endocardium by a broad-based pedunculated stalk.<ref name="pmid7477198" /> | * Morphologically, these [[Lesion|lesions]] tend to be attached to the [[endocardium]] by a broad-based [[pedunculated]] stalk.<ref name="pmid7477198" /> | ||
* In some cases, the attachment to the endocardium can also be without a clear stalk, or sessile.<ref name="pmid7477198" /> | * In some cases, the attachment to the [[endocardium]] can also be without a clear stalk, or [[sessile]].<ref name="pmid7477198" /> | ||
* Cardiac myxomas are non-invasive tumors, thus there is no infiltration to underlying tissues.<ref name="pmid12208428" /> | * Cardiac myxomas are [[Non-invasive (medical)|non-invasive]] [[Tumor|tumors]], thus there is no [[Infiltration (medical)|infiltration]] to underlying [[Tissue (biology)|tissues]].<ref name="pmid12208428">{{cite journal |vauthors=Yoon DH, Roberts W |title=Sex distribution in cardiac myxomas |journal=Am. J. Cardiol. |volume=90 |issue=5 |pages=563–5 |year=2002 |pmid=12208428 |doi= |url=}}</ref> | ||
* Cardiac myxomas are intracavitary tumors. | * Cardiac myxomas are intracavitary [[tumors]]. | ||
* The distribution is normally within the [[interatrial septum]] or adjacent to foramen ovale (75%). | * The distribution is normally within the [[interatrial septum]] or adjacent to [[foramen ovale]] (75%). | ||
* However, they can also be found in other cardiac chambers, such as [[right atrium]] (15%), ventricles (2%) or cardiac valves (rare). | * However, they can also be found in other cardiac chambers, such as [[right atrium]] (15%), ventricles (2%) or cardiac valves (rare). | ||
* Large cardiac myxomas are usually located in [[fossa ovalis]].<ref name="pmid10903697" /> | * Large cardiac myxomas are usually located in [[fossa ovalis]].<ref name="pmid10903697" /> | ||
* The size of the tumor varies from 0.6 to 12 cm, with a mean weight of 40 g. | * The size of the tumor varies from 0.6 to 12 cm, with a mean weight of 40 g. | ||
* [http://www.peir.net Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | * [http://www.peir.net Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
<div align="left"> | <div align="left"> | ||
| Line 58: | Line 57: | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
*On microscopic histopathological analysis, myxoma cells have an ovoid nucleus with large nucleoli, abundant eosinophilic cytoplasm, and indistinct cell borders.<ref name="pmid18350919">{{cite journal |vauthors=Vaideeswar P, Butany JW |title=Benign cardiac tumors of the pluripotent mesenchyme |journal=Semin Diagn Pathol |volume=25 |issue=1 |pages=20–8 |year=2008 |pmid=18350919 |doi= |url=}}</ref> | *On [[microscopic]] [[histopathological]] analysis, myxoma cells have an ovoid [[nucleus]] with large [[nucleoli]], abundant [[eosinophilic]] cytoplasm, and indistinct cell borders.<ref name="pmid18350919">{{cite journal |vauthors=Vaideeswar P, Butany JW |title=Benign cardiac tumors of the pluripotent mesenchyme |journal=Semin Diagn Pathol |volume=25 |issue=1 |pages=20–8 |year=2008 |pmid=18350919 |doi= |url=}}</ref> | ||
*They are usually arranged in perivascular ring structures (typically, infiltrated by lymphocytes and macrophages).<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | *They are usually arranged in perivascular ring structures (typically, infiltrated by [[lymphocytes]] and [[macrophages]]).<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
*The '''Gamna-Bodies''' which consist of [[fibrosis]] and deposition of [[pigments|iron pigments]] are a characteristic finding of myxoma tumors.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | *The '''Gamna-Bodies''' which consist of [[fibrosis]] and deposition of [[pigments|iron pigments]] are a characteristic finding of myxoma tumors.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
*Other frequent histological findings, are hemosiderin within the histiocytes, thrombosis, fibrosis and calcifications.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | *Other frequent histological findings, are [[hemosiderin]] within the [[histiocytes]], [[thrombosis]], [[fibrosis]] and [[Calcification|calcifications]].<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
*In some cases, | *In some cases, [[extramedullary hematopoiesis]] is present and mucin-producing glands can be also seen in the base of the tumor.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
*The extracellular matrix forms an alcian blue-positive myxoid stroma, composed of variable amounts of proteoglycans, elastin and collagen.<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | *The [[extracellular matrix]] forms an [[alcian blue]]-positive myxoid stroma, composed of variable amounts of [[Proteoglycan|proteoglycans]], [[elastin]] and [[collagen]].<ref name="pmid25297937">{{cite journal |vauthors=Di Vito A, Mignogna C, Donato G |title=The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology |journal=Histopathology |volume=66 |issue=3 |pages=321–32 |year=2015 |pmid=25297937 |doi=10.1111/his.12531 |url=}}</ref> | ||
{| | |||
| | |||
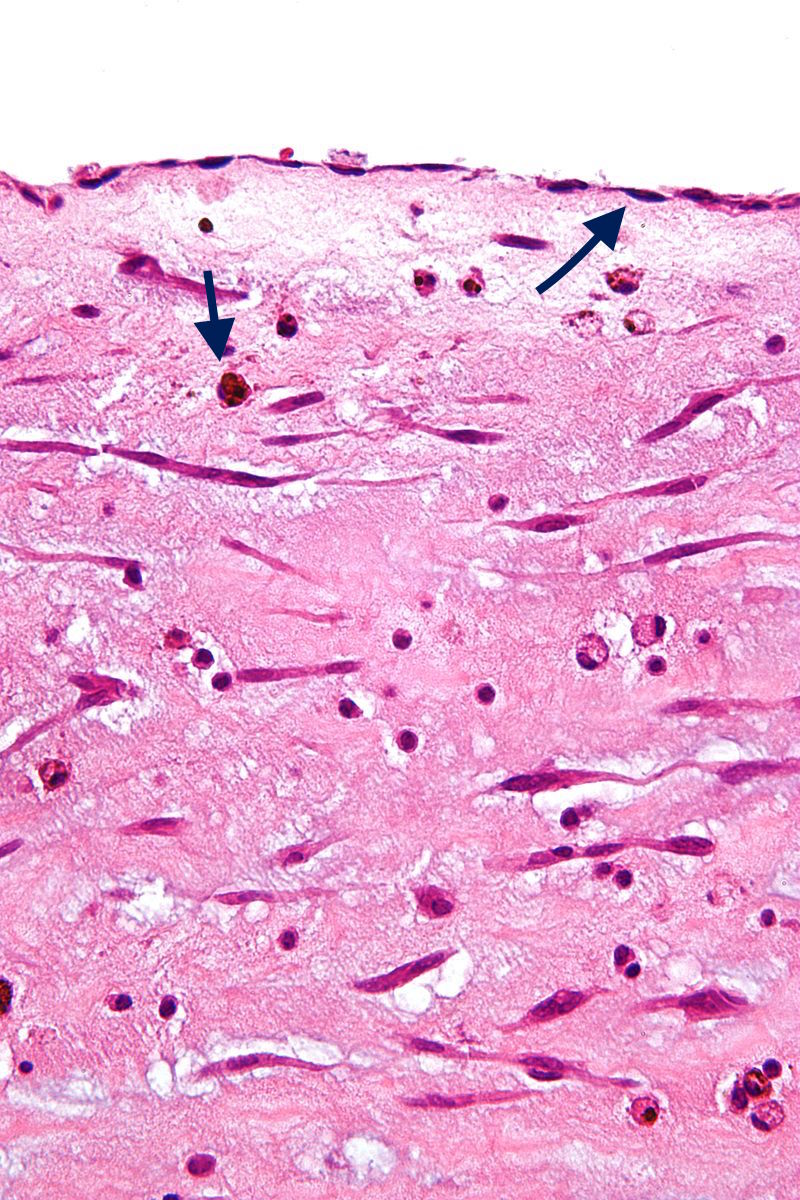
Image:800px-Atrial myxoma edge high mag.jpg|'''Black arrow (top)''': Endothelium '''Black arrow (bottom)''': Hemosiderin macrophage. | [[Image:800px-Atrial myxoma edge high mag.jpg|200px|thumb|none|'''Black arrow (top)''': Endothelium '''Black arrow (bottom)''': Hemosiderin macrophage. [https://upload.wikimedia.org/wikipedia/commons/7/74/Atrial_myxoma_edge_high_mag.jpg Source: Case courtesy by Nephron, via Wikimedia Commons]]] | ||
| | |||
[[Image: Cardiac myxoma mic 2.jpg|200px|thumb|none|'''Gamna Bodies:''' A peculiar form of fibrosis with deposition of iron pigment, identical to that seen in the spleens of patients with sickle cell anemia, is not uncommon in myxoma. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM Ph.D. and published with permission © PEIR, the University of Alabama at Birmingham, Department of Pathology]]] | |||
| | |||
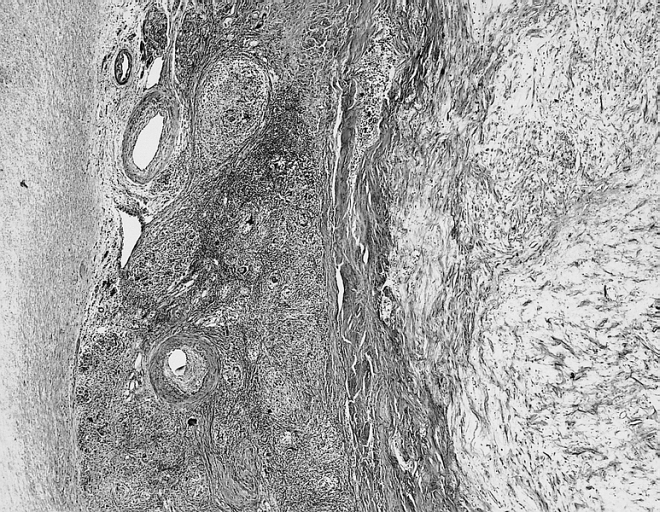
[[Image: Cardiac myxoma mic 3.jpg|200px|thumb|none|'''Cardiac myxoma:''' Common features at the interface with the atrial septum include lymphoid aggregates, smooth muscle bundles, and thick-walled vessels which angiographically may look like neovascularization. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM Ph.D. and published with permission © PEIR, the University of Alabama at Birmingham, Department of Pathology]]] | |||
| | |||
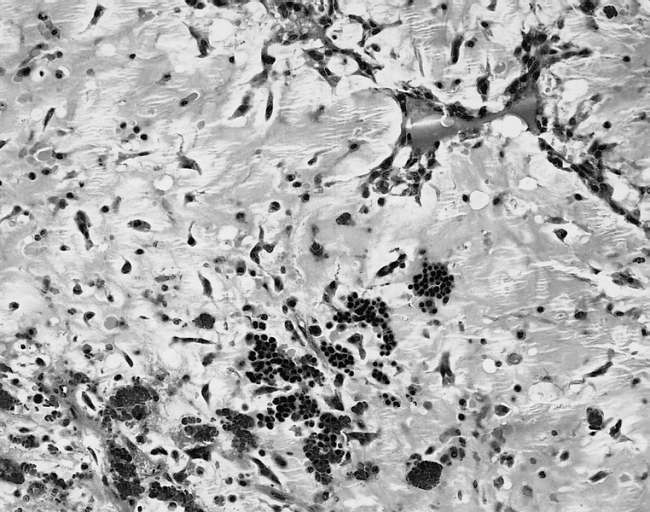
[[Image: Cardiac myxoma mic 4.jpg|200px|thumb|none|'''Cardiac myxoma:''' The extramedullary hematopoiesis seen here is present in about 7 percent of cardiac myxomas. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM Ph.D. and published with permission © PEIR, the University of Alabama at Birmingham, Department of Pathology]]] | |||
| | | | ||
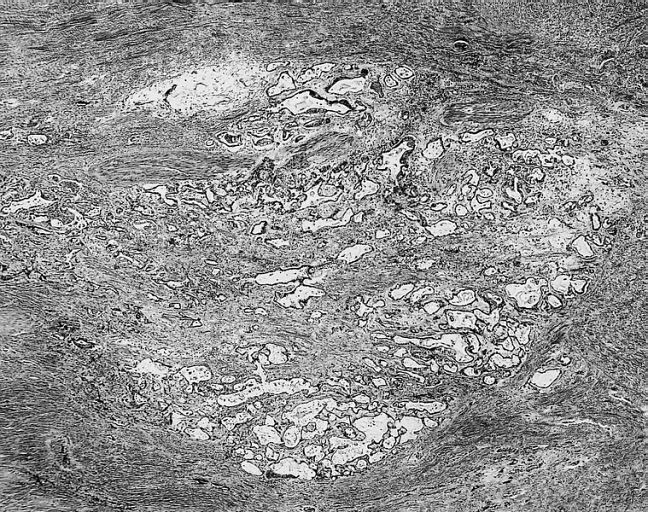
[[ | [[Image:Cardiac myxoma mic 5.jpg|200px|thumb|none|'''Cardiac myxoma:''' Glandular structures are seen in less than 5 percent of cases. In this example, they were limited to the base of the myxoma. [http://www.peir.net Image courtesy of Professor Peter Anderson DVM Ph.D. and published with permission © PEIR, the University of Alabama at Birmingham, Department of Pathology]]] | ||
|} | |} | ||
==Immunohistochemistry== | ==Immunohistochemistry== | ||
*Cardiac myxoma cells exhibit immuno-reactivity mainly for [[calretinin]] (75–100%) followed by vimentin (>50%), NOTCH1, alpha-1 antichymotrypsin and plakophilin- 2.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref> | *Cardiac myxoma cells exhibit immuno-reactivity mainly for [[calretinin]] (75–100%) followed by [[vimentin]] (>50%), [[NOTCH1]], alpha-1 antichymotrypsin and [[Plakophilin-2|plakophilin- 2]].<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref> | ||
*Calretinin plays an important role in the discrimination of mural thrombi and papillary fibroelastoma.<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref> | *[[Calretinin]] plays an important role in the [[discrimination]] of [[Mural thrombus|mural thrombi]] and [[papillary fibroelastoma]].<ref name="pmid11642722">{{cite journal |vauthors=Acebo E, Val-Bernal JF, Gómez-Roman JJ |title=Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma |journal=Histol. Histopathol. |volume=16 |issue=4 |pages=1031–6 |year=2001 |pmid=11642722 |doi= |url=}}</ref> | ||
*Another | *Another [[immunohistochemical]] marker, [[survivin]] (an apoptosis inhibitor) has been detected to play an important role in the development and growth of cardiac myxomas.<ref name="pmid21880190">{{cite journal |vauthors=Lin YS, Jung SM, Wu HH, Shiu TF, Tzai FC, Chu JJ, Lin PJ, Chu PH |title=Survivin expression in cardiac myxoma |journal=Chang Gung Med J |volume=34 |issue=4 |pages=360–6 |year=2011 |pmid=21880190 |doi= |url=}}</ref> | ||
{| style="border: 0px; font-size: 90%; margin: 3px; width: 500px" | {| style="border: 0px; font-size: 90%; margin: 3px; width: 500px" | ||
| Line 111: | Line 100: | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''General aspects'''|| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''General aspects'''|| style="padding: 5px 5px; background: #F5F5F5;" | | ||
:*Isolated cells with irregular cellular borders, mild or no atypia, absence of mitosis | :*Isolated cells with irregular [[cellular]] borders, mild or no [[atypia]], absence of [[mitosis]] | ||
:*Myxoma requires the presence of lepidic cells | :*Myxoma requires the presence of lepidic [[Cell (biology)|cells]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Genetics'''|| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Genetics'''|| style="padding: 5px 5px; background: #F5F5F5;" | | ||
:*[[PRKAR1A]] gene plays an important role in cardiac development and myxomagenesis | :*[[PRKAR1A]] gene plays an important role in [[Heart|cardiac]] development and myxomagenesis | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Gross Pathology'''|| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Gross Pathology'''|| style="padding: 5px 5px; background: #F5F5F5;" | | ||
:*Smooth, lobulated mass can be friable or gelatinous | :*Smooth, lobulated [[mass]] can be friable or gelatinous | ||
:*No infiltration to underlying tissues | :*No [[Infiltration (medical)|infiltration]] to underlying [[Tissue (biology)|tissues]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Micropathology'''|| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Micropathology'''|| style="padding: 5px 5px; background: #F5F5F5;" | | ||
:*Inflammatory | :*[[Inflammatory]] infiltrates with [[hemosiderin]], [[calcification]]s, and [[extramedullary hematopoiesis]] | ||
:*Scattered thin-walled vessels | :*Scattered thin-walled vessels | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Inmunohistochemistry'''|| style="padding: 5px 5px; background: #F5F5F5;" | | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" align="center" | '''Inmunohistochemistry'''|| style="padding: 5px 5px; background: #F5F5F5;" | | ||
:*Calretinin (75–100%) | :*[[Calretinin]] (75–100%) | ||
:*Vimentin (>50%) | :*[[Vimentin]] (>50%) | ||
|} | |} | ||
Latest revision as of 17:08, 9 July 2020
|
Myxoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Myxoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myxoma pathophysiology |
|
Risk calculators and risk factors for Myxoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Sogand Goudarzi, MD [2] Maria Fernanda Villarreal, M.D. [3]Cafer Zorkun, M.D., Ph.D. [4]Ahmad Al Maradni, M.D. [5]
Overview
Cardiac myxoma is a benign intracavitary endocardial mass that represents the most common primary tumor of the heart. Myxoma cells are characterized by undifferentiated mesenchymal cells, which potentially differentiate into many tissues such as blood vessels, glandular structures, and bones. The primary distribution of cardiac myxoma is the left atrium (75%) of the heart; regularly, they tend to be located in the fossa ovalis and endocardium of the atrial septum.
Pathogenesis
Pathogenesis
- Cardiac myxoma arises from remnants of subendocardial vasoformative reserve cells, which are primitive mesenchymal cells that are normally involved in the supportive structure of the endocardium.[1][2][3][4][5]
- The exact pathogenesis of cardiac myxoma is not fully understood.[5]
- It is thought that cardiac myxoma is produced by the neoplastic theory, dysembryoplastic theory, histopathogenesis of glandular cells in myxoma or the thrombotic theory.[6][7]
- The site of tumor attachment, normally the foramen ovale, is considered to be consistent with an origin from multipotent mesenchymal cells or from embryonic rests.[8]
Genetics
- Sporadic cardiac myxomas and familial forms are related with several chromosome and gene alterations which involve cardiac development.[9]
- Inherited myxomas are usually present in Carney complex.[9]
- The development of Carney complex is a result of PRKAR1A gene inactivation mutation that is associated with chromosome 17q24.2-q24.3.[9]
- The gene 17q24.2-q24.3 plays an important role in cardiac development and myxomagenesis. The expression of PRKAR1A causes myxomatous changes in the endocardium.[9]
- The encoded protein of PRKAR1A is a type 1A regulatory subunit of protein kinase A.Inactivating germline mutations of this gene are found in 70% of people with Carney complex.[10]
- Less commonly, the molecular pathogenesis of Carney complex is a variety of genetic changes at chromosome 2p16.[11]
- Both types of Carney complex are autosomal dominant.
- Despite dissimilar genetics, there appears to be no phenotypic difference between PRKAR1A and chromosome 2p16 mutations.[12]
Associated Conditions
- The Carney complex is characterized by myxomatous neoplasms (cardiac, endocrine, cutaneous, and neural), and a host of pigmented lesions of the skin and mucosae, including the rarely occurring epitheloid blue nevus.[13][14][15]
- Approximately 7% of all cardiac myxomas are associated with Carney complex.[16]
Gross Pathology
- On gross pathology, external appearance, consistency size, and weight are extremely variable findings of cardiac myxoma.[17]
- Tumor consistency depends on the quantity and distribution of fibrous tissue and calcification (it can be smooth, lobulated, friable or gelatinous).
- Myxomas are usually described as having a gelatinous, irregular surface.
- Myxomas that have an irregular consistency are more likely to form surface thrombi and embolize.[18]
- Morphologically, these lesions tend to be attached to the endocardium by a broad-based pedunculated stalk.[8]
- In some cases, the attachment to the endocardium can also be without a clear stalk, or sessile.[8]
- Cardiac myxomas are non-invasive tumors, thus there is no infiltration to underlying tissues.[19]
- Cardiac myxomas are intracavitary tumors.
- The distribution is normally within the interatrial septum or adjacent to foramen ovale (75%).
- However, they can also be found in other cardiac chambers, such as right atrium (15%), ventricles (2%) or cardiac valves (rare).
- Large cardiac myxomas are usually located in fossa ovalis.[5]
- The size of the tumor varies from 0.6 to 12 cm, with a mean weight of 40 g.
- Images shown below are courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
-
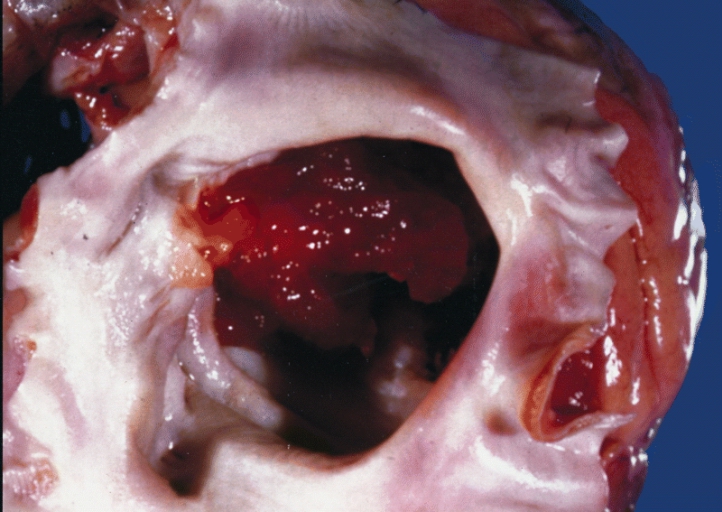
A gelatinous tumor is attached by a narrow pedicle to the atrial septum. The myxoma has an irregular surface and nearly fills the left atrium
-
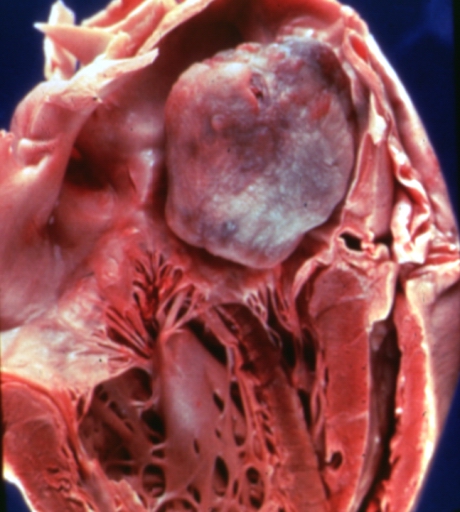
Left atrial myxoma
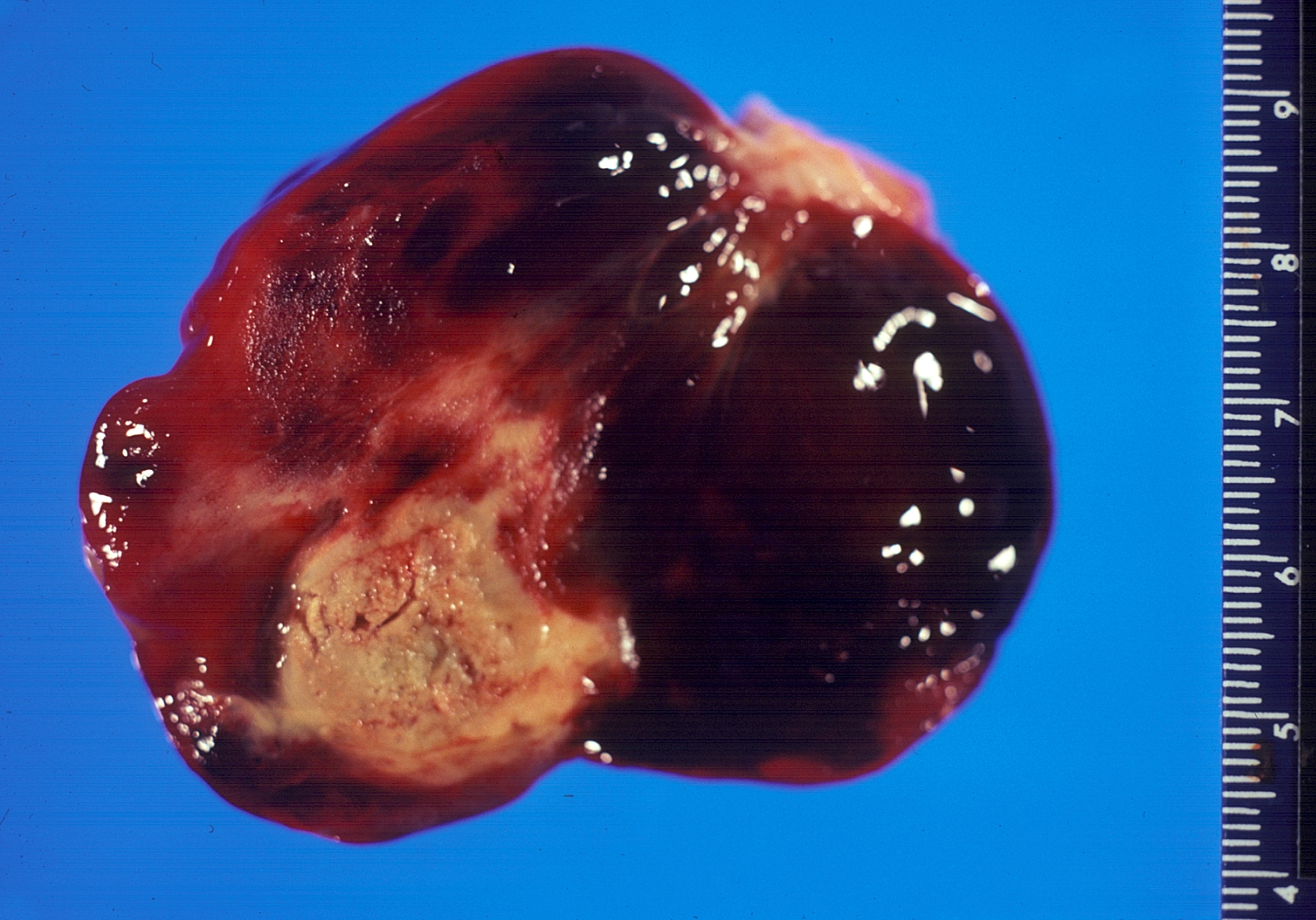
-
Gross pathology atrial myxoma: myxomas are brownish or white and are frequently covered with thrombus
Microscopic Pathology
- On microscopic histopathological analysis, myxoma cells have an ovoid nucleus with large nucleoli, abundant eosinophilic cytoplasm, and indistinct cell borders.[20]
- They are usually arranged in perivascular ring structures (typically, infiltrated by lymphocytes and macrophages).[17]
- The Gamna-Bodies which consist of fibrosis and deposition of iron pigments are a characteristic finding of myxoma tumors.[17]
- Other frequent histological findings, are hemosiderin within the histiocytes, thrombosis, fibrosis and calcifications.[17]
- In some cases, extramedullary hematopoiesis is present and mucin-producing glands can be also seen in the base of the tumor.[17]
- The extracellular matrix forms an alcian blue-positive myxoid stroma, composed of variable amounts of proteoglycans, elastin and collagen.[17]
 |
 |
 |
 |
 |
Immunohistochemistry
- Cardiac myxoma cells exhibit immuno-reactivity mainly for calretinin (75–100%) followed by vimentin (>50%), NOTCH1, alpha-1 antichymotrypsin and plakophilin- 2.[21]
- Calretinin plays an important role in the discrimination of mural thrombi and papillary fibroelastoma.[21]
- Another immunohistochemical marker, survivin (an apoptosis inhibitor) has been detected to play an important role in the development and growth of cardiac myxomas.[22]
| Features | Description |
|---|---|
| General aspects | |
| Genetics | |
| Gross Pathology |
|
| Micropathology |
|
| Inmunohistochemistry |
|
References
- ↑ Bulkley BH, Hutchins GM (1979). "Atrial myxomas: a fifty year review". Am. Heart J. 97 (5): 639–43. PMID 433739.
- ↑ Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR (2000). "Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation". Radiographics. 20 (4): 1073–103, quiz 1110–1, 1112. doi:10.1148/radiographics.20.4.g00jl081073. PMID 10903697.
- ↑ Roscher AA, Kato NS, Quan H, Padmanabhan M (1996). "Intra-atrial myxomas, clinical-pathologic correlation based on two case studies including historical review". J Cardiovasc Surg (Torino). 37 (6 Suppl 1): 131–7. PMID 10064365.
- ↑ Acebo E, Val-Bernal JF, Gómez-Román JJ (2001). "Prichard's structures of the fossa ovalis are not histogenetically related to cardiac myxoma". Histopathology. 39 (5): 529–35. PMID 11737312.
- ↑ 5.0 5.1 5.2 Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR (2000). "Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation". Radiographics. 20 (4): 1073–103, quiz 1110–1, 1112. doi:10.1148/radiographics.20.4.g00jl081073. PMID 10903697.
- ↑ Orlandi A, Ciucci A, Ferlosio A, Genta R, Spagnoli LG, Gabbiani G (2006). "Cardiac myxoma cells exhibit embryonic endocardial stem cell features". J. Pathol. 209 (2): 231–9. doi:10.1002/path.1959. PMID 16508920.
- ↑ Amano J, Kono T, Wada Y, Zhang T, Koide N, Fujimori M, Ito K (2003). "Cardiac myxoma: its origin and tumor characteristics". Ann Thorac Cardiovasc Surg. 9 (4): 215–21. PMID 13129418.
- ↑ 8.0 8.1 8.2 Reynen K (1995). "Cardiac myxomas". N. Engl. J. Med. 333 (24): 1610–7. doi:10.1056/NEJM199512143332407. PMID 7477198.
- ↑ 9.0 9.1 9.2 9.3 Sun Y, Chen X, Sun J, Wen X, Liu X, Zhang Y, Hoffman AR, Hu JF, Gao Y (2015). "A Novel Inherited Mutation in PRKAR1A Abrogates PreRNA Splicing in a Carney Complex Family". Can J Cardiol. 31 (11): 1393–401. doi:10.1016/j.cjca.2015.05.018. PMID 26416542.
- ↑ Correa R, Salpea P, Stratakis CA (October 2015). "Carney complex: an update". Eur. J. Endocrinol. 173 (4): M85–97. doi:10.1530/EJE-15-0209. PMC 4553126. PMID 26130139.
- ↑ Stratakis, Constantine A.; Kirschner, Lawrence S.; Carney, J. Aidan (2001). "Clinical and Molecular Features of the Carney Complex: Diagnostic Criteria and Recommendations for Patient Evaluation". The Journal of Clinical Endocrinology & Metabolism. 86 (9): 4041–4046. doi:10.1210/jcem.86.9.7903. ISSN 0021-972X.
- ↑ Stratakis, Constantine A.; Kirschner, Lawrence S.; Carney, J. Aidan (2001). "Clinical and Molecular Features of the Carney Complex: Diagnostic Criteria and Recommendations for Patient Evaluation". The Journal of Clinical Endocrinology & Metabolism. 86 (9): 4041–4046. doi:10.1210/jcem.86.9.7903. ISSN 0021-972X.
- ↑ Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64(4):270-83.
- ↑ Iglesias C, Torrelo A, Colmenero I, Mediero IG, Zambrano A, Requenca L. Isolated multiple congential epithelioid blue naevus. British Journal of Dermatology 2005;152:391-393.
- ↑ Gaissmaier et al. (letter and response) Carney Complex. Circulation 1999;100 (25); e150 http://circ.ahajournals.org/cgi/reprint/100/25/e150
- ↑ Reynen, K. (1995). "Cardiac Myxomas". New England Journal of Medicine. 333 (24): 1610–1617. doi:10.1056/NEJM199512143332407. PMID 7477198.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 Di Vito A, Mignogna C, Donato G (2015). "The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology". Histopathology. 66 (3): 321–32. doi:10.1111/his.12531. PMID 25297937.
- ↑ He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B; et al. (2015). "Risk factors for embolism in cardiac myxoma: a retrospective analysis". Med Sci Monit. 21: 1146–54. doi:10.12659/MSM.893855. PMC 4418206. PMID 25900256.
- ↑ Yoon DH, Roberts W (2002). "Sex distribution in cardiac myxomas". Am. J. Cardiol. 90 (5): 563–5. PMID 12208428.
- ↑ Vaideeswar P, Butany JW (2008). "Benign cardiac tumors of the pluripotent mesenchyme". Semin Diagn Pathol. 25 (1): 20–8. PMID 18350919.
- ↑ 21.0 21.1 Acebo E, Val-Bernal JF, Gómez-Roman JJ (2001). "Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac myxoma". Histol. Histopathol. 16 (4): 1031–6. PMID 11642722.
- ↑ Lin YS, Jung SM, Wu HH, Shiu TF, Tzai FC, Chu JJ, Lin PJ, Chu PH (2011). "Survivin expression in cardiac myxoma". Chang Gung Med J. 34 (4): 360–6. PMID 21880190.