Myeloproliferative neoplasm pathophysiology: Difference between revisions
No edit summary |
|||
| (72 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
<div style="-webkit-user-select: none;"> | |||
{|class="infobox" style="position: fixed; top: 65%; right: 5px; margin: 0 0 0 0; border: 0; float: right; | |||
|- | |||
| {{#ev:youtube|https://https://www.youtube.com/watch?v=jFxCZ91sDpI|350}} | |||
|- | |||
|} | |||
__NOTOC__ | __NOTOC__ | ||
{{Myeloproliferative disease}} | {{Myeloproliferative disease}} | ||
{{CMG}} | {{CMG}}{{AE}} {{MJK}} {{shyam}} | ||
==Overview== | ==Overview== | ||
The | The pathophysiology of myeloproliferative neoplasms is based on the specific subtype of myeloproliferative neoplasm. Each of the 8 different myeloproliferative neoplasms have a slightly different pathophysiologic basis. Primary cytogenetic abnormalities have not been identified in the majority of myeloproliferative neoplasms. Aberrant activation of [[tyrosine kinase]]s and associated signaling pathways is frequently implicated as the disease-initiating event for many of the myeloproliferative neoplasms. Clinical and pathologic features in the myeloproliferative neoplasms are due to dysregulated proliferation and expansion of [[myeloid]] [[progenitors]] in the [[bone marrow]], resulting in altered populations of [[granulocytes]], [[erythrocytes]], or [[platelets]] in the peripheral blood. | ||
==Pathophysiology== | ==Pathophysiology== | ||
=== | ===[[Polycythemia Vera]]=== | ||
==== Physiology ==== | |||
In order to understand the pathophysiology of [[polycythemia vera]], one must understand normal physiology of [[Red blood cell|erythroid cell]] production. Under normal conditions, the hormone [[erythropoietin]] will bind to the [[erythropoietin]] receptor on [[Red blood cell|erythroid cell]], resulting in activation of the [[JAK-STAT signaling pathway]]. Specifically, the [[erythropoietin receptor]] is associated with the [[JAK2]] protein, and binding of the [[erythropoietin receptor]] by its endogenous ligand ([[erythropoietin]]) will stimulate a basal level of [[Red blood cell|erythroid cell]] production. | |||
==== Pathophysiology ==== | |||
In patients with [[polycythemia vera]], this normal signaling process is excessively activated and dysregulated. The pathophysiology of polycythemia vera is well-defined and is specific to this subcategory of myeloproliferative neoplasm. | |||
* The disease-initiating event is the development of the ''[[Janus kinase]]'' (''[[Janus kinase|JAK2]]'') ''V617F'' point [[mutation]] or ''[[Janus kinase|JAK2]]'' exon 12 [[mutation]]. This results in aberrant activation of ''[[Janus kinase|JAK2]]'' signaling, resulting in excess signal transduction via intracellular ''[[STAT5]]'' signaling. The ''[[Janus kinase|JAK2]]'' mutation results in autonomous [[Red blood cell|erythroid cell]] production. This leads to proliferation of [[Red blood cell|erythroid]] precursors, with resultant increase in [[red blood cell]] production and increase in [[hemoglobin]] content. Greater than 95% of cases of [[polycythemia vera]] are due to the ''[[Janus kinase|JAK2]]'' mutation. | |||
* [[Mutation|Mutations]] in ''[[PI3K|phosphoinositide-3-kinase]]'' ([[PI3K]]) and ''[[AKT]]'' have also been implicated in the pathophysiology of [[polycythemia vera]] but are less common. | |||
''For more information, go to [[Polycythemia vera pathophysiology]].'' | |||
===[[Essential thrombocythemia|Essential Thrombocythemia]]=== | |||
The pathophysiology of [[Essential thrombocytosis|essential thrombocythemia]] is most commonly due to either a [[mutation]] in one of 3 [[Gene|genes]]: ''[[Janus kinase|JAK2]]'', ''CALR'', or ''MPL''.<ref name="pmidhttp://dx.doi.org/10.1182/blood-2013-11-538983">{{cite journal| author=Schmoldt A, Benthe HF, Haberland G| title=Digitoxin metabolism by rat liver microsomes. | journal=Biochem Pharmacol | year= 1975 | volume= 24 | issue= 17 | pages= 1639-41 | pmid=http://dx.doi.org/10.1182/blood-2013-11-538983 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10 }} </ref> | |||
* The pathophysiology of ''[[Janus kinase|JAK2]]''-mutant [[Essential thrombocytosis|essential thrombocythemia]] is similar to ''[[Janus kinase|JAK2]]''-mutant [[polycythemia vera]], except that the abnormal cell is the [[megakaryocyte]] in [[Essential thrombocytosis|essential thrombocythemia]]. The ''[[Janus kinase|JAK2]]'' mutation accounts for 63% of [[Essential thrombocytosis|essential thrombocythemia]] cases. | |||
* The ''CALR'' [[mutation]] is second most common mutation in [[Essential thrombocytosis|essential thrombocythemia]] and accounts for 23% of cases. The pathophysiology of ''CALR''-mutant [[Essential thrombocytosis|essential thrombocythemia]] involves a mutation in [[calreticulin]], which is a [[Chaperone (protein)|chaperone protein]] in the [[endoplasmic reticulum]]. This [[mutation]] results in aberrant activation of the [[thrombopoietin receptor]]. | |||
* The least common, yet well-defined, [[mutation]] in [[Essential thrombocytosis|essential thrombocythemia]] is the ''MPL'' [[mutation]]. This accounts for 4% of [[Essential thrombocytosis|essential thrombocythemia]]. The ''MPL'' [[gene]] encodes the [[thrombopoietin receptor]], and a point [[mutation]] involving a [[tryptophan]] codon in the ''MPL'' [[gene]] causes excess activation of this receptor, resulting in excess [[platelet]] production. | |||
''For more information, go to [[Essential thrombocytosis pathophysiology]].'' | |||
===[[Chronic Myeloid Leukemia]]=== | |||
The pathophysiology of chronic myeloid leukemia involves the [[Philadelphia chromosome]] translocation, in which parts of two chromosomes (the 9<sup>th</sup> and 22<sup>nd</sup> by conventional [[karyotype|karyotypic]] numbering) switch places. As a result, part of the ''BCR'' ("breakpoint cluster region") gene from chromosome 22 is fused with the ''ABL'' gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185. Because ''ABL'' carries a domain that can add phosphate groups to tyrosine residues (a [[tyrosine kinase]]), the ''[[BCR]]-[[ABL]]'' fusion gene product is also a tyrosine kinase. The fused ''[[BCR]]-[[ABL]]'' protein interacts with the interleukin 3beta receptor, which results in excess myeloid cell production. The ''[[BCR]]-[[ABL]]'' transcript is continuously active and does not require activation by other cellular messaging proteins. In turn ''[[BCR]]-[[ABL]]'' activates a cascade of proteins which control the [[cell cycle]], speeding up cell division. Moreover the ''BCR-ABL'' protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the ''[[BCR]]-[[ABL]]'' protein is the pathophysiologic cause of chronic myelogenous leukemia.<ref name="Hehlmann">{{cite journal|title=Chronic myeloid leukaemia|author=Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet|journal=Lancet|volume=370|issue=9584|pages=342-50|date=2007|pmid=17662883}}</ref> | |||
===[[Primary Myelofibrosis]]=== | |||
The pathophysiology of primary myelofibrosis is most commonly due to either a mutation in one of 3 genes: ''JAK2'', ''CALR'', ''MPL''.<ref name="pmidhttp://dx.doi.org/10.1182/blood-2013-11-538983">{{cite journal| author=Schmoldt A, Benthe HF, Haberland G| title=Digitoxin metabolism by rat liver microsomes. | journal=Biochem Pharmacol | year= 1975 | volume= 24 | issue= 17 | pages= 1639-41 | pmid=http://dx.doi.org/10.1182/blood-2013-11-538983 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10 }} </ref> The pathophysiology of ''JAK2''-mutation primary myelofibrosis is similar to ''JAK2''-mutant polycythemia vera, except that the abnormal cell is the megakaryocyte, which produces excess PDGF and TGF-beta and stimulates excess collagen production. The ''JAK2'' mutation accounts for 59% of primary myelofibrosis cases. The ''CALR'' mutation is second most common mutation in primary myelofibrosis and accounts for 27% of cases. The pathophysiology of ''CALR''-mutant primary myelofibrosis involves a mutation in [[calreticulin]], which is a chaperone protein in the endoplasmic reticulum. This mutation results in aberrant activation of the thrombopoietin receptor. The least common, yet well-defined, mutation in essential thrombocythemia is the ''MPL'' mutation. This accounts for 7% of primary myelofibrosis cases. The ''MPL'' gene encodes the thrombopoietin receptor, and a point mutation involving a tryptophan codon in the ''MPL'' gene causes excess activation of this receptor, resulting in excess megakaryocyte-mediated PDGF and TGF-beta production, which creates bone marrow fibrosis. | |||
=== | ===[[Chronic Neutrophilic Leukemia]]=== | ||
The pathophysiology of [[chronic neutrophilic leukemia]] involves a point mutation in the ''colony-stimulating factor 3 receptor'' (''CSF3R'') gene. The point mutation replaces [[isoleucine]] for [[threonine]] at the 618th position (''T618I''). This point mutation is pathognomonic for [[chronic neutrophilic leukemia]], though it is a rare mutation overall and accounts for only a few hundred cases in the United States. It is found in 83% of patients with [[chronic neutrophilic leukemia]].<ref name="pmid26366092">{{cite journal| author=Menezes J, Cigudosa JC| title=Chronic neutrophilic leukemia: a clinical perspective. | journal=Onco Targets Ther | year= 2015 | volume= 8 | issue= | pages= 2383-90 | pmid=26366092 | doi=10.2147/OTT.S49688 | pmc=4562747 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26366092 }} </ref> This mutation results in aberrant activation of the receptor, which stimulating [[neutrophil]] production. Although this mutation is the most frequent driver mutation for [[chronic neutrophilic leukemia]], other mutations that have been described in chronic neutrophilic leukemia include SET binding protein 1 (''SETBP1'') mutations, which are also found in atypical [[chronic myeloid leukemia]]. This mutation contributes to the pathophysiology of [[chronic neutrophilic leukemia]] by altering DNA replication. | |||
===[[Chronic Eosinophilic Leukemia]]=== | |||
The pathophysiology of [[chronic eosinophilic leukemia]] most commonly involves rearrangements affecting the platelet-derived growth factor receptors (''PDGFRA'' and ''PDGFRB'').<ref name="pmid23982058">{{cite journal| author=Legrand F, Renneville A, MacIntyre E, Mastrilli S, Ackermann F, Cayuela JM et al.| title=The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases. | journal=Medicine (Baltimore) | year= 2013 | volume= 92 | issue= 5 | pages= e1-e9 | pmid=23982058 | doi=10.1097/MD.0b013e3182a71eba | pmc=4553979 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23982058 }} </ref> Upon deletion of the intervening ''CHIC2'' locus on chromosome 4, the ''FIP1L1'' locus (located upstream of ''PDGFRA'') is juxtaposed to the ''PDGFRA'' locus, and ''FIP1L1'' drives the expression of ''PDGFRA''. This is the most frequent clonal event in hypereosinophilic syndrome.<ref name="pmid23982058">{{cite journal| author=Legrand F, Renneville A, MacIntyre E, Mastrilli S, Ackermann F, Cayuela JM et al.| title=The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases. | journal=Medicine (Baltimore) | year= 2013 | volume= 92 | issue= 5 | pages= e1-e9 | pmid=23982058 | doi=10.1097/MD.0b013e3182a71eba | pmc=4553979 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23982058 }} </ref> Excess PDGFR expression results in stimulation of eosinophil production. Other genes that are known to be involved in chronic eosinophilic leukemia include fibroblast growth factor receptor 1 (''FGFR1'') and ''PCM1-JAK2''. Eosinophils can deposit in various organs, including the liver and heart, and this can create end-organ damage in chronic eosinophilic leukemia. | |||
===[[Myeloproliferative Neoplasm, Unclassifiable]]=== | |||
There is no specific pathophysiologic basis for myeloproliferative neoplasm, unclassifiable. However, the final pathophysiologic event is the stimulation of cells of the myeloid lineage, similar to other subtypes of myeloproliferative neoplasm. | |||
=== | ===[[Mastocytosis]]=== | ||
The pathophysiology of mastocytosis, or mast cell neoplasm, has been largely unknown for decades. It was later discovered the mast cell arises from the common myeloid progenitor. The most common molecular mutation in mastocytosis is the ''c-kit'' ''D816V'' mutation. The ''D816V'' mutation is found in more than 80% of patients with mastocytosis.<ref name="pmid29051803">{{cite journal| author=Gallogly MM, Lazarus HM, Cooper BW| title=Midostaurin: a novel therapeutic agent for patients with FLT3-mutated acute myeloid leukemia and systemic mastocytosis. | journal=Ther Adv Hematol | year= 2017 | volume= 8 | issue= 9 | pages= 245-261 | pmid=29051803 | doi=10.1177/2040620717721459 | pmc=5639976 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29051803 }} </ref> Under normal conditions, the c-kit protein (also known as CD117) allows for expansion of hematopoietic cells, which is highly regulated. In mastocytosis, aberrant ''c-kit'' activation results in excess and uncontrolled production of myeloid-derived cells, such as mast cells. c-kit is a tyrosine kinase that signals via PI3K and mTOR, and this leads to cell proliferation. Mast cells accumulate in the bone marrow, skin, and other organs. End-organ damage commonly occurs in systemic mastocytosis.<ref name="pmid29051803">{{cite journal| author=Gallogly MM, Lazarus HM, Cooper BW| title=Midostaurin: a novel therapeutic agent for patients with FLT3-mutated acute myeloid leukemia and systemic mastocytosis. | journal=Ther Adv Hematol | year= 2017 | volume= 8 | issue= 9 | pages= 245-261 | pmid=29051803 | doi=10.1177/2040620717721459 | pmc=5639976 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=29051803 }} </ref> | |||
==== | ==Gallery== | ||
{| | |||
|[[image:Bcr-abl.jpg|thumb|center]] | |||
|[[Philadelphia chromosome]]. A piece of [[chromosome 9]] and a piece of [[chromosome 22]] break off and trade places. The [[BCR]]/[[Abl gene|ABL]] gene is formed on [[chromosome 22]] where the piece of [[chromosome 9]] attaches. The changed [[chromosome 22]] is called the [[Philadelphia chromosome]].<ref name="cancergov">National Cancer Institute. Physician Data Query Database 2015.http://www.cancer.gov/types/leukemia/patient/cml-treatment-pdq</ref> | |||
|- | |||
|[[image:CDR526538-571.jpg|thumb|center]] | |||
|[[Blood cell]] development. A blood [[stem cell]] goes through several steps to become a [[red blood cell]], [[platelet]], or [[white blood cell]].<ref name="cancergov">National Cancer Institute. Physician Data Query Database 2015.http://www.cancer.gov/types/leukemia/patient/cml-treatment-pdq</ref> | |||
|- | |||
|} | |||
==References== | ==References== | ||
| Line 29: | Line 66: | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Hematology]] | [[Category:Hematology]] | ||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
Latest revision as of 14:57, 30 October 2019
| https://https://www.youtube.com/watch?v=jFxCZ91sDpI%7C350}} |
|
Myeloproliferative Neoplasm Microchapters |
|
Differentiating myeloproliferative neoplasm from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Myeloproliferative neoplasm pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myeloproliferative neoplasm pathophysiology |
|
Directions to Hospitals Treating Myeloproliferative neoplasm |
|
Risk calculators and risk factors for Myeloproliferative neoplasm pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Mohamad Alkateb, MBBCh [2] Shyam Patel [3]
Overview
The pathophysiology of myeloproliferative neoplasms is based on the specific subtype of myeloproliferative neoplasm. Each of the 8 different myeloproliferative neoplasms have a slightly different pathophysiologic basis. Primary cytogenetic abnormalities have not been identified in the majority of myeloproliferative neoplasms. Aberrant activation of tyrosine kinases and associated signaling pathways is frequently implicated as the disease-initiating event for many of the myeloproliferative neoplasms. Clinical and pathologic features in the myeloproliferative neoplasms are due to dysregulated proliferation and expansion of myeloid progenitors in the bone marrow, resulting in altered populations of granulocytes, erythrocytes, or platelets in the peripheral blood.
Pathophysiology
Polycythemia Vera
Physiology
In order to understand the pathophysiology of polycythemia vera, one must understand normal physiology of erythroid cell production. Under normal conditions, the hormone erythropoietin will bind to the erythropoietin receptor on erythroid cell, resulting in activation of the JAK-STAT signaling pathway. Specifically, the erythropoietin receptor is associated with the JAK2 protein, and binding of the erythropoietin receptor by its endogenous ligand (erythropoietin) will stimulate a basal level of erythroid cell production.
Pathophysiology
In patients with polycythemia vera, this normal signaling process is excessively activated and dysregulated. The pathophysiology of polycythemia vera is well-defined and is specific to this subcategory of myeloproliferative neoplasm.
- The disease-initiating event is the development of the Janus kinase (JAK2) V617F point mutation or JAK2 exon 12 mutation. This results in aberrant activation of JAK2 signaling, resulting in excess signal transduction via intracellular STAT5 signaling. The JAK2 mutation results in autonomous erythroid cell production. This leads to proliferation of erythroid precursors, with resultant increase in red blood cell production and increase in hemoglobin content. Greater than 95% of cases of polycythemia vera are due to the JAK2 mutation.
- Mutations in phosphoinositide-3-kinase (PI3K) and AKT have also been implicated in the pathophysiology of polycythemia vera but are less common.
For more information, go to Polycythemia vera pathophysiology.
Essential Thrombocythemia
The pathophysiology of essential thrombocythemia is most commonly due to either a mutation in one of 3 genes: JAK2, CALR, or MPL.[1]
- The pathophysiology of JAK2-mutant essential thrombocythemia is similar to JAK2-mutant polycythemia vera, except that the abnormal cell is the megakaryocyte in essential thrombocythemia. The JAK2 mutation accounts for 63% of essential thrombocythemia cases.
- The CALR mutation is second most common mutation in essential thrombocythemia and accounts for 23% of cases. The pathophysiology of CALR-mutant essential thrombocythemia involves a mutation in calreticulin, which is a chaperone protein in the endoplasmic reticulum. This mutation results in aberrant activation of the thrombopoietin receptor.
- The least common, yet well-defined, mutation in essential thrombocythemia is the MPL mutation. This accounts for 4% of essential thrombocythemia. The MPL gene encodes the thrombopoietin receptor, and a point mutation involving a tryptophan codon in the MPL gene causes excess activation of this receptor, resulting in excess platelet production.
For more information, go to Essential thrombocytosis pathophysiology.
Chronic Myeloid Leukemia
The pathophysiology of chronic myeloid leukemia involves the Philadelphia chromosome translocation, in which parts of two chromosomes (the 9th and 22nd by conventional karyotypic numbering) switch places. As a result, part of the BCR ("breakpoint cluster region") gene from chromosome 22 is fused with the ABL gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185. Because ABL carries a domain that can add phosphate groups to tyrosine residues (a tyrosine kinase), the BCR-ABL fusion gene product is also a tyrosine kinase. The fused BCR-ABL protein interacts with the interleukin 3beta receptor, which results in excess myeloid cell production. The BCR-ABL transcript is continuously active and does not require activation by other cellular messaging proteins. In turn BCR-ABL activates a cascade of proteins which control the cell cycle, speeding up cell division. Moreover the BCR-ABL protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the BCR-ABL protein is the pathophysiologic cause of chronic myelogenous leukemia.[2]
Primary Myelofibrosis
The pathophysiology of primary myelofibrosis is most commonly due to either a mutation in one of 3 genes: JAK2, CALR, MPL.[1] The pathophysiology of JAK2-mutation primary myelofibrosis is similar to JAK2-mutant polycythemia vera, except that the abnormal cell is the megakaryocyte, which produces excess PDGF and TGF-beta and stimulates excess collagen production. The JAK2 mutation accounts for 59% of primary myelofibrosis cases. The CALR mutation is second most common mutation in primary myelofibrosis and accounts for 27% of cases. The pathophysiology of CALR-mutant primary myelofibrosis involves a mutation in calreticulin, which is a chaperone protein in the endoplasmic reticulum. This mutation results in aberrant activation of the thrombopoietin receptor. The least common, yet well-defined, mutation in essential thrombocythemia is the MPL mutation. This accounts for 7% of primary myelofibrosis cases. The MPL gene encodes the thrombopoietin receptor, and a point mutation involving a tryptophan codon in the MPL gene causes excess activation of this receptor, resulting in excess megakaryocyte-mediated PDGF and TGF-beta production, which creates bone marrow fibrosis.
Chronic Neutrophilic Leukemia
The pathophysiology of chronic neutrophilic leukemia involves a point mutation in the colony-stimulating factor 3 receptor (CSF3R) gene. The point mutation replaces isoleucine for threonine at the 618th position (T618I). This point mutation is pathognomonic for chronic neutrophilic leukemia, though it is a rare mutation overall and accounts for only a few hundred cases in the United States. It is found in 83% of patients with chronic neutrophilic leukemia.[3] This mutation results in aberrant activation of the receptor, which stimulating neutrophil production. Although this mutation is the most frequent driver mutation for chronic neutrophilic leukemia, other mutations that have been described in chronic neutrophilic leukemia include SET binding protein 1 (SETBP1) mutations, which are also found in atypical chronic myeloid leukemia. This mutation contributes to the pathophysiology of chronic neutrophilic leukemia by altering DNA replication.
Chronic Eosinophilic Leukemia
The pathophysiology of chronic eosinophilic leukemia most commonly involves rearrangements affecting the platelet-derived growth factor receptors (PDGFRA and PDGFRB).[4] Upon deletion of the intervening CHIC2 locus on chromosome 4, the FIP1L1 locus (located upstream of PDGFRA) is juxtaposed to the PDGFRA locus, and FIP1L1 drives the expression of PDGFRA. This is the most frequent clonal event in hypereosinophilic syndrome.[4] Excess PDGFR expression results in stimulation of eosinophil production. Other genes that are known to be involved in chronic eosinophilic leukemia include fibroblast growth factor receptor 1 (FGFR1) and PCM1-JAK2. Eosinophils can deposit in various organs, including the liver and heart, and this can create end-organ damage in chronic eosinophilic leukemia.
Myeloproliferative Neoplasm, Unclassifiable
There is no specific pathophysiologic basis for myeloproliferative neoplasm, unclassifiable. However, the final pathophysiologic event is the stimulation of cells of the myeloid lineage, similar to other subtypes of myeloproliferative neoplasm.
Mastocytosis
The pathophysiology of mastocytosis, or mast cell neoplasm, has been largely unknown for decades. It was later discovered the mast cell arises from the common myeloid progenitor. The most common molecular mutation in mastocytosis is the c-kit D816V mutation. The D816V mutation is found in more than 80% of patients with mastocytosis.[5] Under normal conditions, the c-kit protein (also known as CD117) allows for expansion of hematopoietic cells, which is highly regulated. In mastocytosis, aberrant c-kit activation results in excess and uncontrolled production of myeloid-derived cells, such as mast cells. c-kit is a tyrosine kinase that signals via PI3K and mTOR, and this leads to cell proliferation. Mast cells accumulate in the bone marrow, skin, and other organs. End-organ damage commonly occurs in systemic mastocytosis.[5]
Gallery
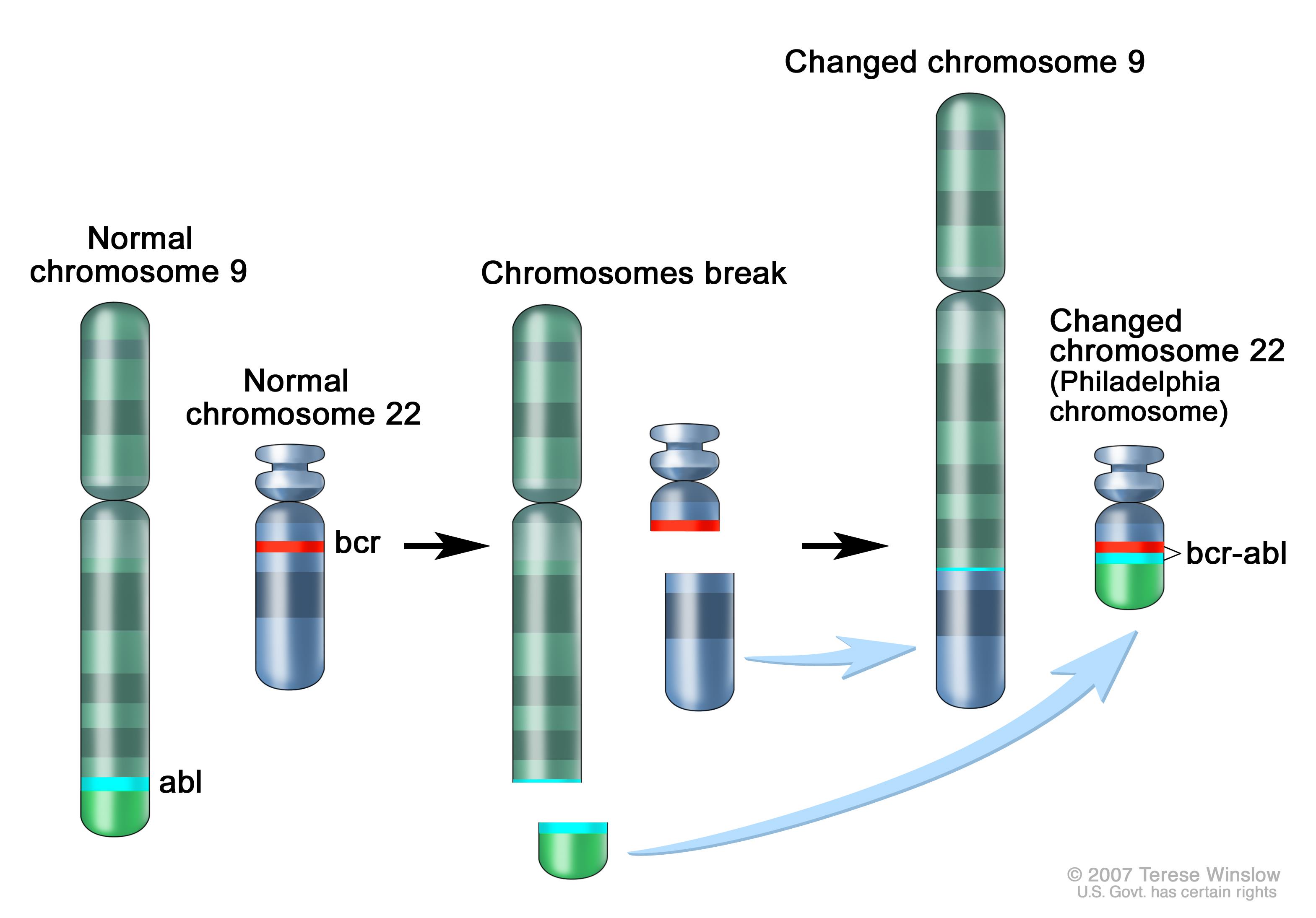 |
Philadelphia chromosome. A piece of chromosome 9 and a piece of chromosome 22 break off and trade places. The BCR/ABL gene is formed on chromosome 22 where the piece of chromosome 9 attaches. The changed chromosome 22 is called the Philadelphia chromosome.[6] |
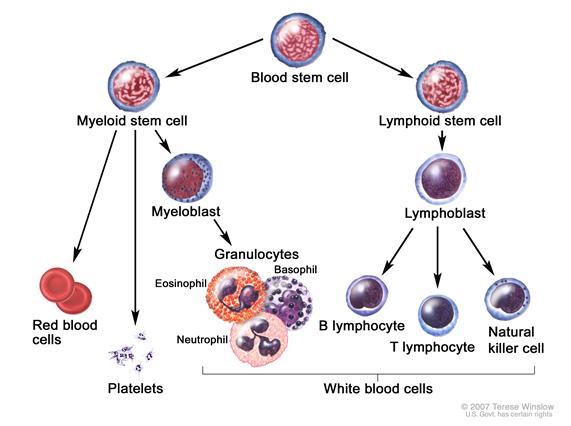 |
Blood cell development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell.[6] |
References
- ↑ 1.0 1.1 Schmoldt A, Benthe HF, Haberland G (1975). "Digitoxin metabolism by rat liver microsomes". Biochem Pharmacol. 24 (17): 1639–41. PMID http://dx.doi.org/10.1182/blood-2013-11-538983 Check
|pmid=value (help). - ↑ Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. PMID 17662883.
- ↑ Menezes J, Cigudosa JC (2015). "Chronic neutrophilic leukemia: a clinical perspective". Onco Targets Ther. 8: 2383–90. doi:10.2147/OTT.S49688. PMC 4562747. PMID 26366092.
- ↑ 4.0 4.1 Legrand F, Renneville A, MacIntyre E, Mastrilli S, Ackermann F, Cayuela JM; et al. (2013). "The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases". Medicine (Baltimore). 92 (5): e1–e9. doi:10.1097/MD.0b013e3182a71eba. PMC 4553979. PMID 23982058.
- ↑ 5.0 5.1 Gallogly MM, Lazarus HM, Cooper BW (2017). "Midostaurin: a novel therapeutic agent for patients with FLT3-mutated acute myeloid leukemia and systemic mastocytosis". Ther Adv Hematol. 8 (9): 245–261. doi:10.1177/2040620717721459. PMC 5639976. PMID 29051803.
- ↑ 6.0 6.1 National Cancer Institute. Physician Data Query Database 2015.http://www.cancer.gov/types/leukemia/patient/cml-treatment-pdq