Loxapine (oral): Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{SG}} | |||
|genericName=loxapine succinate | |||
|aOrAn=an | |||
|drugClass=antipsychotic | |||
|indicationType=treatment | |||
|indication=schizophrenia | |||
|hasBlackBoxWarning=Yes | |||
|adverseReactions=Taste sense altered, sedation, pharyngitis. | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">WARNING</span> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Increased Mortality in Elderly Patients with Dementia-Related Psychosis </span></i> Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Loxapine is not approved for the treatment of patients with dementia-related psychosis | |||
|fdaLIADAdult=====Schizophrenia==== | |||
*Initial dosage of 10 mg twice daily | |||
*Severely disturbed patients initial dosage up to a total of 50 mg daily may be desirable. | |||
*Dosage should then be increased fairly rapidly over the first seven to ten days until there is effective control of symptoms of schizophrenia. The usual therapeutic and maintenance range is 60 mg to 100 mg daily. | |||
*Some patients respond to lower dosage and others require higher dosage. | |||
*Daily dosage higher than 250 mg is not recommended. | |||
*For maintenance therapy, dosage should be reduced to the lowest level compatible with symptom control; many patients have been maintained satisfactorily at dosages in the range of 20 to 60 mg daily. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Loxapine in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Loxapine in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Loxapine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Loxapine in pediatric patients. | |||
|contraindications=Loxapine is contraindicated in comatose or severe drug-induced depressed states (alcohol, barbiturates, narcotics, etc.). | |||
Loxapine is contraindicated in individuals with known hypersensitivity to dibenzoxazepines. | |||
|warnings=Increased Mortality in Elderly Patients with Dementia-Related Psychosis | |||
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Loxapine is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING). | |||
Tardive Dyskinesia | |||
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. | |||
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses. | |||
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown. | |||
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically. | |||
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome. (See ADVERSE REACTIONS and INFORMATION FOR PATIENTS sections). | |||
Neuroleptic Malignant Syndrome (NMS) | |||
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). | |||
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology. | |||
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS. | |||
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported. | |||
Loxapine, like other antipsychotics, may impair mental and/or physical abilities, especially during the first few days of therapy. Therefore, ambulatory patients should be warned about activities requiring alertness (e.g., operating vehicles or machinery) and about concomitant use of alcohol and other CNS depressants. | |||
Loxapine has not been evaluated for the management of behavioral complications in patients with mental retardation, and therefore, it cannot be recommended. | |||
Leukopenia, Neutropenia and Agranulocytosis | |||
In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents. | |||
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a preexisting low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Loxapine Succinate Capsules USP at the first sign of a decline in WBC in the absence of other causative factors. | |||
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue Loxapine Succinate Capsules USP and have their WBC followed until recovery. | |||
|clinicalTrials=CNS Effects: Manifestations of adverse effects on the central nervous system, other than extrapyramidal effects, have been seen infrequently. Drowsiness, usually mild, may occur at the beginning of therapy or when dosage is increased. It usually subsides with continued loxapine therapy. The incidence of sedation has been less than that of certain aliphatic phenothiazines and slightly more than the piperazine phenothiazines. Dizziness, faintness, staggering gait, shuffling gait, muscle twitching, weakness, insomnia, agitation, tension, seizures, akinesia, slurred speech, numbness, and confusional states have been reported. Neuroleptic malignant syndrome (NMS) has been reported (see WARNINGS). | |||
Extrapyramidal Symptoms - Neuromuscular (extrapyramidal) reactions during the administration of loxapine have been reported frequently, often during the first few days of treatment. In most patients, these reactions involved parkinsonian-like symptoms such as tremor, rigidity, excessive salivation, and masked facies. Akathisia (motor restlessness) also has been reported relatively frequently. These symptoms are usually not severe and can be controlled by reduction of loxapine dosage or by administration of antiparkinson drugs in usual dosage. | |||
Dystonia - Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups. | |||
Persistent Tardive Dyskinesia - As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high-dose therapy, especially females. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movement of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities. | |||
There is no known effective treatment for tardive dyskinesia; antiparkinson agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been suggested that fine vermicular movements of the tongue may be an early sign of the syndrome, and if the medication is stopped at that time the syndrome may not develop. | |||
Cardiovascular Effects: Tachycardia, hypotension, hypertension, orthostatic hypotension, lightheadedness, and syncope have been reported. | |||
A few cases of ECG changes similar to those seen with phenothiazines have been reported. It is not known whether these were related to loxapine administration. | |||
Hematologic: Rarely, agranulocytosis, thrombocytopenia, leukopenia. | |||
Skin: Dermatitis, edema (puffiness of face), pruritus, rash, alopecia, and seborrhea have been reported with loxapine. | |||
Anticholinergic Effects: Dry mouth, nasal congestion, constipation, blurred vision, urinary retention, and paralytic ileus have occurred. | |||
Gastrointestinal: Nausea and vomiting have been reported in some patients. Hepatocellular injury (i.e., SGOT/SGPT elevation) has been reported in association with loxapine administration and rarely, jaundice and/or hepatitis questionably related to loxapine treatment. | |||
Other Adverse Reactions: Weight gain, weight loss, dyspnea, ptosis, hyperpyrexia, flushed facies, headache, paresthesia, and polydipsia have been reported in some patients. Rarely, galactorrhea, amenorrhea, gynecomastia, and menstrual irregularity of uncertain etiology have been reported. | |||
|drugInteractions=There have been rare reports of significant respiratory depression, stupor and/or hypotension with the concomitant use of loxapine and lorazepam. | |||
The risk of using loxapine in combination with CNS-active drugs has not been systematically evaluated. Therefore, caution is advised if the concomitant administration of loxapine and CNS-active drugs is required. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Non-teratogenic Effects | |||
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization. | |||
Loxapine Succinate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
Safe use of loxapine during pregnancy or lactation has not been established; therefore, its use in pregnancy, in nursing mothers, or in women of childbearing potential requires that the benefits of treatment be weighed against the possible risks to mother and child. No embryotoxicity or teratogenicity was observed in studies in rats, rabbits, or dogs although, with the exception of one rabbit study, the highest dosage was only two times the maximum recommended human dose and in some studies it was below this dose. Perinatal studies have shown renal papillary abnormalities in offspring of rats treated from mid-pregnancy with doses of 0.6 and 1.8 mg/kg, doses which approximate the usual human dose but which are considerably below the maximum recommended human dose. | |||
|useInNursing=The extent of the excretion of loxapine or its metabolites in human milk is not known. However, loxapine and its metabolites have been shown to be transported into the milk of lactating dogs. Loxapine administration to nursing women should be avoided if clinically possible. | |||
|useInPed=Safety and effectiveness of loxapine in pediatric patients have not been established. | |||
|overdose=Signs and symptoms of overdosage will depend on the amount ingested and individual patient tolerance. As would be expected from the pharmacologic actions of the drug, the clinical findings may range from mild depression of the CNS and cardiovascular systems to profound hypotension, respiratory depression, and unconsciousness. The possibility of occurrence of extrapyramidal symptoms and/or convulsive seizures should be kept in mind. Renal failure following loxapine overdosage has also been reported. | |||
The treatment of overdosage is essentially symptomatic and supportive. Early gastric lavage and extended dialysis might be expected to be beneficial. Centrally-acting emetics may have little effect because of the antiemetic action of loxapine. In addition, emesis should be avoided because of the possibility of aspiration of vomitus. Avoid analeptics, such as pentylenetetrazol, which may cause convulsions. Severe hypotension might be expected to respond to the administration of norepinephrine or phenylephrine. EPINEPHRINE SHOULD NOT BE USED SINCE ITS USE IN A PATIENT WITH PARTIAL ADRENERGIC BLOCKADE MAY FURTHER LOWER THE BLOOD PRESSURE. Severe extrapyramidal reactions should be treated with anticholinergic antiparkinson agents or diphenhydramine hydrochloride, and anticonvulsant therapy should be initiated as indicated. Additional measures include oxygen and intravenous fluids. | |||
|drugBox={{drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 408581688 | |||
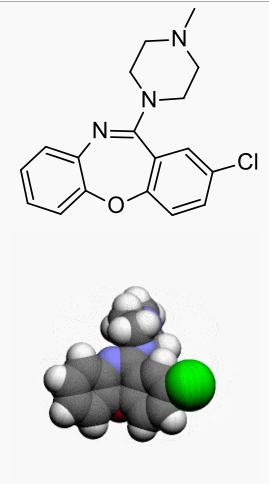
| IUPAC_name = 2-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine | |||
| image = Loxapine chemical structure.png | |||
| width = 250 | |||
<!--Clinical data--> | |||
| tradename = Loxapac, Loxitane, Adasuve | |||
| Drugs.com = {{drugs.com|monograph|loxapine-succinate}} | |||
| MedlinePlus = a682311 | |||
| DailyMedID = 50e11732-7387-452d-b3e6-db3a431d5c4a | |||
| licence_EU = Adasuve | |||
| licence_US = Loxapine | |||
| pregnancy_US = C | |||
| legal_US = Rx-only | |||
| legal_AU = S4 | |||
| routes_of_administration = Inhalation, oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 96.8%<ref name = "DrugPoint" /> | |||
| metabolism = Liver, extensive; active metabolites include [[amoxapine]] and 8-hydroxyloxapine. Inhibits P-glycoprotein and is a substrate of [[CYP1A2]], [[CYP3A4]] and [[CYP2D6]]<ref name = "DrugPoint" /> | |||
| elimination_half-life = Oral, 4 hours; Inhalation, 7.61 hours <ref name = "DrugPoint" /> | |||
| excretion = Majority are excreted within 24 hours. Main route through urine(conjugated metabolites); Small amounts through the faeces(unconjugated metabolites) | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 1977-10-2 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = AH01 | |||
| ATC_supplemental = | |||
| PubChem = 3964 | |||
| IUPHAR_ligand = 205 | |||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| DrugBank = DB00408 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3827 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = LER583670J | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02340 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 50841 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 831 | |||
<!--Chemical data--> | |||
| C=18 | H=18 | Cl=1 | N=3 | O=1 | |||
| molecular_weight = 327.808 g/mol | |||
| smiles = Clc2ccc1Oc4c(/N=C(\c1c2)N3CCN(C)CC3)cccc4 | |||
| InChI = 1/C18H18ClN3O/c1-21-8-10-22(11-9-21)18-14-12-13(19)6-7-16(14)23-17-5-3-2-4-15(17)20-18/h2-7,12H,8-11H2,1H3 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H18ClN3O/c1-21-8-10-22(11-9-21)18-14-12-13(19)6-7-16(14)23-17-5-3-2-4-15(17)20-18/h2-7,12H,8-11H2,1H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = XJGVXQDUIWGIRW-UHFFFAOYSA-N | |||
| melting_point = 109 | |||
| melting_high = 110 | |||
}} | |||
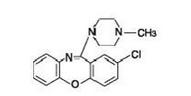
|structure=Loxapine, a dibenzoxazepine compound, represents a subclass of tricyclic antipsychotic agents, chemically distinct from the thioxanthenes, butyrophenones, and phenothiazines. Chemically, it is 2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4]oxazepine. It is present as the succinate salt. | |||
[[File:Loxapine.png|none|350px]] | |||
|PD=Pharmacologically, loxapine is an antipsychotic for which the exact mode of action has not been established. However, changes in the level of excitability of subcortical inhibitory areas have been observed in several animal species in association with such manifestations of tranquilization as calming effects and suppression of aggressive behavior. | |||
In normal human volunteers, signs of sedation were seen within 20 to 30 minutes after administration, were most pronounced within one and one-half to three hours, and lasted through 12 hours. Similar timing of primary pharmacologic effects was seen in animals. | |||
|PK=Absorption of loxapine following oral or parenteral administration is virtually complete. The drug is removed rapidly from the plasma and distributed in tissues. Animal studies suggest an initial preferential distribution in lungs, brain, spleen, heart, and kidney. Loxapine is metabolized extensively and is excreted mainly in the first 24 hours. Metabolites are excreted in the urine in the form of conjugates and in the feces unconjugated. | |||
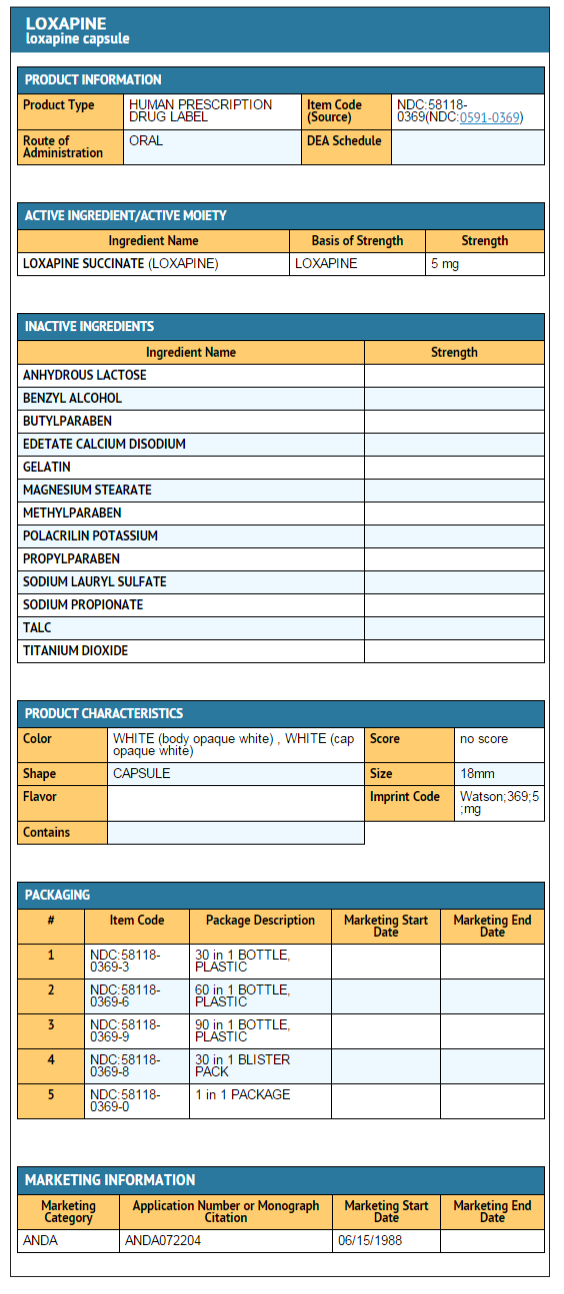
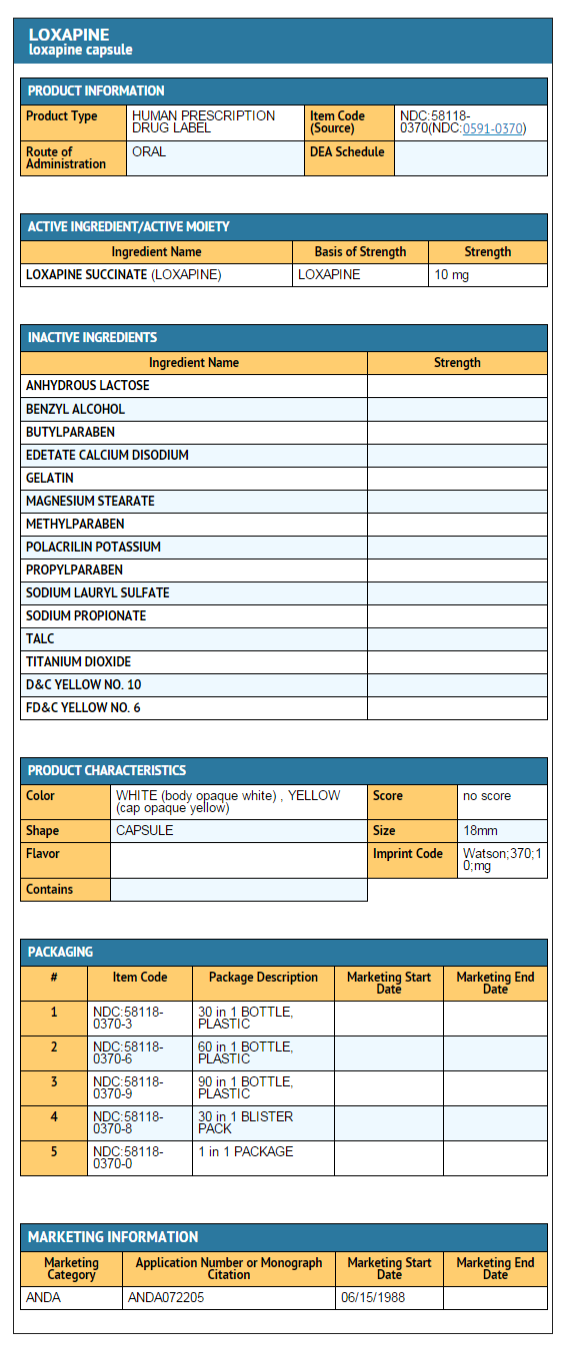
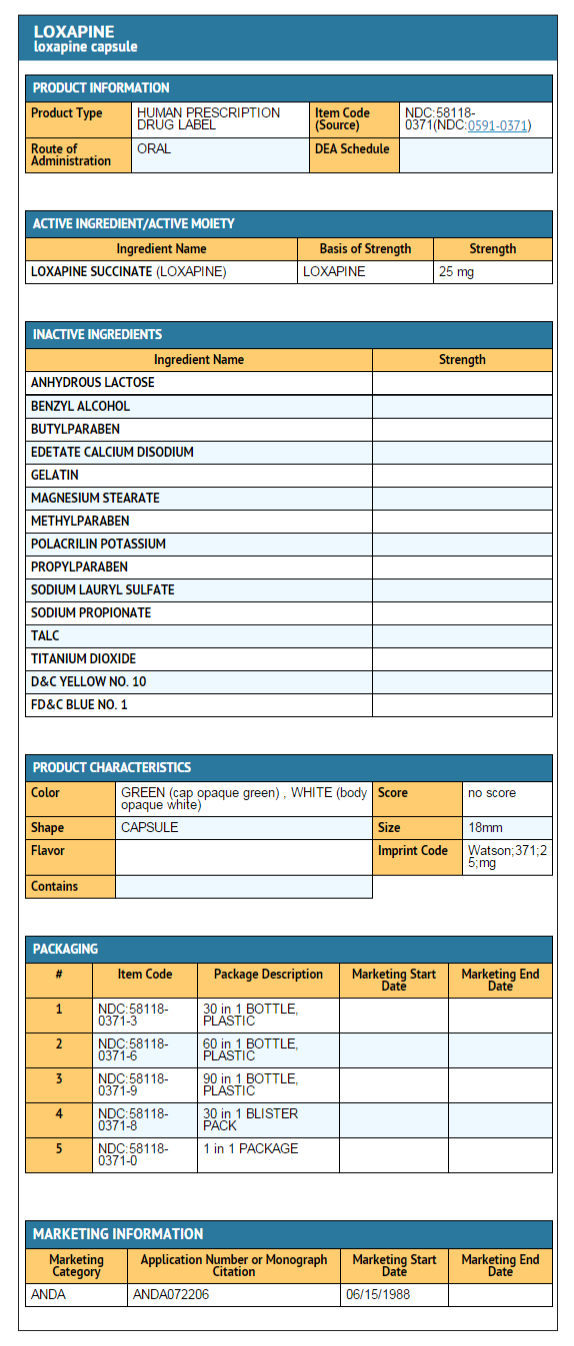
|howSupplied=Loxapine Capsules USP are available in the following strengths: | |||
*Loxapine Succinate USP 6.8 mg equivalent to 5 mg loxapine, black ink, hard shell, opaque, with a white body and cap, printed with Watson 369 on one half and 5 mg on the other, are supplied in bottles of 100. | |||
*Loxapine Succinate USP 13.6 mg equivalent to 10 mg loxapine, black ink, hard shell, opaque, with a white body and yellow cap, printed with Watson 370 on one half and 10 mg on the other, are supplied in bottles of 100. | |||
*Loxapine Succinate USP 34.0 mg equivalent to 25 mg loxapine, black ink, hard shell, opaque, with a white body and green cap, printed with Watson 371 on one half and 25 mg on the other, are supplied in bottles of 100. | |||
*Loxapine Succinate USP 68.1 mg equivalent to 50 mg loxapine, black ink, hard shell, opaque, with a white body and blue cap, printed with Watson 372 on one half and 50 mg on the other, are supplied in bottles of 100. | |||
|storage=*Store at 20°-25°C (68°-77°F). | |||
|packLabel=[[File:Loxapine FDA label.png|none|450px]] | |||
[[File:Loxapine FDA label 10mg.png|none|450px]] | |||
[[File:Loxapine FDA label 25mg.png|none|450px]] | |||
[[File:Loxapine FDA label 50mg.png|none|450px]] | |||
|fdaPatientInfo=Given the likelihood that some patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided. | |||
|alcohol=Alcohol-Loxapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=*Adasuve | |||
}} | |||
{{LabelImage | |||
|fileName=Loxapine package 1.png | |||
}} | |||
{{LabelImage | |||
|fileName=Loxapine package 2.png | |||
}} | |||
{{LabelImage | |||
|fileName=Loxapine package 3.png | |||
}} | |||
{{LabelImage | |||
|fileName=Loxapine package 4.png | |||
}} | |||
{{drugbox | {{drugbox | ||
| IUPAC_name = 2-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine | | IUPAC_name = 2-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine | ||
Revision as of 19:49, 26 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Loxapine is not approved for the treatment of patients with dementia-related psychosis
|
Overview
Loxapine (oral) is an antipsychotic that is FDA approved for the treatment of schizophrenia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Taste sense altered, sedation, pharyngitis..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Schizophrenia
- Initial dosage of 10 mg twice daily
- Severely disturbed patients initial dosage up to a total of 50 mg daily may be desirable.
- Dosage should then be increased fairly rapidly over the first seven to ten days until there is effective control of symptoms of schizophrenia. The usual therapeutic and maintenance range is 60 mg to 100 mg daily.
- Some patients respond to lower dosage and others require higher dosage.
- Daily dosage higher than 250 mg is not recommended.
- For maintenance therapy, dosage should be reduced to the lowest level compatible with symptom control; many patients have been maintained satisfactorily at dosages in the range of 20 to 60 mg daily.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loxapine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Loxapine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Loxapine (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loxapine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Loxapine in pediatric patients.
Contraindications
Loxapine is contraindicated in comatose or severe drug-induced depressed states (alcohol, barbiturates, narcotics, etc.).
Loxapine is contraindicated in individuals with known hypersensitivity to dibenzoxazepines.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Loxapine is not approved for the treatment of patients with dementia-related psychosis
|
Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Loxapine is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Tardive Dyskinesia Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome. (See ADVERSE REACTIONS and INFORMATION FOR PATIENTS sections).
Neuroleptic Malignant Syndrome (NMS) A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Loxapine, like other antipsychotics, may impair mental and/or physical abilities, especially during the first few days of therapy. Therefore, ambulatory patients should be warned about activities requiring alertness (e.g., operating vehicles or machinery) and about concomitant use of alcohol and other CNS depressants.
Loxapine has not been evaluated for the management of behavioral complications in patients with mental retardation, and therefore, it cannot be recommended.
Leukopenia, Neutropenia and Agranulocytosis In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a preexisting low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Loxapine Succinate Capsules USP at the first sign of a decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue Loxapine Succinate Capsules USP and have their WBC followed until recovery.
Adverse Reactions
Clinical Trials Experience
CNS Effects: Manifestations of adverse effects on the central nervous system, other than extrapyramidal effects, have been seen infrequently. Drowsiness, usually mild, may occur at the beginning of therapy or when dosage is increased. It usually subsides with continued loxapine therapy. The incidence of sedation has been less than that of certain aliphatic phenothiazines and slightly more than the piperazine phenothiazines. Dizziness, faintness, staggering gait, shuffling gait, muscle twitching, weakness, insomnia, agitation, tension, seizures, akinesia, slurred speech, numbness, and confusional states have been reported. Neuroleptic malignant syndrome (NMS) has been reported (see WARNINGS).
Extrapyramidal Symptoms - Neuromuscular (extrapyramidal) reactions during the administration of loxapine have been reported frequently, often during the first few days of treatment. In most patients, these reactions involved parkinsonian-like symptoms such as tremor, rigidity, excessive salivation, and masked facies. Akathisia (motor restlessness) also has been reported relatively frequently. These symptoms are usually not severe and can be controlled by reduction of loxapine dosage or by administration of antiparkinson drugs in usual dosage.
Dystonia - Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Persistent Tardive Dyskinesia - As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high-dose therapy, especially females. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movement of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities.
There is no known effective treatment for tardive dyskinesia; antiparkinson agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been suggested that fine vermicular movements of the tongue may be an early sign of the syndrome, and if the medication is stopped at that time the syndrome may not develop.
Cardiovascular Effects: Tachycardia, hypotension, hypertension, orthostatic hypotension, lightheadedness, and syncope have been reported.
A few cases of ECG changes similar to those seen with phenothiazines have been reported. It is not known whether these were related to loxapine administration.
Hematologic: Rarely, agranulocytosis, thrombocytopenia, leukopenia.
Skin: Dermatitis, edema (puffiness of face), pruritus, rash, alopecia, and seborrhea have been reported with loxapine.
Anticholinergic Effects: Dry mouth, nasal congestion, constipation, blurred vision, urinary retention, and paralytic ileus have occurred.
Gastrointestinal: Nausea and vomiting have been reported in some patients. Hepatocellular injury (i.e., SGOT/SGPT elevation) has been reported in association with loxapine administration and rarely, jaundice and/or hepatitis questionably related to loxapine treatment.
Other Adverse Reactions: Weight gain, weight loss, dyspnea, ptosis, hyperpyrexia, flushed facies, headache, paresthesia, and polydipsia have been reported in some patients. Rarely, galactorrhea, amenorrhea, gynecomastia, and menstrual irregularity of uncertain etiology have been reported.
Postmarketing Experience
There is limited information regarding Loxapine (oral) Postmarketing Experience in the drug label.
Drug Interactions
There have been rare reports of significant respiratory depression, stupor and/or hypotension with the concomitant use of loxapine and lorazepam.
The risk of using loxapine in combination with CNS-active drugs has not been systematically evaluated. Therefore, caution is advised if the concomitant administration of loxapine and CNS-active drugs is required.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Loxapine Succinate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Safe use of loxapine during pregnancy or lactation has not been established; therefore, its use in pregnancy, in nursing mothers, or in women of childbearing potential requires that the benefits of treatment be weighed against the possible risks to mother and child. No embryotoxicity or teratogenicity was observed in studies in rats, rabbits, or dogs although, with the exception of one rabbit study, the highest dosage was only two times the maximum recommended human dose and in some studies it was below this dose. Perinatal studies have shown renal papillary abnormalities in offspring of rats treated from mid-pregnancy with doses of 0.6 and 1.8 mg/kg, doses which approximate the usual human dose but which are considerably below the maximum recommended human dose.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Loxapine (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Loxapine (oral) during labor and delivery.
Nursing Mothers
The extent of the excretion of loxapine or its metabolites in human milk is not known. However, loxapine and its metabolites have been shown to be transported into the milk of lactating dogs. Loxapine administration to nursing women should be avoided if clinically possible.
Pediatric Use
Safety and effectiveness of loxapine in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Loxapine (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Loxapine (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Loxapine (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Loxapine (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Loxapine (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Loxapine (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Loxapine (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Loxapine (oral) Administration in the drug label.
Monitoring
There is limited information regarding Loxapine (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Loxapine (oral) and IV administrations.
Overdosage
Signs and symptoms of overdosage will depend on the amount ingested and individual patient tolerance. As would be expected from the pharmacologic actions of the drug, the clinical findings may range from mild depression of the CNS and cardiovascular systems to profound hypotension, respiratory depression, and unconsciousness. The possibility of occurrence of extrapyramidal symptoms and/or convulsive seizures should be kept in mind. Renal failure following loxapine overdosage has also been reported.
The treatment of overdosage is essentially symptomatic and supportive. Early gastric lavage and extended dialysis might be expected to be beneficial. Centrally-acting emetics may have little effect because of the antiemetic action of loxapine. In addition, emesis should be avoided because of the possibility of aspiration of vomitus. Avoid analeptics, such as pentylenetetrazol, which may cause convulsions. Severe hypotension might be expected to respond to the administration of norepinephrine or phenylephrine. EPINEPHRINE SHOULD NOT BE USED SINCE ITS USE IN A PATIENT WITH PARTIAL ADRENERGIC BLOCKADE MAY FURTHER LOWER THE BLOOD PRESSURE. Severe extrapyramidal reactions should be treated with anticholinergic antiparkinson agents or diphenhydramine hydrochloride, and anticonvulsant therapy should be initiated as indicated. Additional measures include oxygen and intravenous fluids.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Loxapac, Loxitane, Adasuve |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682311 |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Inhalation, oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 96.8%[1] |
| Metabolism | Liver, extensive; active metabolites include amoxapine and 8-hydroxyloxapine. Inhibits P-glycoprotein and is a substrate of CYP1A2, CYP3A4 and CYP2D6[1] |
| Elimination half-life | Oral, 4 hours; Inhalation, 7.61 hours [1] |
| Excretion | Majority are excreted within 24 hours. Main route through urine(conjugated metabolites); Small amounts through the faeces(unconjugated metabolites) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H18ClN3O |
| Molar mass | 327.808 g/mol |
| 3D model (JSmol) | |
| Melting point | 109 to 110 °C (Expression error: Unrecognized word "to". °F) |
| |
| |
| | |
Mechanism of Action
There is limited information regarding Loxapine (oral) Mechanism of Action in the drug label.
Structure
Loxapine, a dibenzoxazepine compound, represents a subclass of tricyclic antipsychotic agents, chemically distinct from the thioxanthenes, butyrophenones, and phenothiazines. Chemically, it is 2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4]oxazepine. It is present as the succinate salt.
Pharmacodynamics
Pharmacologically, loxapine is an antipsychotic for which the exact mode of action has not been established. However, changes in the level of excitability of subcortical inhibitory areas have been observed in several animal species in association with such manifestations of tranquilization as calming effects and suppression of aggressive behavior.
In normal human volunteers, signs of sedation were seen within 20 to 30 minutes after administration, were most pronounced within one and one-half to three hours, and lasted through 12 hours. Similar timing of primary pharmacologic effects was seen in animals.
Pharmacokinetics
Absorption of loxapine following oral or parenteral administration is virtually complete. The drug is removed rapidly from the plasma and distributed in tissues. Animal studies suggest an initial preferential distribution in lungs, brain, spleen, heart, and kidney. Loxapine is metabolized extensively and is excreted mainly in the first 24 hours. Metabolites are excreted in the urine in the form of conjugates and in the feces unconjugated.
Nonclinical Toxicology
There is limited information regarding Loxapine (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Loxapine (oral) Clinical Studies in the drug label.
How Supplied
Loxapine Capsules USP are available in the following strengths:
- Loxapine Succinate USP 6.8 mg equivalent to 5 mg loxapine, black ink, hard shell, opaque, with a white body and cap, printed with Watson 369 on one half and 5 mg on the other, are supplied in bottles of 100.
- Loxapine Succinate USP 13.6 mg equivalent to 10 mg loxapine, black ink, hard shell, opaque, with a white body and yellow cap, printed with Watson 370 on one half and 10 mg on the other, are supplied in bottles of 100.
- Loxapine Succinate USP 34.0 mg equivalent to 25 mg loxapine, black ink, hard shell, opaque, with a white body and green cap, printed with Watson 371 on one half and 25 mg on the other, are supplied in bottles of 100.
- Loxapine Succinate USP 68.1 mg equivalent to 50 mg loxapine, black ink, hard shell, opaque, with a white body and blue cap, printed with Watson 372 on one half and 50 mg on the other, are supplied in bottles of 100.
Storage
- Store at 20°-25°C (68°-77°F).
Images
Drug Images
{{#ask: Page Name::Loxapine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Loxapine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Given the likelihood that some patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
Precautions with Alcohol
Alcohol-Loxapine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Adasuve
Look-Alike Drug Names
There is limited information regarding Loxapine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Loxapine (oral) |Label Name=Loxapine package 1.png
}}
{{#subobject:
|Label Page=Loxapine (oral) |Label Name=Loxapine package 2.png
}}
{{#subobject:
|Label Page=Loxapine (oral) |Label Name=Loxapine package 3.png
}}
{{#subobject:
|Label Page=Loxapine (oral) |Label Name=Loxapine package 4.png
}}
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | Oral-4 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H18ClN3O |
| Molar mass | 327.808 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Loxapine (sold as Loxapac®, Loxitane®) is a typical antipsychotic medication, used primarily in the treatment of schizophrenia. It is a member of the dibenzoxazepine class and as a dibenzazepine derivative, it is structurally related to clozapine (which belongs to the chemically closely akin class of dibenzodiazepines). Several researchers have argued that Loxapine may behave as an atypical antipsychotic (PMID 10340686).
Loxapine may be metabolized by N-demethylation to amoxapine, a tetracyclic antidepressant (PMID 1860915).
Side effects
The most significant side-effects of loxapine are excessive salivation and indifference to surroundings. Loxapine, if administered to normal persons causes emotional quietening and insensitivity. In persons with psychosis, it may control aggressive behaviour and restlessness, and reduce the severity of hallucinations and delusions. Other Side effects include tardive dyskinesia, neuroleptic malignant syndrome, extrapyramidal side effects, tremor, gynecomastia and sedation.
Dosage
The typical starting dosage is 10mg twice daily; usual dose range 30-50mg twice daily; maximum recommended dosage is 250mg per day.
External links
- Product monograph from Medscape (free registration required).
- Pages with script errors
- Pages with non-numeric formatnum arguments
- Pages with reference errors
- Template:drugs.com link with non-standard subpage
- Articles with changed DrugBank identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Pages with broken file links
- Infobox drug articles without a structure image
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Typical antipsychotics
- Drugs