Hydroxyprogesterone caproate: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{AJ}} | |authorTag={{AJ}} | ||
|genericName=Hydroxyprogesterone | |genericName=Hydroxyprogesterone caproate | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass= [[progestin]] and [[endocrine|endocrine-metabolic]] agent | |drugClass=[[progestin]] and [[endocrine|endocrine-metabolic]] agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=indicated to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]]. | |indication=indicated to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]]. | ||
| Line 9: | Line 9: | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=Used to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]]. The effectiveness of Makena is based on improvement in the proportion of women who [[delivery|delivered]] <37 weeks of [[gestation]]. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in [[neonatal]] [[mortality]] and [[morbidity]]. | |fdaLIADAdult=* Used to reduce the [[risk]] of [[preterm birth]] in women with a singleton [[pregnancy]] who have a history of singleton [[preterm birth|spontaneous preterm birth]]. The effectiveness of Makena is based on improvement in the proportion of women who [[delivery|delivered]] <37 weeks of [[gestation]]. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in [[neonatal]] [[mortality]] and [[morbidity]]. | ||
* Limitation of use: While there are many risk factors for [[preterm birth]], [[safety]] and [[efficacy]] of Makena has been demonstrated only in women with a prior spontaneous singleton [[preterm birth]]. It is not intended for use in women with multiple [[gestations]] or other risk factors for [[preterm birth]]. | * Limitation of use: While there are many risk factors for [[preterm birth]], [[safety]] and [[efficacy]] of Makena has been demonstrated only in women with a prior spontaneous singleton [[preterm birth]]. It is not intended for use in women with multiple [[gestations]] or other risk factors for [[preterm birth]]. | ||
==== | ====Dosing Information==== | ||
Administer [[intramuscularly]] at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider | |||
Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]] | * Administer [[intramuscularly]] at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider | ||
* Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]] | |||
Continue administration once weekly until week 37 (through 36 weeks, 6 days) of [[gestation]] or [[delivery]], whichever occurs first. | Continue administration once weekly until week 37 (through 36 weeks, 6 days) of [[gestation]] or [[delivery]], whichever occurs first. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in adult patients. | |||
|offLabelAdultNoGuideSupport=[[Amenorrhea]] | =====Preparation and Administration===== | ||
[[Endometrial carcinoma]] | |||
[[Estrogen]] measurement, [[Endogenous]]; [[Diagnosis]] | * Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Makena is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy. | ||
|fdaLIADPed= | |||
* Instructions for administration: | |||
:* Clean the vial top with an alcohol swab before use. | |||
:* Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle. | |||
:* Change the needle to a 21 gauge 1 1/2 inch needle. | |||
:* After preparing the skin, inject in the upper outer quadrant of the gluteus maximus. The solution is viscous and oily. Slow injection (over one minute or longer) is recommended. | |||
:* Applying pressure to the injection site may minimize bruising and swelling. | |||
Discard any unused product 5 weeks after first use. | |||
|offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in adult patients. | |||
|offLabelAdultNoGuideSupport=* [[Amenorrhea]] | |||
* [[Endometrial carcinoma]] | |||
* [[Estrogen]] measurement, [[Endogenous]]; [[Diagnosis]] | |||
|fdaLIADPed=There is limited information regarding <i>FDA-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Hydroxyprogesterone caproate in pediatric patients. | ||
|contraindications=Current or history of [[thrombosis]] or [[thromboembolic disorders]] | |contraindications=* Do not use Makena in women with any of the following conditions: | ||
* Known or suspected [[breast cancer]], other [[breast cancer|hormone-sensitive cancer]], or history of these conditions | :* Current or history of [[thrombosis]] or [[thromboembolic disorders]] | ||
* Undiagnosed abnormal [[vaginal bleeding]] unrelated to [[pregnancy]] | :* Known or suspected [[breast cancer]], other [[breast cancer|hormone-sensitive cancer]], or history of these conditions | ||
* [[Cholestatic jaundice|Cholestatic jaundice of pregnancy]] | :* Undiagnosed abnormal [[vaginal bleeding]] unrelated to [[pregnancy]] | ||
* [[Liver Tumors|Liver tumors]], [[benign]] or [[malignant]], or [[liver disease|active liver disease]] | :* [[Cholestatic jaundice|Cholestatic jaundice of pregnancy]] | ||
* [[hypertension|Uncontrolled hypertension]] | :* [[Liver Tumors|Liver tumors]], [[benign]] or [[malignant]], or [[liver disease|active liver disease]] | ||
:* [[hypertension|Uncontrolled hypertension]] | |||
|warnings=====Thromboembolic Disorders==== | |warnings=====Thromboembolic Disorders==== | ||
Discontinue Makena if an [[arterial]] or [[deep vein thrombosis|deep venous thrombotic]] or [[thromboembolic event]] occurs. | * Discontinue Makena if an [[arterial]] or [[deep vein thrombosis|deep venous thrombotic]] or [[thromboembolic event]] occurs. | ||
====Allergic Reactions==== | ====Allergic Reactions==== | ||
[[Allergic reactions]], including [[urticaria]],[[ pruritus]] and [[angioedema]], have been reported with use of Makena or with other products containing castor oil. Consider discontinuing the drug if such reactions occur. | * [[Allergic reactions]], including [[urticaria]],[[ pruritus]] and [[angioedema]], have been reported with use of Makena or with other products containing castor oil. Consider discontinuing the drug if such reactions occur. | ||
====Decrease in Glucose Tolerance==== | ====Decrease in Glucose Tolerance==== | ||
A decrease in [[glucose tolerance]] has been observed in some patients on [[progestin]] treatment. The mechanism of this decrease is not known. Carefully monitor [[diabetic|prediabetic]] and [[diabetic]] women while they are receiving Makena. | * A decrease in [[glucose tolerance]] has been observed in some patients on [[progestin]] treatment. The mechanism of this decrease is not known. Carefully monitor [[diabetic|prediabetic]] and [[diabetic]] women while they are receiving Makena. | ||
====Fluid Retention==== | ====Fluid Retention==== | ||
Because progestational drugs may cause some degree of [[fluid retention]], carefully monitor women with conditions that might be influenced by this effect (e.g., [[preeclampsia]], [[epilepsy]], [[migraine]], [[asthma]], [[cardiac]] or [[renal dysfunction]]). | * Because progestational drugs may cause some degree of [[fluid retention]], carefully monitor women with conditions that might be influenced by this effect (e.g., [[preeclampsia]], [[epilepsy]], [[migraine]], [[asthma]], [[cardiac]] or [[renal dysfunction]]). | ||
====Depression==== | ====Depression==== | ||
Monitor women who have a history of clinical [[depression]] and discontinue Makena if clinical [[depression]] recurs. | * Monitor women who have a history of clinical [[depression]] and discontinue Makena if clinical [[depression]] recurs. | ||
====Jaundice==== | ====Jaundice==== | ||
Carefully monitor women who develop [[jaundice]] while receiving Makena and consider whether the benefit of use warrants continuation. | * Carefully monitor women who develop [[jaundice]] while receiving Makena and consider whether the benefit of use warrants continuation. | ||
====Hypertension==== | ====Hypertension==== | ||
Carefully monitor women who develop [[hypertension]] while receiving Makena and consider whether the benefit of use warrants continuation. | * Carefully monitor women who develop [[hypertension]] while receiving Makena and consider whether the benefit of use warrants continuation. | ||
|clinicalTrials=Because [[clinical trials]] are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because [[clinical trials]] are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
In a vehicle ([[placebo]])-controlled [[clinical trial]] of 463 [[pregnant]] women at risk for spontaneous [[preterm delivery]] based on [[obstetrical]] history, 310 received 250 mg of Makena and 153 received a vehicle formulation containing no drug by a weekly [[intramuscular injection]] beginning at 16 to 20 weeks of [[gestation]] and continuing until 37 weeks of [[gestation]] or [[delivery]], whichever occurred first. | In a vehicle ([[placebo]])-controlled [[clinical trial]] of 463 [[pregnant]] women at risk for spontaneous [[preterm delivery]] based on [[obstetrical]] history, 310 received 250 mg of Makena and 153 received a vehicle formulation containing no drug by a weekly [[intramuscular injection]] beginning at 16 to 20 weeks of [[gestation]] and continuing until 37 weeks of [[gestation]] or [[delivery]], whichever occurred first. | ||
| Line 74: | Line 87: | ||
[[Pulmonary embolus]] in one subject and [[Injection site reaction|injection site]] [[cellulitis]] in another subject were reported as serious adverse reactions in Makena-treated subjects. | [[Pulmonary embolus]] in one subject and [[Injection site reaction|injection site]] [[cellulitis]] in another subject were reported as serious adverse reactions in Makena-treated subjects. | ||
|postmarketing=The following adverse reactions have been identified during postapproval use of Makena. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=* The following adverse reactions have been identified during postapproval use of Makena. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
* Body as a whole: Local [[injection site reactions]] (including [[erythema]], [[urticaria]], [[rash]], [[irritation]], [[hypersensitivity]], warmth); [[fatigue]]; [[fever]]; [[hot flashes]]/[[flushes]] | * Body as a whole: | ||
* Digestive disorders: [[Vomiting]] | :* Local [[injection site reactions]] (including [[erythema]], [[urticaria]], [[rash]], [[irritation]], [[hypersensitivity]], warmth); [[fatigue]]; [[fever]]; [[hot flashes]]/[[flushes]] | ||
* Infections: [[Urinary tract infection]] | * Digestive disorders: | ||
* Nervous system disorders: [[Headache]], [[dizziness]] | :* [[Vomiting]] | ||
* Pregnancy, puerperium and perinatal conditions: [[Cervical incompetence]], [[premature rupture of membranes]] | * Infections: | ||
:* [[Urinary tract infection]] | |||
* Nervous system disorders: | |||
:* [[Headache]], [[dizziness]] | |||
* Pregnancy, puerperium and perinatal conditions: | |||
:* [[Cervical incompetence]], [[premature rupture of membranes]] | |||
* Reproductive system and breast disorders: [[Cervical dilation]], [[Cervical|shortened cervix]] | * Reproductive system and breast disorders: [[Cervical dilation]], [[Cervical|shortened cervix]] | ||
* Respiratory disorders: [[Dyspnea]], [[chest discomfort]] | * Respiratory disorders: | ||
* Skin: [[Rash]] | :* [[Dyspnea]], [[chest discomfort]] | ||
|drugInteractions=[[Cytochrome P450]] (CYP) enzymes: An in vitro inhibition study using human [[liver]] [[microsomes]] and CYP isoform-selective substrates indicated that hydroxyprogesterone caproate increased the metabolic rate of [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] by approximately 80%, 150%, and 80%, respectively. However, in another in vitro study using human [[hepatocytes]] under conditions where the prototypical inducers or inhibitors caused the anticipated increases or decreases in CYP enzyme activities, hydroxyprogesterone caproate did not induce or inhibit [[CYP1A2]], [[CYP2A6]], or [[CYP2B6]] activity. Overall, the findings indicate that hydroxyprogesterone caproate has minimal potential for [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] related drug-drug interactions at the clinically relevant concentrations. | * Skin: | ||
:* [[Rash]] | |||
|drugInteractions=* [[Cytochrome P450]] (CYP) enzymes: An in vitro inhibition study using human [[liver]] [[microsomes]] and CYP isoform-selective substrates indicated that hydroxyprogesterone caproate increased the metabolic rate of [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] by approximately 80%, 150%, and 80%, respectively. However, in another in vitro study using human [[hepatocytes]] under conditions where the prototypical inducers or inhibitors caused the anticipated increases or decreases in CYP enzyme activities, hydroxyprogesterone caproate did not induce or inhibit [[CYP1A2]], [[CYP2A6]], or [[CYP2B6]] activity. Overall, the findings indicate that hydroxyprogesterone caproate has minimal potential for [[CYP1A2]], [[CYP2A6]], and [[CYP2B6]] related drug-drug interactions at the clinically relevant concentrations. | |||
In vitro data indicated that therapeutic concentration of hydroxyprogesterone caproate is not likely to inhibit the activity of [[CYP2C8]], [[CYP2C9]], [[CYP2C19]], [[CYP2D6]], [[CYP2E1]], and [[CYP3A4]]. | * In vitro data indicated that therapeutic concentration of hydroxyprogesterone caproate is not likely to inhibit the activity of [[CYP2C8]], [[CYP2C9]], [[CYP2C19]], [[CYP2D6]], [[CYP2E1]], and [[CYP3A4]]. | ||
|FDAPregCat=B | |FDAPregCat=B | ||
|useInPregnancyFDA=There are no adequate and well-controlled studies of Makena use in women during the [[first trimester of pregnancy]]. Data from a vehicle ([[placebo]])-controlled [[clinical trial]] of 310 [[pregnant]] women who received Makena at weekly doses of 250 mg by [[intramuscular injection]] in their [[pregnancy|second and third trimesters]]1, as well as long-term (2-5 years) follow-up safety data on 194 of their infants 2, did not demonstrate any [[teratogenic]] risks to infants from in utero exposure to Makena. | |useInPregnancyFDA=* There are no adequate and well-controlled studies of Makena use in women during the [[first trimester of pregnancy]]. Data from a vehicle ([[placebo]])-controlled [[clinical trial]] of 310 [[pregnant]] women who received Makena at weekly doses of 250 mg by [[intramuscular injection]] in their [[pregnancy|second and third trimesters]]1, as well as long-term (2-5 years) follow-up safety data on 194 of their infants 2, did not demonstrate any [[teratogenic]] risks to infants from in utero exposure to Makena. | ||
Reproduction studies have been performed in mice and rats at doses up to 95 and 5, respectively, times the human dose and have revealed no evidence of impaired [[fertility]] or harm to the [[fetus]] due to Makena. | * Reproduction studies have been performed in mice and rats at doses up to 95 and 5, respectively, times the human dose and have revealed no evidence of impaired [[fertility]] or harm to the [[fetus]] due to Makena. | ||
Makena administration produced embryolethality in rhesus monkeys but not in cynomolgus monkeys exposed to 1 and 10 times the human dose equivalent every 7 days between days 20 and 146 of [[gestation]]. There were no [[teratogenic]] effects in either species. | * Makena administration produced embryolethality in rhesus monkeys but not in cynomolgus monkeys exposed to 1 and 10 times the human dose equivalent every 7 days between days 20 and 146 of [[gestation]]. There were no [[teratogenic]] effects in either species. | ||
|useInLaborDelivery=Makena is not intended for use to stop active [[preterm labor]]. The effect of Makena in active [[labor]] is unknown. | |useInLaborDelivery=* Makena is not intended for use to stop active [[preterm labor]]. The effect of Makena in active [[labor]] is unknown. | ||
|useInNursing=Discontinue Makena at 37 weeks of gestation or upon [[delivery]]. Detectable amounts of progestins have been identified in the milk of mothers receiving progestin treatment. Many studies have found no adverse effects of progestins on breastfeeding performance, or on the health, growth, or development of the [[infant]] | |useInNursing=* Discontinue Makena at 37 weeks of gestation or upon [[delivery]]. Detectable amounts of progestins have been identified in the milk of mothers receiving progestin treatment. Many studies have found no adverse effects of progestins on breastfeeding performance, or on the health, growth, or development of the [[infant]] | ||
|useInPed=Makena is not indicated for use in children. Safety and effectiveness in [[pediatric]] patients less than 16 years of age have not been established. A small number of women under age 18 years were studied; safety and efficacy are expected to be the same in women aged 16 years and above as for users 18 years and older. | |useInPed=* Makena is not indicated for use in children. Safety and effectiveness in [[pediatric]] patients less than 16 years of age have not been established. A small number of women under age 18 years were studied; safety and efficacy are expected to be the same in women aged 16 years and above as for users 18 years and older. | ||
|useInGeri=Makena is not intended for use in [[postmenopausal]] women. Safety and effectiveness in [[postmenopausal]] women have not been established. | |useInGeri=* Makena is not intended for use in [[postmenopausal]] women. Safety and effectiveness in [[postmenopausal]] women have not been established. | ||
|useInRenalImpair=No studies have been conducted to examine the [[pharmacokinetics]] of Makena in patients with [[renal impairment]]. | |useInRenalImpair=No studies have been conducted to examine the [[pharmacokinetics]] of Makena in patients with [[renal impairment]]. | ||
|useInHepaticImpair=No studies have been conducted to examine the pharmacokinetics of Makena in patients with hepatic impairment. Makena is extensively metabolized and [[hepatic impairment]] may reduce the elimination of Makena. | |useInHepaticImpair=* No studies have been conducted to examine the pharmacokinetics of Makena in patients with hepatic impairment. Makena is extensively metabolized and [[hepatic impairment]] may reduce the elimination of Makena. | ||
|administration=Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Makena is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy. | |administration=* Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Makena is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy. | ||
====Instructions for administration==== | ====Instructions for administration==== | ||
| Line 109: | Line 129: | ||
Applying pressure to the [[injection site]] may minimize [[bruising]] and [[swelling]]. | Applying pressure to the [[injection site]] may minimize [[bruising]] and [[swelling]]. | ||
Discard any unused product 5 weeks after first use. | Discard any unused product 5 weeks after first use. | ||
| | |mechAction=* Hydroxyprogesterone caproate is a synthetic [[progestin]]. The mechanism by which hydroxyprogesterone caproate reduces the risk recurrent preterm birth is not known. | ||
|structure=[[File:hydroxyprogesterone caproate structure.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|PD=No specific pharmacodynamic studies were conducted with Makena. | |PD=No specific pharmacodynamic studies were conducted with Makena. | ||
|PK=Absorption: | |PK======Absorption:===== | ||
* Peak serum levels of hydroxyprogesterone caproate appeared after 3-7 days in non-pregnant female subjects following a single [[intramuscular injection]] of 1000 mg hydroxyprogesterone caproate. Based on [[pharmacokinetic]] analysis of five non-pregnant female subjects who received a single [[intramuscular]] administration of 1000 mg hydroxyprogesterone caproate, the mean (±SD) Cmax is estimated to be 27.8 (±5.3) ng/mL, and the [[Tmax]] is estimated to be 4.6 (±1.7) days. The elimination half-life of hydroxyprogesterone caproate was 7.8 (±3.0) days. Once-weekly intramuscular administration of 1000 mg hydroxyprogesterone caproate to non-pregnant women resulted in trough concentration of 60.0 (±14) ng/mL after 13 weeks. The [[pharmacokinetics]] of the 250 mg dose of hydroxyprogesterone caproate has not been evaluated. | |||
=====Distribution:===== | |||
* Hydroxyprogesterone caproate binds extensively to [[plasma proteins]] including albumin and [[corticosteroid]] binding [[globulins]]. | |||
=====Metabolism:===== | |||
The | * In vitro studies have shown that hydroxyprogesterone caproate can be [[metabolized]] by human [[hepatocytes]], both by phase I and phase II reactions. Hydroxyprogesterone caproate undergoes extensive reduction, [[hydroxylation]] and [[conjugation]]. The conjugated [[metabolites]] include [[sulfated]], [[glucuronidated]] and [[acetylated]] products. In vitro data indicate that the [[metabolism]] of hydroxyprogesterone caproate is predominantly mediated by [[CYP3A4]] and [[CYP3A5]]. The in vitro data indicate that the caproate group is retained during [[metabolism]] of hydroxyprogesterone caproate. | ||
=====Excretion:===== | |||
* Both [[conjugated]] metabolites and free [[steroids]] are excreted in the [[urine]] and [[feces]], with the [[conjugated]] [[metabolites]] being prominent. Following intramuscular administration to pregnant women at 10-12 weeks gestation, approximately 50% of a dose was recovered in the [[feces]] and approximately 30% recovered in the [[urine]]. | |||
|clinicalStudies======Clinical Trial to Evaluate Reduction of Risk of Preterm Birth===== | |||
* In a multicenter, randomized, double-blind, vehicle (placebo)-controlled clinical trial, the safety and effectiveness of Makena for the reduction of the risk of spontaneous [[preterm birth]] was studied in women with a singleton [[pregnancy]] (age 16 to 43 years) who had a documented history of singleton [[preterm birth|spontaneous preterm birth]] (defined as [[delivery]] at less than 37 weeks of [[gestation]] following spontaneous [[preterm labor]] or [[premature rupture of membranes]]).1 At the time of randomization (between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]]), an [[ultrasound]] examination had confirmed [[gestational]] age and no known [[fetal]] [[anomaly]]. Women were excluded for prior [[progesterone]] treatment or [[heparin]] therapy during the current [[pregnancy]], a history of [[thromboembolic disease]], or maternal/obstetrical complications (such as current or planned [[cerclage]], hypertension requiring medication, or a [[seizure]] disorder). | |||
[[ | |||
* A total of 463 [[pregnant]] women were randomized to receive either Makena (N=310) or vehicle (N=153) at a dose of 250 mg administered weekly by [[intramuscular]] injection starting between 16 weeks, 0 days and 20 weeks, 6 days of [[gestation]], and continuing until 37 weeks of [[gestation]] or [[delivery]]. Demographics of the Makena-treated women were similar to those in the control group, and included: 59.0% Black, 25.5% Caucasian, 13.9% Hispanic and 0.6% Asian. The mean body mass index was 26.9 kg/m2. | |||
* The proportions of women in each treatment arm who delivered at <37 (the primary study endpoint), <35, and <32 weeks of [[gestation]] are displayed in Table 4. | |||
[[File:table4.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:* Four Makena-treated subjects were lost to follow-up. They were counted as deliveries at their [[gestational]] ages at time of last contact (184, 220, 343 and 364 weeks). | |||
:* Adjusted for interim analysis. | |||
Makena | * Compared to controls, treatment with Makena reduced the proportion of women who [[delivery|delivered]] [[preterm]] at <37 weeks. The proportions of women [[delivery|delivering]] at <35 and < 32 weeks also were lower among women treated with Makena. The upper bounds of the confidence intervals for the treatment difference at < 35 and <32 weeks were close to zero. Inclusion of zero in a confidence interval would indicate the treatment difference is not statistically significant. Compared to the other [[gestational]] ages evaluated, the number of [[preterm]] births at <32 weeks was limited. | ||
* After adjusting for time in the study, 7.5% of Makena-treated subjects delivered prior to 25 weeks compared to 4.7% of control subjects; see Figure 1. | |||
[[File:graphn.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* The rates of [[fetal]] and [[neonatal]] deaths in each treatment arm are displayed in Table 5. Due to the higher rate of [[miscarriages]] and [[stillbirths]] in the Makena arm, there was no overall survival difference demonstrated in this [[clinical trial]]. | |||
[[File:table5.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Makena | :* A Four of the 310 Makena-treated subjects were lost to follow-up and [[stillbirth]] or [[neonatal]] status could not be determined | ||
:* Percentages are based on the number of enrolled subjects and not adjusted for time on drug | |||
:* Percentage adjusted for the number of at risk subjects (n=209 for Makena, n=107 for control) enrolled at <20 weeks [[gestation]]. | |||
Makena | * A composite [[neonatal]] [[morbidity]]/[[mortality]] index evaluated adverse outcomes in livebirths. It was based on the number of [[neonates]] who died or experienced [[respiratory distress syndrome]], [[bronchopulmonary dysplasia]], grade 3 or 4 [[intraventricular hemorrhage]], proven [[sepsis]], or [[necrotizing enterocolitis]]. Although the proportion of neonates who experienced 1 or more events was numerically lower in the Makena arm (11.9% vs. 17.2%), the number of adverse outcomes was limited and the difference between arms was not statistically significant. | ||
=====Infant Follow-Up Safety Study===== | |||
* [[Infants]] born to women enrolled in this study, and who survived to be discharged from the nursery, were eligible for participation in a follow-up safety study. Of 348 eligible offspring, 79.9% enrolled: 194 children of Makena-treated women and 84 children of control subjects. The primary endpoint was the score on the Ages & Stages Questionnaire (ASQ), which evaluates communication, gross motor, fine motor, problem solving, and personal/social parameters. The proportion of children whose scores met the screening threshold for [[developmental]] delay in each developmental domain was similar for each treatment group. | |||
|howSupplied=Makena (NDC 64011-243-01) is supplied as 5 mL of a [[sterile]] solution in a multidose glass vial. | |||
Each 5 mL vial contains hydroxyprogesterone caproate USP, 250 mg/mL (25% w/v), in [[castor oil]] USP (28.6% v/v) and [[benzyl benzoate]] USP (46% v/v) with the preservative [[benzyl alcohol]] NF (2% v/v). | |||
Single unit carton: Contains one 5 mL multidose vial of Makena (250 mg/mL) containing 1250 mg of hydroxyprogesterone caproate. | |||
|storage=* Store at controlled room temperature [15°-30°C (59°-86°F)]. Use within 5 weeks after first use. | |||
(250 mg/mL) | |||
Store at controlled | |||
room temperature | |||
15°-30°C (59°-86°F) | |||
5 weeks after first use. | |||
* Caution: Protect vial from light. Store vial in its box. Store upright. | |||
|alcohol=Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:03, 31 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hydroxyprogesterone caproate is a progestin and endocrine-metabolic agent that is FDA approved for the treatment of indicated to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth.. Common adverse reactions include injection site reactions, pain, swelling, pruritus, nodule, urticaria, nausea, and diarrhea..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. The effectiveness of Makena is based on improvement in the proportion of women who delivered <37 weeks of gestation. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in neonatal mortality and morbidity.
- Limitation of use: While there are many risk factors for preterm birth, safety and efficacy of Makena has been demonstrated only in women with a prior spontaneous singleton preterm birth. It is not intended for use in women with multiple gestations or other risk factors for preterm birth.
Dosing Information
- Administer intramuscularly at a dose of 250 mg (1 mL) once weekly (every 7 days) by a healthcare provider
- Begin treatment between 16 weeks, 0 days and 20 weeks, 6 days of gestation
Continue administration once weekly until week 37 (through 36 weeks, 6 days) of gestation or delivery, whichever occurs first.
Preparation and Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Makena is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy.
- Instructions for administration:
- Clean the vial top with an alcohol swab before use.
- Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle.
- Change the needle to a 21 gauge 1 1/2 inch needle.
- After preparing the skin, inject in the upper outer quadrant of the gluteus maximus. The solution is viscous and oily. Slow injection (over one minute or longer) is recommended.
- Applying pressure to the injection site may minimize bruising and swelling.
Discard any unused product 5 weeks after first use.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyprogesterone caproate in adult patients.
Non–Guideline-Supported Use
- Amenorrhea
- Endometrial carcinoma
- Estrogen measurement, Endogenous; Diagnosis
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Label Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxyprogesterone caproate in pediatric patients.
Contraindications
- Do not use Makena in women with any of the following conditions:
- Current or history of thrombosis or thromboembolic disorders
- Known or suspected breast cancer, other hormone-sensitive cancer, or history of these conditions
- Undiagnosed abnormal vaginal bleeding unrelated to pregnancy
- Cholestatic jaundice of pregnancy
- Liver tumors, benign or malignant, or active liver disease
- Uncontrolled hypertension
Warnings
Thromboembolic Disorders
- Discontinue Makena if an arterial or deep venous thrombotic or thromboembolic event occurs.
Allergic Reactions
- Allergic reactions, including urticaria,pruritus and angioedema, have been reported with use of Makena or with other products containing castor oil. Consider discontinuing the drug if such reactions occur.
Decrease in Glucose Tolerance
- A decrease in glucose tolerance has been observed in some patients on progestin treatment. The mechanism of this decrease is not known. Carefully monitor prediabetic and diabetic women while they are receiving Makena.
Fluid Retention
- Because progestational drugs may cause some degree of fluid retention, carefully monitor women with conditions that might be influenced by this effect (e.g., preeclampsia, epilepsy, migraine, asthma, cardiac or renal dysfunction).
Depression
- Monitor women who have a history of clinical depression and discontinue Makena if clinical depression recurs.
Jaundice
- Carefully monitor women who develop jaundice while receiving Makena and consider whether the benefit of use warrants continuation.
Hypertension
- Carefully monitor women who develop hypertension while receiving Makena and consider whether the benefit of use warrants continuation.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a vehicle (placebo)-controlled clinical trial of 463 pregnant women at risk for spontaneous preterm delivery based on obstetrical history, 310 received 250 mg of Makena and 153 received a vehicle formulation containing no drug by a weekly intramuscular injection beginning at 16 to 20 weeks of gestation and continuing until 37 weeks of gestation or delivery, whichever occurred first.
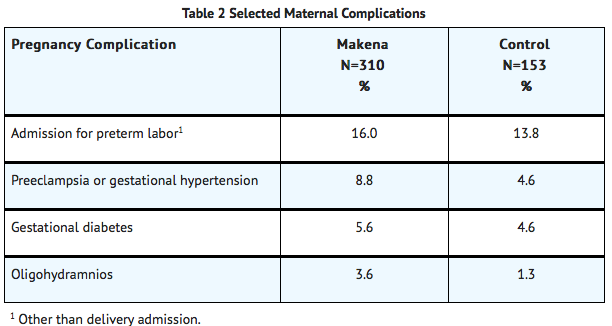
Certain pregnancy-related fetal and maternal complications or events were numerically increased in the Makena-treated subjects as compared to control subjects, including miscarriage and stillbirth, admission for preterm labor, preeclampsia or gestational hypertension, gestational diabetes, and oligohydramnios.
1 N = Total number of subjects enrolled prior to 20 weeks 0 days 2 N = Total number of subjects at risk ≥ 20 weeks
Common Adverse Reactions
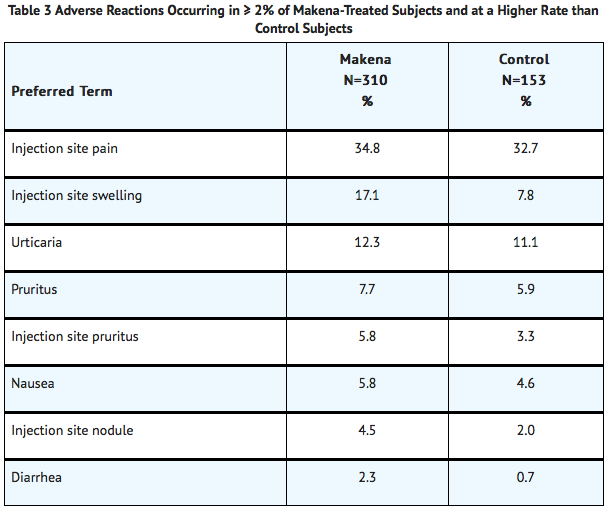
The most common adverse reaction was injection site pain, which was reported after at least one injection by 34.8% of the Makena group and 32.7% of the control group. Table 3 lists adverse reactions that occurred in ≥2% of subjects and at a higher rate in the Makena group than in the control group.
In the clinical trial, 2.2% of subjects receiving Makena were reported as discontinuing therapy due to adverse reactions compared to 2.6% of control subjects. The most common adverse reactions that led to discontinuation in both groups were urticaria and injection site pain/swelling (1% each).
Pulmonary embolus in one subject and injection site cellulitis in another subject were reported as serious adverse reactions in Makena-treated subjects.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Makena. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Body as a whole:
- Local injection site reactions (including erythema, urticaria, rash, irritation, hypersensitivity, warmth); fatigue; fever; hot flashes/flushes
- Digestive disorders:
- Infections:
- Nervous system disorders:
- Pregnancy, puerperium and perinatal conditions:
- Reproductive system and breast disorders: Cervical dilation, shortened cervix
- Respiratory disorders:
- Skin:
Drug Interactions
- Cytochrome P450 (CYP) enzymes: An in vitro inhibition study using human liver microsomes and CYP isoform-selective substrates indicated that hydroxyprogesterone caproate increased the metabolic rate of CYP1A2, CYP2A6, and CYP2B6 by approximately 80%, 150%, and 80%, respectively. However, in another in vitro study using human hepatocytes under conditions where the prototypical inducers or inhibitors caused the anticipated increases or decreases in CYP enzyme activities, hydroxyprogesterone caproate did not induce or inhibit CYP1A2, CYP2A6, or CYP2B6 activity. Overall, the findings indicate that hydroxyprogesterone caproate has minimal potential for CYP1A2, CYP2A6, and CYP2B6 related drug-drug interactions at the clinically relevant concentrations.
- In vitro data indicated that therapeutic concentration of hydroxyprogesterone caproate is not likely to inhibit the activity of CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Makena use in women during the first trimester of pregnancy. Data from a vehicle (placebo)-controlled clinical trial of 310 pregnant women who received Makena at weekly doses of 250 mg by intramuscular injection in their second and third trimesters1, as well as long-term (2-5 years) follow-up safety data on 194 of their infants 2, did not demonstrate any teratogenic risks to infants from in utero exposure to Makena.
- Reproduction studies have been performed in mice and rats at doses up to 95 and 5, respectively, times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Makena.
- Makena administration produced embryolethality in rhesus monkeys but not in cynomolgus monkeys exposed to 1 and 10 times the human dose equivalent every 7 days between days 20 and 146 of gestation. There were no teratogenic effects in either species.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hydroxyprogesterone caproate in women who are pregnant.
Labor and Delivery
- Makena is not intended for use to stop active preterm labor. The effect of Makena in active labor is unknown.
Nursing Mothers
- Discontinue Makena at 37 weeks of gestation or upon delivery. Detectable amounts of progestins have been identified in the milk of mothers receiving progestin treatment. Many studies have found no adverse effects of progestins on breastfeeding performance, or on the health, growth, or development of the infant
Pediatric Use
- Makena is not indicated for use in children. Safety and effectiveness in pediatric patients less than 16 years of age have not been established. A small number of women under age 18 years were studied; safety and efficacy are expected to be the same in women aged 16 years and above as for users 18 years and older.
Geriatic Use
- Makena is not intended for use in postmenopausal women. Safety and effectiveness in postmenopausal women have not been established.
Gender
There is no FDA guidance on the use of Hydroxyprogesterone caproate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hydroxyprogesterone caproate with respect to specific racial populations.
Renal Impairment
No studies have been conducted to examine the pharmacokinetics of Makena in patients with renal impairment.
Hepatic Impairment
- No studies have been conducted to examine the pharmacokinetics of Makena in patients with hepatic impairment. Makena is extensively metabolized and hepatic impairment may reduce the elimination of Makena.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hydroxyprogesterone caproate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hydroxyprogesterone caproate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Makena is a clear, yellow solution. Do not use if solid particles appear or if the solution is cloudy.
Instructions for administration
Clean the vial top with an alcohol swab before use. Draw up 1 mL of drug into a 3 mL syringe with an 18 gauge needle. Change the needle to a 21 gauge 1 1/2 inch needle. After preparing the skin, inject in the upper outer quadrant of the gluteus maximus. The solution is viscous and oily. Slow injection (over one minute or longer) is recommended. Applying pressure to the injection site may minimize bruising and swelling. Discard any unused product 5 weeks after first use.
Monitoring
There is limited information regarding Hydroxyprogesterone caproate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hydroxyprogesterone caproate and IV administrations.
Overdosage
There is limited information regarding Hydroxyprogesterone caproate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Hydroxyprogesterone caproate Pharmacology in the drug label.
Mechanism of Action
- Hydroxyprogesterone caproate is a synthetic progestin. The mechanism by which hydroxyprogesterone caproate reduces the risk recurrent preterm birth is not known.
Structure
Pharmacodynamics
No specific pharmacodynamic studies were conducted with Makena.
Pharmacokinetics
Absorption:
- Peak serum levels of hydroxyprogesterone caproate appeared after 3-7 days in non-pregnant female subjects following a single intramuscular injection of 1000 mg hydroxyprogesterone caproate. Based on pharmacokinetic analysis of five non-pregnant female subjects who received a single intramuscular administration of 1000 mg hydroxyprogesterone caproate, the mean (±SD) Cmax is estimated to be 27.8 (±5.3) ng/mL, and the Tmax is estimated to be 4.6 (±1.7) days. The elimination half-life of hydroxyprogesterone caproate was 7.8 (±3.0) days. Once-weekly intramuscular administration of 1000 mg hydroxyprogesterone caproate to non-pregnant women resulted in trough concentration of 60.0 (±14) ng/mL after 13 weeks. The pharmacokinetics of the 250 mg dose of hydroxyprogesterone caproate has not been evaluated.
Distribution:
- Hydroxyprogesterone caproate binds extensively to plasma proteins including albumin and corticosteroid binding globulins.
Metabolism:
- In vitro studies have shown that hydroxyprogesterone caproate can be metabolized by human hepatocytes, both by phase I and phase II reactions. Hydroxyprogesterone caproate undergoes extensive reduction, hydroxylation and conjugation. The conjugated metabolites include sulfated, glucuronidated and acetylated products. In vitro data indicate that the metabolism of hydroxyprogesterone caproate is predominantly mediated by CYP3A4 and CYP3A5. The in vitro data indicate that the caproate group is retained during metabolism of hydroxyprogesterone caproate.
Excretion:
- Both conjugated metabolites and free steroids are excreted in the urine and feces, with the conjugated metabolites being prominent. Following intramuscular administration to pregnant women at 10-12 weeks gestation, approximately 50% of a dose was recovered in the feces and approximately 30% recovered in the urine.
Nonclinical Toxicology
There is limited information regarding Hydroxyprogesterone caproate Nonclinical Toxicology in the drug label.
Clinical Studies
Clinical Trial to Evaluate Reduction of Risk of Preterm Birth
- In a multicenter, randomized, double-blind, vehicle (placebo)-controlled clinical trial, the safety and effectiveness of Makena for the reduction of the risk of spontaneous preterm birth was studied in women with a singleton pregnancy (age 16 to 43 years) who had a documented history of singleton spontaneous preterm birth (defined as delivery at less than 37 weeks of gestation following spontaneous preterm labor or premature rupture of membranes).1 At the time of randomization (between 16 weeks, 0 days and 20 weeks, 6 days of gestation), an ultrasound examination had confirmed gestational age and no known fetal anomaly. Women were excluded for prior progesterone treatment or heparin therapy during the current pregnancy, a history of thromboembolic disease, or maternal/obstetrical complications (such as current or planned cerclage, hypertension requiring medication, or a seizure disorder).
- A total of 463 pregnant women were randomized to receive either Makena (N=310) or vehicle (N=153) at a dose of 250 mg administered weekly by intramuscular injection starting between 16 weeks, 0 days and 20 weeks, 6 days of gestation, and continuing until 37 weeks of gestation or delivery. Demographics of the Makena-treated women were similar to those in the control group, and included: 59.0% Black, 25.5% Caucasian, 13.9% Hispanic and 0.6% Asian. The mean body mass index was 26.9 kg/m2.
- The proportions of women in each treatment arm who delivered at <37 (the primary study endpoint), <35, and <32 weeks of gestation are displayed in Table 4.
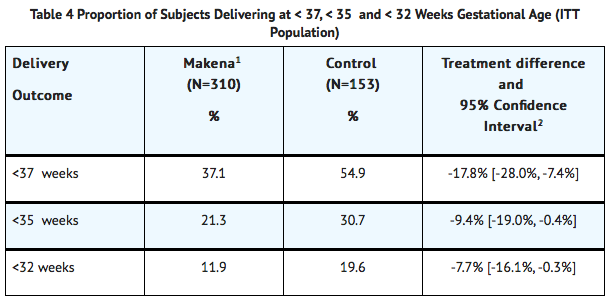
- Four Makena-treated subjects were lost to follow-up. They were counted as deliveries at their gestational ages at time of last contact (184, 220, 343 and 364 weeks).
- Adjusted for interim analysis.
- Compared to controls, treatment with Makena reduced the proportion of women who delivered preterm at <37 weeks. The proportions of women delivering at <35 and < 32 weeks also were lower among women treated with Makena. The upper bounds of the confidence intervals for the treatment difference at < 35 and <32 weeks were close to zero. Inclusion of zero in a confidence interval would indicate the treatment difference is not statistically significant. Compared to the other gestational ages evaluated, the number of preterm births at <32 weeks was limited.
- After adjusting for time in the study, 7.5% of Makena-treated subjects delivered prior to 25 weeks compared to 4.7% of control subjects; see Figure 1.
- The rates of fetal and neonatal deaths in each treatment arm are displayed in Table 5. Due to the higher rate of miscarriages and stillbirths in the Makena arm, there was no overall survival difference demonstrated in this clinical trial.
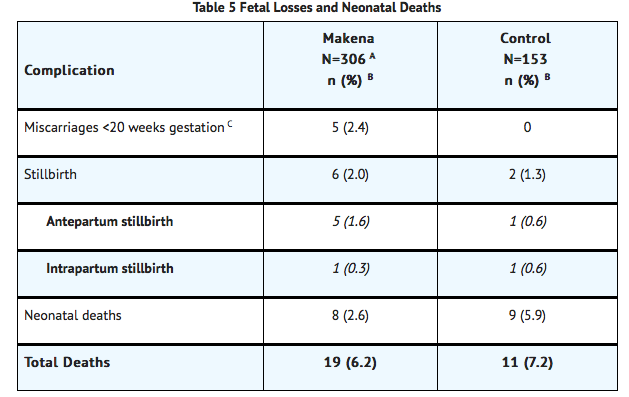
- A Four of the 310 Makena-treated subjects were lost to follow-up and stillbirth or neonatal status could not be determined
- Percentages are based on the number of enrolled subjects and not adjusted for time on drug
- Percentage adjusted for the number of at risk subjects (n=209 for Makena, n=107 for control) enrolled at <20 weeks gestation.
- A composite neonatal morbidity/mortality index evaluated adverse outcomes in livebirths. It was based on the number of neonates who died or experienced respiratory distress syndrome, bronchopulmonary dysplasia, grade 3 or 4 intraventricular hemorrhage, proven sepsis, or necrotizing enterocolitis. Although the proportion of neonates who experienced 1 or more events was numerically lower in the Makena arm (11.9% vs. 17.2%), the number of adverse outcomes was limited and the difference between arms was not statistically significant.
Infant Follow-Up Safety Study
- Infants born to women enrolled in this study, and who survived to be discharged from the nursery, were eligible for participation in a follow-up safety study. Of 348 eligible offspring, 79.9% enrolled: 194 children of Makena-treated women and 84 children of control subjects. The primary endpoint was the score on the Ages & Stages Questionnaire (ASQ), which evaluates communication, gross motor, fine motor, problem solving, and personal/social parameters. The proportion of children whose scores met the screening threshold for developmental delay in each developmental domain was similar for each treatment group.
How Supplied
Makena (NDC 64011-243-01) is supplied as 5 mL of a sterile solution in a multidose glass vial.
Each 5 mL vial contains hydroxyprogesterone caproate USP, 250 mg/mL (25% w/v), in castor oil USP (28.6% v/v) and benzyl benzoate USP (46% v/v) with the preservative benzyl alcohol NF (2% v/v).
Single unit carton: Contains one 5 mL multidose vial of Makena (250 mg/mL) containing 1250 mg of hydroxyprogesterone caproate.
Storage
- Store at controlled room temperature [15°-30°C (59°-86°F)]. Use within 5 weeks after first use.
- Caution: Protect vial from light. Store vial in its box. Store upright.
Images
Drug Images
{{#ask: Page Name::Hydroxyprogesterone caproate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Hydroxyprogesterone caproate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Hydroxyprogesterone caproate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Hydroxyprogesterone caproate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Hydroxyprogesterone caproate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Hydroxyprogesterone caproate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.