Growth hormone deficiency pathophysiology: Difference between revisions
| Line 4: | Line 4: | ||
==Overview == | ==Overview == | ||
The [[Somatotrophs|somatotroph cells]] of the [[Anterior pituitary gland|anterior pituitary organ]] | The [[Somatotrophs|somatotroph cells]] of the [[Anterior pituitary gland|anterior pituitary organ]] synthesize Growth hormone (GH). The most widely studied impact of growth hormone is increasing [[weight]]. GH causes [[epiphyseal plate]] broadening and [[ligament]] development. GH deficiency brings about changes in the [[physiology]] of various frameworks of the body, showing as modified [[lipid digestion]], expanded subcutaneous instinctive fat and diminished bulk. The hereditary premise of inborn growth hormone deficiency relies upon numerous qualities, for instance, POU1F1 quality transformations are the most widely recognized hereditary reason for the joined pituitary hormone lack. Quality erasures, frameshift transformations, and jabber changes of GH1 quality have been portrayed as reasons for familial GHD. | ||
==Pathophysiology== | ==Pathophysiology== | ||
Revision as of 12:16, 25 October 2017
|
Growth hormone deficiency Microchapters |
|
Differentiating Growth hormone deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Growth hormone deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Growth hormone deficiency pathophysiology |
|
Risk calculators and risk factors for Growth hormone deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohammed Abdelwahed M.D[2]
Overview
The somatotroph cells of the anterior pituitary organ synthesize Growth hormone (GH). The most widely studied impact of growth hormone is increasing weight. GH causes epiphyseal plate broadening and ligament development. GH deficiency brings about changes in the physiology of various frameworks of the body, showing as modified lipid digestion, expanded subcutaneous instinctive fat and diminished bulk. The hereditary premise of inborn growth hormone deficiency relies upon numerous qualities, for instance, POU1F1 quality transformations are the most widely recognized hereditary reason for the joined pituitary hormone lack. Quality erasures, frameshift transformations, and jabber changes of GH1 quality have been portrayed as reasons for familial GHD.
Pathophysiology
- The somatotroph cells of the anterior pituitary gland produce growth hormone.[1]
- They are regulated by two hypothalamic hormones; GH-releasing hormone (GHRH) stimulates and somatostatin inhibits them.
- GH effect is increasing body mass:
- GH increases total body protein content and is associated with an increase in amino acid incorporation into cartilage and bone.[2]
- GH stimulates lipolysis decreasing total body fat content.
- GH also increases bone mass by stimulating skeletal insulin-like growth factor-I and causing hypertrophy of osteoblasts, bone remodeling, and mineralization. GH decreases expression of adipocyte maturation regulators (C/EBPα, PPARγ) and prominent genes related to lipid synthesis such as FAS and FABP. GH treatment increased the mRNA expression of adiponectin and UCP1 in mature adipocytes.[3]
- GH causes epiphyseal plate widening and cartilage growth.
- GH deficiency results in alterations in the physiology of different systems of the body, manifesting as altered lipid metabolism, increased subcutaneous visceral fat, decreased muscle mass, decreased bone density, low exercise performance, and reduced quality of life.
Regulation of growth hormone secretion
- The secretion of growth hormone is controlled by a complex regulatory system. Primarily, it is controlled by two hormones; GH-releasing hormone and somatostatin.
- The adenylate cyclase-cyclic AMP-protein kinase A plays a major role in the control of GH secretion by GH-releasing hormone.
- GH gene expression is also of importance in determining the GH responses.
- GH secretion is pulsatile; between pulses, serum GH concentration may be undetectable. It is thought that the pulses of GH release are mediated by the reduction in inhibition by somatostatin with an increase of GHRH. [11,21].
- The glucocorticoids play a principal role in the functional maturation of GH cells in the fetal pituitary glands, inducing GH and GHRH-receptor gene expression, and establish the GH secretory system.
- During puberty, there is a temporary increase in GH secretion with a subsequent return to the normal values in early adulthood.(2)

Molecular effects of growth hormone on cells
- Growth hormone acts by binding to the receptor homodimer in the liver.
- The receptor consists of an extracellular ligand-binding domain and a cytoplasmic signaling part.
- Its action is to stimulate hepatic synthesis and secretion of insulin-like growth factor-1 (IGF-1).
- IGF-1 is a protein responsible for most of the growth-promoting activities of GH.
- IGF-1 directly inhibits GH secretion and GH receptor function by a negative feedback effect.
- GH stimulates cell proliferation in osteoblasts. Human trabecular osteoblasts produce mainly IGF-II, IGFBP-3 and fewer quantities of IGF-I in culture.
- IGFs and their binding proteins may exert important regulatory effects on the GH effect on osteoblasts.
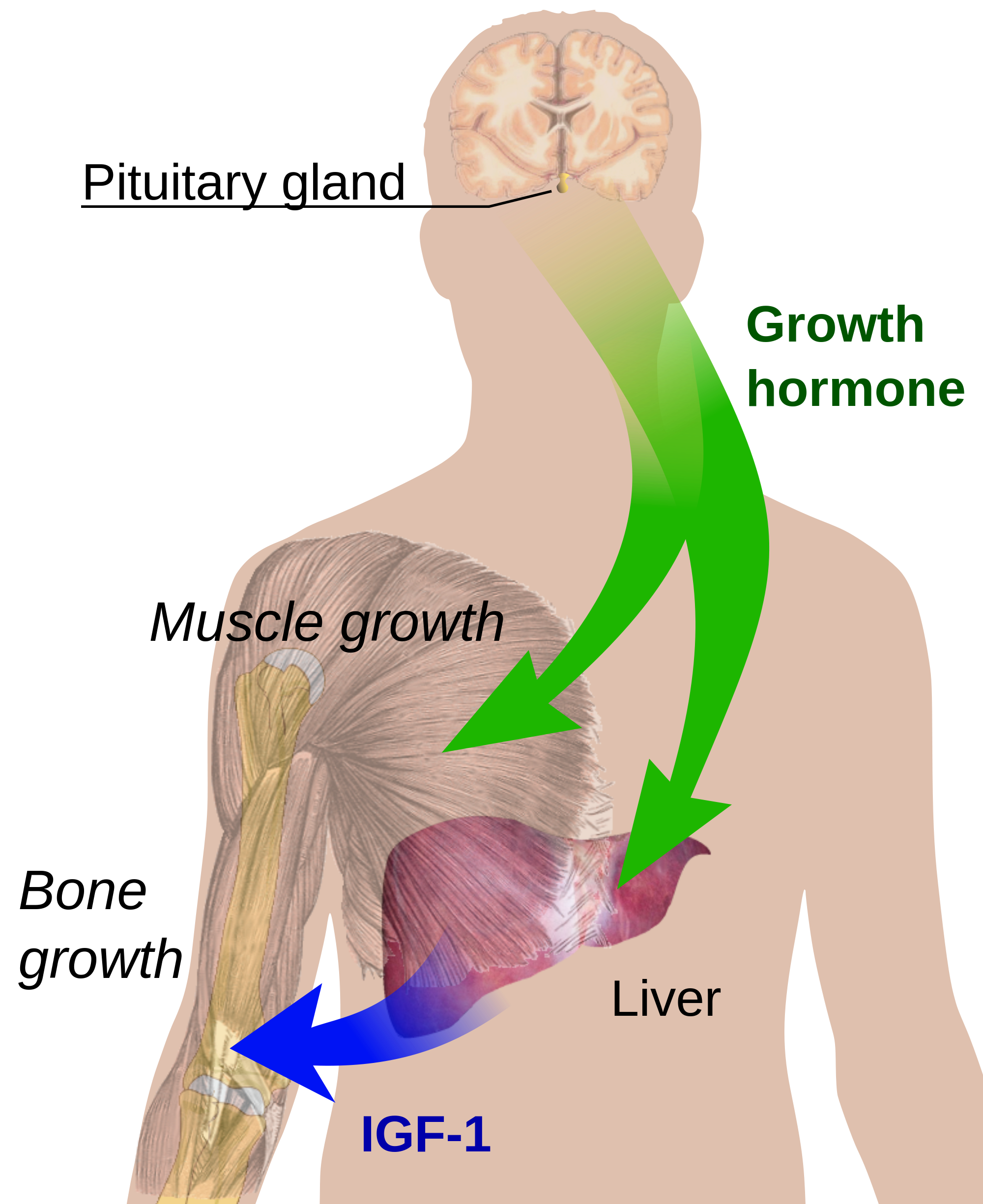
- A single GH molecule binds with two GH receptor molecules, followed by activation of JAK2 tyrosine kinase by phosphorylation.
- Phosphorylation of JAK2 leads to phosphorylation of intracellular proteins called STAT proteins, MAP kinase activation, and induction of gene expression.
- These STAT proteins are phosphorylated by JAK2 and directly translocated to the cell nucleus, where they play the major control of GH-specific gene effects by binding to nuclear DNA.
- STAT5 plays an important role in the regulation of expression of some genes in the liver cells.
- A defect in GH-mediated JAK-STAT signal transduction could be a cause of the GH resistance.

Genetic basis of growth hormone deficiency
POU1F1 gene mutations
- It is the most common known genetic cause of the combined pituitary hormone deficiency.[4]
- It is responsible for pituitary-specific transcription of genes for GH, prolactin, thyrotropin, and the growth hormone-releasing hormone (GHRH) receptor.[5]
- PROP1 mutations result in failure to activate POU1F1/Pit1 gene expression and probably cause pituitary hypoplasia.[6]
GH1 gene mutations
- GH1 gene encoding GH is located on chromosome 17.
- Gene deletions, frameshift mutations, and nonsense mutations of GH1 have been described as causes of familial GHD.
Syndrome of bioinactive GH
- Bioinactive GH has the main symptoms and signs of isolated GHD with normal basal GH levels and low insulin-like growth factor I concentrations.[7]
GH receptor signal transduction
- It is essential for normal signaling of the GH receptor. Mutations in the gene encoding signal transducer decrease the response of receptors to GH.[8]
IGF-I gene mutations
- Mutations in the gene encoding IGF-I cause a unique syndrome of GHD.[9]
- Patients with IGF-I gene mutations have prenatal growth failure, microcephaly, significant neurocognitive deficits, and sensorineural hearing loss.
Defective stabilization of circulating IGF-I
- Acid-labile subunit is important for the stabilization of the IGF-I.
- Mutations in the gene coding for it causes less stable and subsequently less effect.[10]
IGF-I receptor mutations
- Mutations in the gene encoding the receptor for the IGF-I result in partial loss of function of the IGF-I receptor.[11]
References
- ↑ Cuttler L (1996). "The regulation of growth hormone secretion". Endocrinol Metab Clin North Am. 25 (3): 541–71. PMID 8879986.
- ↑ MURPHY WR, DAUGHADAY WH, HARTNETT C (1956). "The effect of hypophysectomy and growth hormone on the incorporation of labeled sulfate into tibial epiphyseal and nasal cartilage of the rat". J Lab Clin Med. 47 (5): 715–22. PMID 13319878.
- ↑ Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M; et al. (2005). "Endocrine control of body composition in infancy, childhood, and puberty". Endocr Rev. 26 (1): 114–46. doi:10.1210/er.2003-0038. PMID 15689575.
- ↑ Ziemnicka K, Budny B, Drobnik K, Baszko-Błaszyk D, Stajgis M, Katulska K; et al. (2016). "Two coexisting heterozygous frameshift mutations in PROP1 are responsible for a different phenotype of combined pituitary hormone deficiency". J Appl Genet. 57 (3): 373–81. doi:10.1007/s13353-015-0328-z. PMC 4963446. PMID 26608600.
- ↑ Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG (1990). "Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1". Nature. 347 (6293): 528–33. doi:10.1038/347528a0. PMID 1977085.
- ↑ Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O'Connell SM; et al. (1998). "Mutations in PROP1 cause familial combined pituitary hormone deficiency". Nat Genet. 18 (2): 147–9. doi:10.1038/ng0298-147. PMID 9462743.
- ↑ Besson A, Salemi S, Deladoëy J, Vuissoz JM, Eblé A, Bidlingmaier M; et al. (2005). "Short stature caused by a biologically inactive mutant growth hormone (GH-C53S)". J Clin Endocrinol Metab. 90 (5): 2493–9. doi:10.1210/jc.2004-1838. PMID 15713716.
- ↑ Hwa V, Camacho-Hübner C, Little BM, David A, Metherell LA, El-Khatib N; et al. (2007). "Growth hormone insensitivity and severe short stature in siblings: a novel mutation at the exon 13-intron 13 junction of the STAT5b gene". Horm Res. 68 (5): 218–24. doi:10.1159/000101334. PMID 17389811.
- ↑ Batey L, Moon JE, Yu Y, Wu B, Hirschhorn JN, Shen Y; et al. (2014). "A novel deletion of IGF1 in a patient with idiopathic short stature provides insight Into IGF1 haploinsufficiency". J Clin Endocrinol Metab. 99 (1): E153–9. doi:10.1210/jc.2013-3106. PMC 3879666. PMID 24243634.
- ↑ Domené HM, Hwa V, Argente J, Wit JM, Wit JM, Camacho-Hübner C; et al. (2009). "Human acid-labile subunit deficiency: clinical, endocrine and metabolic consequences". Horm Res. 72 (3): 129–41. doi:10.1159/000232486. PMID 19729943.
- ↑ Kawashima Y, Higaki K, Fukushima T, Hakuno F, Nagaishi J, Hanaki K; et al. (2012). "Novel missense mutation in the IGF-I receptor L2 domain results in intrauterine and postnatal growth retardation". Clin Endocrinol (Oxf). 77 (2): 246–54. doi:10.1111/j.1365-2265.2012.04357.x. PMID 22309212.