Goodpasture syndrome pathophysiology: Difference between revisions
No edit summary |
|||
| Line 12: | Line 12: | ||
==Overview== | ==Overview== | ||
The [[pathogenesis]] of Goodpasture syndrome includes the presence of [[Autoantibody|autoantibodies]] directed against the [[glomerular]] and/or [[alveolar]] [[Basement membrane|basement membrane.]] As with many [[autoimmune]] conditions, the precise cause of Goodpasture’s Syndrome is not yet known. It is believed to be a [[type II hypersensitivity]] reaction to Goodpasture’s [[antigens]] on the [[cells]] of the [[glomeruli]] of the [[kidneys]] and the [[Pulmonary alveolus|pulmonary alveoli]]. The [[basement membrane]] (including a triple chain [[type IV collagen]]) lining the [[alveoli]] and [[glomeruli]] are particularly damaged in patients suffering from Goodpasture syndrome. The [[immune system]] misreads the as foreign, which leads to attack and destruction, as it would happen in case of an invading pathogen. The target [[antigen]] that has the strongest pathogenic effect on [[Goodpasture disease|anti-GBM disease]] is the non-collagenous 1 domain of alpha-3 [[type IV collagen]]. There is strong correlation of [[Anti-glomerular basement membrane antibody|anti-glomerular basement membrane]] disease with allele HLA DRB1-1501. This [[allele]] is associated in causing [[renal injury]]. On gross pathology, Goodpasture syndrome with lung involvement may present with diffuse [[pulmonary hemorrhage]]. On microscopic [[histopathological]] analysis, early focal proliferative changes that display [[necrosis]] and crescent formation with an inflamed [[interstitial]] are seen. Under direct immunofluorescence, linear [[immunoglobulin G]] deposits are found encompassing the [[glomerular basement membrane]] and at times the distal [[tubular]] portion of the [[Basement membrane|basement membrane.]] | |||
==Pathogenesis== | ==Pathogenesis== | ||
Revision as of 16:12, 3 May 2018
| Title |
| https://https://www.youtube.com/watch?v=vAX2UMA8Oek%7C350}} |
|
Goodpasture syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Goodpasture syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Goodpasture syndrome pathophysiology |
|
Risk calculators and risk factors for Goodpasture syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Ali Poyan Mehr, M.D. [2];Associate Editor(s)-in-Chief: Krzysztof Wierzbicki M.D. [3] Akshun Kalia M.B.B.S.[4]
Overview
The pathogenesis of Goodpasture syndrome includes the presence of autoantibodies directed against the glomerular and/or alveolar basement membrane. As with many autoimmune conditions, the precise cause of Goodpasture’s Syndrome is not yet known. It is believed to be a type II hypersensitivity reaction to Goodpasture’s antigens on the cells of the glomeruli of the kidneys and the pulmonary alveoli. The basement membrane (including a triple chain type IV collagen) lining the alveoli and glomeruli are particularly damaged in patients suffering from Goodpasture syndrome. The immune system misreads the as foreign, which leads to attack and destruction, as it would happen in case of an invading pathogen. The target antigen that has the strongest pathogenic effect on anti-GBM disease is the non-collagenous 1 domain of alpha-3 type IV collagen. There is strong correlation of anti-glomerular basement membrane disease with allele HLA DRB1-1501. This allele is associated in causing renal injury. On gross pathology, Goodpasture syndrome with lung involvement may present with diffuse pulmonary hemorrhage. On microscopic histopathological analysis, early focal proliferative changes that display necrosis and crescent formation with an inflamed interstitial are seen. Under direct immunofluorescence, linear immunoglobulin G deposits are found encompassing the glomerular basement membrane and at times the distal tubular portion of the basement membrane.
Pathogenesis
Goodpasture syndrome is an autoimmune condition resulting from antibodies against the glomerular and alveolar basement membrane. It is thought Goodpasture syndrome is the result of type II hypersensitivity reaction which leads to generation of antibodies which bind antigenic proteins of glomerular and alveolar basement membrane. The antigen-antibody complex leads to activation of complement system and tissue destruction.[1][2]
Anatomy
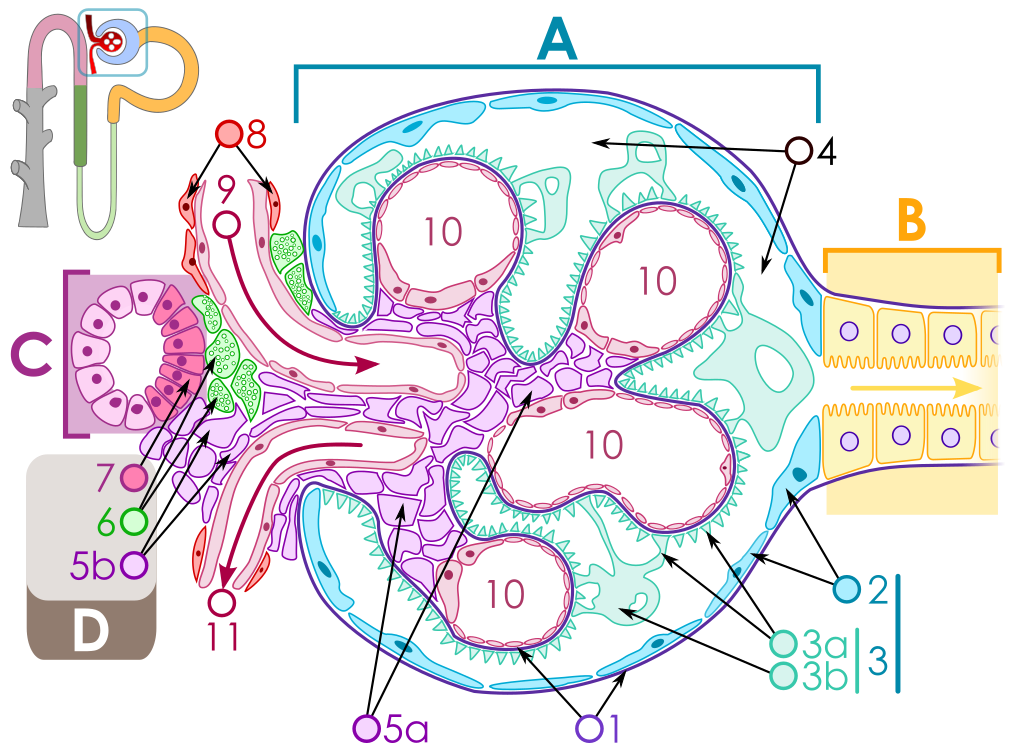
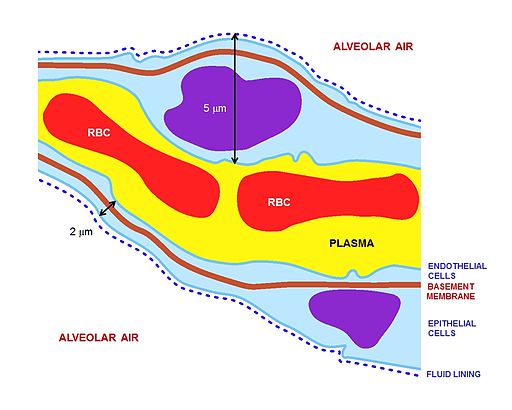
The key for the renal corpuscle figure is: A – Renal corpuscle, B – Proximal tubule, C – Distal convoluted tubule, D – Juxtaglomerular apparatus, 1. Basement membrane (Basal lamina), 2. Bowman's capsule – parietal layer, 3. Bowman's capsule – visceral layer, 3a. Pedicels (Foot processes from podocytes), 3b. Podocyte, 4. Bowman's space (urinary space), 5a. Mesangium – Intraglomerular cell, 5b. Mesangium – Extraglomerular cell, 6. Granular cells (Juxtaglomerular cells), 7. Macula densa, 8. Myocytes (smooth muscle), 9. Afferent arteriole, 10. Glomerulus Capillaries, 11. Efferent arteriole.
Pathophysiology
- The basement membrane (in general) is primarily made up of type IV collagen.
- The type IV collagen is made of three alpha subunits of collagen, which form a triple helix.
- The alpha subunits of type IV collagen can be of six different types and named as alpha1 to alpha6.
- Each alpha subunit is further bound to three components namely:
- The amino terminus of alpha chain is bound by a short 7S domain.
- The carboxyl terminus is bound by a triple helix of alpha chains.
- Lastly the alpha chain has a non-collagenous component.
- The autoantibodies in Goodpasture syndrome is directed against the non-collagenous components of the alpha3 chain of type IV collagen.
- The target antigen that has the strongest pathogenic effect on anti-GBM disease is the non-collagenous 1 domain of alpha-3 type IV collagen.[1]
- There are two dominant epitopes such as epitope A (17-31) and epitope B(127-141) and are seen in the alpha-3 non-collagenous 1 domain of type IV collagen. The antibodies do not normally bind unless a change has occurred in the non-cross linked hexamers or trimers.
- In Goodpasture syndrome, these epitopes undergo a transitional change in the non-cross linked hexamers or trimers. This allows antibodies to be bound to these epitopes.[3]
- The pathogenic role of epitopes such as epitope A, has been shown to affect the function of the renals, especially of the alpha-3 type IV collagen of non-collagenous 1 domain.
- Epitope B however is not able to induce Goodpasture syndrome by itself.
- In addition, antibodies may be directed against other components of alpha3 chain.
- Even though the basement membrane of various tissues are similar in structure, the antibodies are reactive only against glomerular and alveolar membranes.
- This can be attributed to the fact that glomerular and alveolar basement membrane have greater expression and availability of epitopes for antibody binding. Moreover, the structure of glomerular and alveolar membrane is such that it promotes easy access to antibodies in these places.
- Under pathological conditions seen with any type of lung injury, vascular permeability of the respiratory membrane is increased which further promotes antibody accumulation in the alveolar basement membrane.
- The cause of renal injury is due to anti-glomerular basement membrane antibodies ability to bind and activate complement system and proteases, resulting in an interruption between the filtration barrier and the Bowman’s capsule.
- The interruption of the Bowman's capsule and filtration barrier, induces crescent shaped antigen-antibody complex formation in the renal corpuscles, invoking proteinuria and activating T cells (CD4+ and CD8+), macrophages, and neutrophils.[4][5]
- Conditions such as oxidation, nitrosylation, glycation, increased body temperature, or proteolytic cleavage can further lead to increased antigen-antibody cross reaction and early symptom onset.[6]
- It has been shown that proteolytic cleavage of disulfide bonds in non-cross linked hexamers has shown greater affinity for Goodpasture syndrome antibodies to bind.
- Environmental factors also contribute to the disease such as exposures to hydrocarbons, exposure to formaldehyde or smoking tobacco.
- Smoking tobacco for instance causes inhibition of sulfilimine bond substances. Inhibition of the sulfilimine bond substances cause non-cross linked hexamers to form.[7]
Genes
Genes involved in the pathogenesis of Goodpasture syndrome include certain alleles of human leukocyte antigen (HLA).[2] [8]
- HLA-B27 has been found to be more frequently associated with severe nephritic form of Goodpasture syndrome.
- Other alleles associated with Goodpasture syndrome include increased frequency of HLA-DR15 and DRB1*03, DRB1*04 and a decreased frequency of DRB1*01 and DRB1*07.
- Goodpasture disease is also strongly associated with the DRB1*1501 and to DRB1*1502 allele.
- The allele DRB1*1501 is primarily associated with causing renal injury and estimated to be present in 80% of patients with Goodpasture syndrome,
- Recent studies have shown that DRB1*1501 allele is found in approximately one third caucasian patients with Goodpasture syndrome.
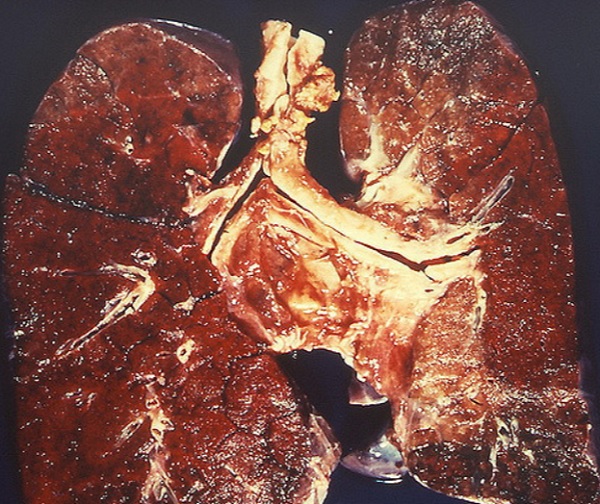
Gross Pathology
On gross pathology, Goddpasture syndrome with lung involvement may present with diffuse pulmonary hemorrhage.
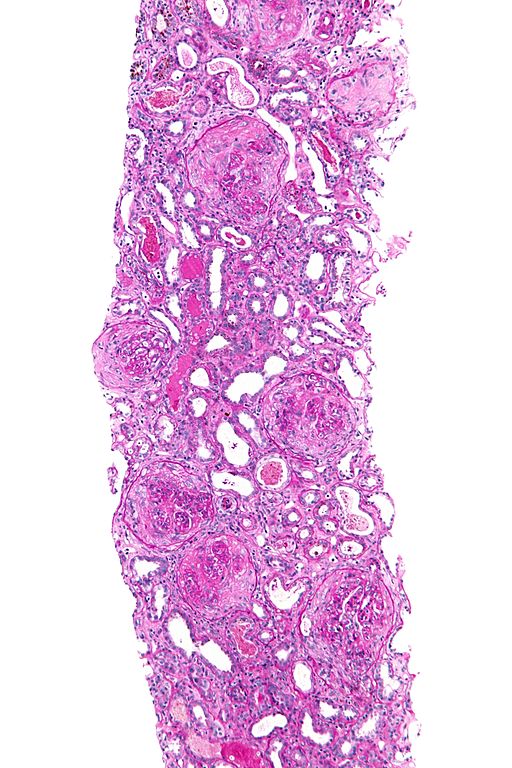
Microscopic Pathology
On microscopic histopathological analysis, findings include:[9][10][11][12]
- In kidneys, early focal proliferative changes that display necrosis and crescent formation (in 75% of glomeruli) with an inflamed interstitial are seen.
- Linear staining of IgG & C3 along the GBM (characteristic) with renal tubular necrosis and loss (tubular portion of the basement membrane).
- Other features of proliferative or necrotizing glomerulonephritis.
- In lungs, findings include neutrophilic capillaritis, hyaline membrane (in alveoli) and diffuse alveolar hemorrhage.
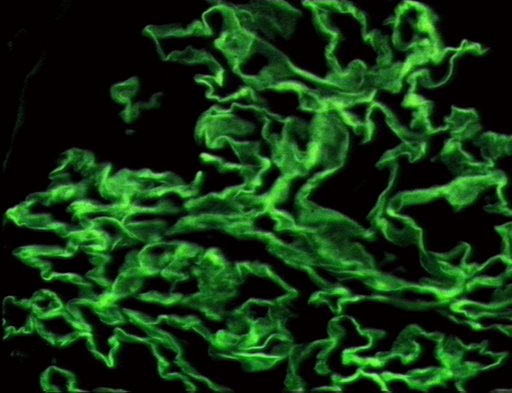
- The following are images of the microscopic pathology of crescent glomerulonephritis and the immunofluorescence of linear IgG and C3 deposition.
References
- ↑ 1.0 1.1 Zhao J, Cui Z, Yang R, Jia XY, Zhang Y, Zhao MH (November 2009). "Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity". Kidney Int. 76 (10): 1108–15. doi:10.1038/ki.2009.348. PMID 19741587.
- ↑ 2.0 2.1 Xie LJ, Cui Z, Jia XY, Chen Z, Liu XR, Zhao MH (July 2015). "Coexistence of Anti-Glomerular Basement Membrane Antibodies and Anti-Neutrophil Cytoplasmic Antibodies in a Child With Human Leukocyte Antigen Susceptibility and Detailed Antibody Description: A Case Report". Medicine (Baltimore). 94 (29): e1179. doi:10.1097/MD.0000000000001179. PMID 26200622.
- ↑ Chen JL, Hu SY, Jia XY, Zhao J, Yang R, Cui Z, Zhao MH (January 2013). "Association of epitope spreading of antiglomerular basement membrane antibodies and kidney injury". Clin J Am Soc Nephrol. 8 (1): 51–8. doi:10.2215/CJN.05140512. PMC 3531658. PMID 23085731.
- ↑ Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (June 2003). "Alport's syndrome, Goodpasture's syndrome, and type IV collagen". N. Engl. J. Med. 348 (25): 2543–56. doi:10.1056/NEJMra022296. PMID 12815141.
- ↑ Cui Z, Zhao J, Jia XY, Zhu SN, Zhao MH (April 2011). "Clinical features and outcomes of anti-glomerular basement membrane disease in older patients". Am. J. Kidney Dis. 57 (4): 575–82. doi:10.1053/j.ajkd.2010.09.022. PMID 21168945.
- ↑ Peto P, Salama AD (January 2011). "Update on antiglomerular basement membrane disease". Curr Opin Rheumatol. 23 (1): 32–7. doi:10.1097/BOR.0b013e328341009f. PMID 21124085.
- ↑ Pedchenko V, Bondar O, Fogo AB, Vanacore R, Voziyan P, Kitching AR, Wieslander J, Kashtan C, Borza DB, Neilson EG, Wilson CB, Hudson BG (July 2010). "Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis". N. Engl. J. Med. 363 (4): 343–54. doi:10.1056/NEJMoa0910500. PMC 4144421. PMID 20660402.
- ↑ Couser WG (2016). "Pathogenesis and treatment of glomerulonephritis-an update". J Bras Nefrol. 38 (1): 107–22. doi:10.5935/0101-2800.20160016. PMID 27049372.
- ↑ Frankel SK, Cosgrove GP, Fischer A, Meehan RT, Brown KK (February 2006). "Update in the diagnosis and management of pulmonary vasculitis". Chest. 129 (2): 452–65. doi:10.1378/chest.129.2.452. PMID 16478866.
- ↑ Zhao J, Yan Y, Cui Z, Yang R, Zhao MH (June 2009). "The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity". Hum. Immunol. 70 (6): 425–9. doi:10.1016/j.humimm.2009.04.004. PMID 19364515.
- ↑ University of Pittsburgh Medical Center Pathology. www.path.upmc.edu/cases/case541.html Accessed on Novermber 2nd 2016
- ↑ Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Pagliuca G; et al. (2015). "Goodpasture's syndrome: a clinical update". Autoimmun Rev. 14 (3): 246–53. doi:10.1016/j.autrev.2014.11.006. PMID 25462583.
- ↑ http://picasaweb.google.com/mcmumbi/USMLEIIImages