Gastrointestinal varices pathophysiology: Difference between revisions
| (8 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Gastrointestinal varices}} | {{Gastrointestinal varices}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} {{HK}} | ||
==Overview== | ==Overview== | ||
Varices arise from [[Hemodynamics|hemodynamic]] disturbance between the systemic and [[portal venous system]]. The majority of [[venous]] drainage of the [[gastrointestinal system]] occurs via the [[portal venous system]]. Whenever there is an interruption of drainage through the [[Portal venous system|portal system]] (for example due to [[cirrhosis]]), the [[Blood vessel|vessels]] contributing to the [[Portocaval anastomoses|porto-caval shunts]] become more prominent due to increased pressure gradient. The interruption in [[blood flow]] leads to the creation collateral [[Blood vessel|vessels]] that involve [[veins]] of the [[esophagus]], [[stomach]], [[pelvis]] ([[hemorrhoids]]), [[retroperitoneum]], [[liver]], [[abdominal wall]], and other areas. | |||
==Pathophysiology== | ==Pathophysiology== | ||
Varices arise from hemodynamic disturbance between the systemic and portal venous system. The majority of venous drainage of the gastrointestinal system occurs via the portal venous system. Whenever there is an interruption of drainage through the portal system (for example due to cirrhosis), the vessels contributing to the porto-caval shunts become more prominent due to increased pressure gradient. The interruption in blood flow leads to the creation collateral vessels that involve veins of the esophagus, stomach, pelvis (hemorrhoids), retroperitoneum, liver, abdominal wall, and other areas.<ref name="urlAnatomy - The Gastrointestinal Circulation - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK53099/ |title=Anatomy - The Gastrointestinal Circulation - NCBI Bookshelf |format= |work= |accessdate=}}</ref><ref name="pmid2181704">{{cite journal |vauthors=Mahl TC, Groszmann RJ |title=Pathophysiology of portal hypertension and variceal bleeding |journal=Surg. Clin. North Am. |volume=70 |issue=2 |pages=251–66 |year=1990 |pmid=2181704 |doi= |url=}}</ref> | Varices arise from [[Hemodynamics|hemodynamic]] disturbance between the systemic and [[portal venous system]]. The majority of [[venous]] drainage of the [[gastrointestinal system]] occurs via the [[portal venous system]]. Whenever there is an interruption of drainage through the [[Portal venous system|portal system]] (for example due to [[cirrhosis]]), the [[vessels]] contributing to the [[Portocaval anastomoses|porto-caval shunts]] become more prominent due to increased pressure gradient. The interruption in [[blood flow]] leads to the creation collateral [[Blood vessel|vessels]] that involve veins of the [[esophagus]], [[stomach]], [[pelvis]] ([[hemorrhoids]]), [[retroperitoneum]], [[liver]], [[abdominal wall]], and other areas.<ref name="urlAnatomy - The Gastrointestinal Circulation - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK53099/ |title=Anatomy - The Gastrointestinal Circulation - NCBI Bookshelf |format= |work= |accessdate=}}</ref><ref name="pmid2181704">{{cite journal |vauthors=Mahl TC, Groszmann RJ |title=Pathophysiology of portal hypertension and variceal bleeding |journal=Surg. Clin. North Am. |volume=70 |issue=2 |pages=251–66 |year=1990 |pmid=2181704 |doi= |url=}}</ref> | ||
=== Esophageal varices === | === Esophageal varices === | ||
Esophageal varices are a major complication of portal hypertension (increased blood pressure in the portal venous system). In order to understand the mechanism leading to the development of esophageal varices, it is important to understand the normal vascular architecture and venous drainage of the esophagus.<ref name="pmid22666604">{{cite journal |vauthors=Maruyama H, Yokosuka O |title=Pathophysiology of portal hypertension and esophageal varices |journal=Int J Hepatol |volume=2012 |issue= |pages=895787 |year=2012 |pmid=22666604 |pmc=3362051 |doi=10.1155/2012/895787 |url=}}</ref> | Esophageal varices are a major complication of [[portal hypertension]] (increased [[blood pressure]] in the [[portal venous system]]). In order to understand the mechanism leading to the development of esophageal varices, it is important to understand the normal [[vascular]] architecture and [[venous]] drainage of the [[esophagus]].<ref name="pmid22666604">{{cite journal |vauthors=Maruyama H, Yokosuka O |title=Pathophysiology of portal hypertension and esophageal varices |journal=Int J Hepatol |volume=2012 |issue= |pages=895787 |year=2012 |pmid=22666604 |pmc=3362051 |doi=10.1155/2012/895787 |url=}}</ref> | ||
'''Vascular architecture and venous drainage of esophagus''' | '''Vascular architecture and venous drainage of esophagus''' | ||
* Vascular resistance increases against portal blood flow in cirrhosis, | * [[Vascular resistance]] increases against [[portal]] [[blood flow]] in [[cirrhosis]], non-[[Cirrhosis|cirrhotic]] [[portal]] [[fibrosis]], idiopathic [[portal hypertension]], extra-[[hepatic]] [[portal vein]] obstruction, [[Budd-Chiari syndrome]], and other [[Portal hypertension|portal hypertensive]] disorders, inducing congestion of [[blood]] in the [[Splenic vein|splenic]] and mesenteric veins that lie upstream of the portal trunk<ref name="pmid6023778">{{cite journal |vauthors=Moreno AH, Burchell AR, Rousselot LM, Panke WF, Slafsky F, Burke JH |title=Portal blood flow in cirrhosis of the liver |journal=J. Clin. Invest. |volume=46 |issue=3 |pages=436–45 |year=1967 |pmid=6023778 |pmc=297064 |doi=10.1172/JCI105545 |url=}}</ref><ref name="pmid20066733">{{cite journal |vauthors=Ponziani FR, Zocco MA, Campanale C, Rinninella E, Tortora A, Di Maurizio L, Bombardieri G, De Cristofaro R, De Gaetano AM, Landolfi R, Gasbarrini A |title=Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment |journal=World J. Gastroenterol. |volume=16 |issue=2 |pages=143–55 |year=2010 |pmid=20066733 |pmc=2806552 |doi= |url=}}</ref><ref name="pmid9462648">{{cite journal |vauthors=Tanaka M, Wanless IR |title=Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules |journal=Hepatology |volume=27 |issue=2 |pages=488–96 |year=1998 |pmid=9462648 |doi=10.1002/hep.510270224 |url=}}</ref> | ||
* The major vessels draining blood from the esophagus include, the left gastric | * The major vessels draining blood from the [[esophagus]] include, the [[Left gastric vein|left gastric]] and less frequently [[short gastric veins]]<ref name="pmid21423905">{{cite journal |vauthors=Adithan S, Venkatesan B, Sundarajan E, Kate V, Kalayarasan R |title=Color Doppler evaluation of left gastric vein hemodynamics in cirrhosis with portal hypertension and its correlation with esophageal varices and variceal bleed |journal=Indian J Radiol Imaging |volume=20 |issue=4 |pages=289–93 |year=2010 |pmid=21423905 |pmc=3056627 |doi=10.4103/0971-3026.73541 |url=}}</ref><ref name="pmid22568419">{{cite journal |vauthors=Rebibo L, Chivot C, Fuks D, Sabbagh C, Yzet T, Regimbeau JM |title=Three-dimensional computed tomography analysis of the left gastric vein in a pancreatectomy |journal=HPB (Oxford) |volume=14 |issue=6 |pages=414–21 |year=2012 |pmid=22568419 |pmc=3384867 |doi=10.1111/j.1477-2574.2012.00468.x |url=}}</ref> | ||
'''Porto-caval collaterals in esophagus''' | '''Porto-caval collaterals in esophagus''' | ||
* Portal hypertension develops due to the formation of porto-collateral circulation<ref name="pmid3953799">{{cite journal |vauthors=Sikuler E, Groszmann RJ |title=Interaction of flow and resistance in maintenance of portal hypertension in a rat model |journal=Am. J. Physiol. |volume=250 |issue=2 Pt 1 |pages=G205–12 |year=1986 |pmid=3953799 |doi= |url=}}</ref> | * [[Portal hypertension]] develops due to the formation of porto-collateral circulation<ref name="pmid3953799">{{cite journal |vauthors=Sikuler E, Groszmann RJ |title=Interaction of flow and resistance in maintenance of portal hypertension in a rat model |journal=Am. J. Physiol. |volume=250 |issue=2 Pt 1 |pages=G205–12 |year=1986 |pmid=3953799 |doi= |url=}}</ref> | ||
* Dilatation and hypertrophy of preexisting vascular channels lead to the formation of these collateral channels<ref name="pmid25755456">{{cite journal |vauthors=Sharma M, Rameshbabu CS |title=Collateral pathways in portal hypertension |journal=J Clin Exp Hepatol |volume=2 |issue=4 |pages=338–52 |year=2012 |pmid=25755456 |pmc=3940321 |doi=10.1016/j.jceh.2012.08.001 |url=}}</ref> | * Dilatation and [[Hypertrophy (medical)|hypertrophy]] of preexisting [[vascular]] channels lead to the formation of these collateral channels<ref name="pmid25755456">{{cite journal |vauthors=Sharma M, Rameshbabu CS |title=Collateral pathways in portal hypertension |journal=J Clin Exp Hepatol |volume=2 |issue=4 |pages=338–52 |year=2012 |pmid=25755456 |pmc=3940321 |doi=10.1016/j.jceh.2012.08.001 |url=}}</ref> | ||
* Collaterals develop according to the increased portal pressure, and minimum threshold level of hepatic-venous portal | * Collaterals develop according to the increased portal pressure, and minimum threshold level of [[hepatic]]-[[venous]] portal gradient may be 10 mmHg for the development of porto-systemic collaterals and esophageal varices<ref name="pmid18695309">{{cite journal |vauthors=Kumar A, Sharma P, Sarin SK |title=Hepatic venous pressure gradient measurement: time to learn! |journal=Indian J Gastroenterol |volume=27 |issue=2 |pages=74–80 |year=2008 |pmid=18695309 |doi= |url=}}</ref> | ||
'''Role of hepatic vasodilators''' | '''Role of hepatic vasodilators''' | ||
'''(a) Nitric Oxide (NO)''' | '''(a) Nitric Oxide (NO)''' | ||
* Nitric oxide (NO) acts as an intra-hepatic vasodilator<ref name="pmid24819865">{{cite journal |vauthors=Simmonds MJ, Detterich JA, Connes P |title=Nitric oxide, vasodilation and the red blood cell |journal=Biorheology |volume=51 |issue=2-3 |pages=121–34 |year=2014 |pmid=24819865 |doi=10.3233/BIR-140653 |url=}}</ref><ref name="pmid12602508">{{cite journal |vauthors=González-Abraldes J, García-Pagán JC, Bosch J |title=Nitric oxide and portal hypertension |journal=Metab Brain Dis |volume=17 |issue=4 |pages=311–24 |year=2002 |pmid=12602508 |doi= |url=}}</ref> | * [[Nitric oxide]] ([[Nitric oxide|NO]]) acts as an intra-[[hepatic]] [[vasodilator]]<ref name="pmid24819865">{{cite journal |vauthors=Simmonds MJ, Detterich JA, Connes P |title=Nitric oxide, vasodilation and the red blood cell |journal=Biorheology |volume=51 |issue=2-3 |pages=121–34 |year=2014 |pmid=24819865 |doi=10.3233/BIR-140653 |url=}}</ref><ref name="pmid12602508">{{cite journal |vauthors=González-Abraldes J, García-Pagán JC, Bosch J |title=Nitric oxide and portal hypertension |journal=Metab Brain Dis |volume=17 |issue=4 |pages=311–24 |year=2002 |pmid=12602508 |doi= |url=}}</ref> | ||
* The levels of NO are decreased in patients suffering from chronic liver disease<ref name="pmid10643626">{{cite journal |vauthors=Wiest R, Groszmann RJ |title=Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=411–26 |year=1999 |pmid=10643626 |doi=10.1055/s-2007-1007129 |url=}}</ref> | * The levels of [[Nitric oxide|NO]] are decreased in patients suffering from [[chronic liver disease]]<ref name="pmid10643626">{{cite journal |vauthors=Wiest R, Groszmann RJ |title=Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=411–26 |year=1999 |pmid=10643626 |doi=10.1055/s-2007-1007129 |url=}}</ref> | ||
* This leads to an imbalance between the endogenous vasodilators and vasoconstrictors inside the hepatic vascular tree | * This leads to an imbalance between the endogenous [[vasodilators]] and [[vasoconstrictors]] inside the [[hepatic]] [[Vascular|vascular tree]] | ||
* Reduced levels of hepatic NO production may contribute to the increased | * Reduced levels of hepatic [[Nitric oxide|NO]] production may contribute to the increased intra-[[hepatic]] vascular resistance in [[cirrhosis]], thereby worsening [[portal hypertension]]<ref name="urlNitric Oxide - Hepatic Circulation - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK53065/ |title=Nitric Oxide - Hepatic Circulation - NCBI Bookshelf |format= |work= |accessdate=}}</ref> | ||
* NO-dependent apoptosis maintains the hepatic sinusoidal homeostasis | * [[Nitric oxide|NO]]-dependent [[apoptosis]] maintains the [[hepatic]] [[Sinusoid (blood vessel)|sinusoidal]] [[homeostasis]] | ||
* NO also leads to apoptosis of hepatic stellate | * [[Nitric oxide|NO]] also leads to apoptosis of [[hepatic]] [[Stellate cell|stellate cells]] through a signaling mechanism that involves [[mitochondria]], and a decreased level of [[Nitric oxide|NO]] may lead to a disturbance of the intra-[[hepatic]] [[homeostasis]]<ref name="pmid18459124">{{cite journal |vauthors=Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH |title=Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species |journal=Hepatology |volume=47 |issue=6 |pages=1983–93 |year=2008 |pmid=18459124 |pmc=2562502 |doi=10.1002/hep.22285 |url=}}</ref> | ||
'''(b) Glucagon''' | '''(b) Glucagon''' | ||
* Glucagon is a hormonal vasodilator which is associated with increased blood flow in the splanchnic bed and portal hypertension<ref name="pmid21160999">{{cite journal |vauthors=Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J |title=Physiopathology of splanchnic vasodilation in portal hypertension |journal=World J Hepatol |volume=2 |issue=6 |pages=208–20 |year=2010 |pmid=21160999 |pmc=2999290 |doi=10.4254/wjh.v2.i6.208 |url=}}</ref> | * [[Glucagon]] is a [[hormonal]] [[vasodilator]] which is associated with increased [[blood flow]] in the [[splanchnic]] bed and [[portal hypertension]]<ref name="pmid21160999">{{cite journal |vauthors=Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J |title=Physiopathology of splanchnic vasodilation in portal hypertension |journal=World J Hepatol |volume=2 |issue=6 |pages=208–20 |year=2010 |pmid=21160999 |pmc=2999290 |doi=10.4254/wjh.v2.i6.208 |url=}}</ref> | ||
* Plasma glucagon levels are increased in cirrhotic patients due to decreased hepatic clearance of glucagon as well as an increased secretion of glucagon by | * [[Blood plasma|Plasma]] [[glucagon]] levels are increased in [[Cirrhosis|cirrhotic]] patients due to decreased [[hepatic]] clearance of [[glucagon]] as well as an increased [[secretion]] of [[glucagon]] by [[pancreatic]] [[alpha cells]]<ref name="pmid21160999">{{cite journal |vauthors=Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J |title=Physiopathology of splanchnic vasodilation in portal hypertension |journal=World J Hepatol |volume=2 |issue=6 |pages=208–20 |year=2010 |pmid=21160999 |pmc=2999290 |doi=10.4254/wjh.v2.i6.208 |url=}}</ref><ref name="pmid8175150">{{cite journal |vauthors=Gomis R, Fernández-Alvarez J, Pizcueta P, Fernández M, Casamitjana R, Bosch J, Rodés J |title=Impaired function of pancreatic islets from rats with portal hypertension resulting from cirrhosis and partial portal vein ligation |journal=Hepatology |volume=19 |issue=5 |pages=1257–61 |year=1994 |pmid=8175150 |doi= |url=}}</ref> | ||
* Hyperglucagonemia may play a part in splanchnic vasodilatation of chronic portal hypertension<ref name="pmid26266087">{{cite journal |vauthors=Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P |title=Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans |journal=Mol Metab |volume=4 |issue=8 |pages=551–60 |year=2015 |pmid=26266087 |pmc=4529499 |doi=10.1016/j.molmet.2015.06.001 |url=}}</ref><ref name="Tibblin1970">{{cite journal|last1=Tibblin|first1=Sten|title=Splanchnic Hemodynamic Responses to Glucagon|journal=Archives of Surgery|volume=100|issue=1|year=1970|pages=84|issn=0004-0010|doi=10.1001/archsurg.1970.01340190086020}}</ref><ref name="pmid10643627">{{cite journal |vauthors=García-Pagán JC, Escorsell A, Moitinho E, Bosch J |title=Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=427–38 |year=1999 |pmid=10643627 |doi= |url=}}</ref> | * Hyperglucagonemia may play a part in [[splanchnic]] [[vasodilatation]] of chronic [[portal hypertension]]<ref name="pmid26266087">{{cite journal |vauthors=Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P |title=Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans |journal=Mol Metab |volume=4 |issue=8 |pages=551–60 |year=2015 |pmid=26266087 |pmc=4529499 |doi=10.1016/j.molmet.2015.06.001 |url=}}</ref><ref name="Tibblin1970">{{cite journal|last1=Tibblin|first1=Sten|title=Splanchnic Hemodynamic Responses to Glucagon|journal=Archives of Surgery|volume=100|issue=1|year=1970|pages=84|issn=0004-0010|doi=10.1001/archsurg.1970.01340190086020}}</ref><ref name="pmid10643627">{{cite journal |vauthors=García-Pagán JC, Escorsell A, Moitinho E, Bosch J |title=Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=427–38 |year=1999 |pmid=10643627 |doi= |url=}}</ref> | ||
'''(c) Prostacyclin''' | '''(c) Prostacyclin''' | ||
* Prostacyclin is an endogenous vasodilator<ref name="pmid17555364">{{cite journal |vauthors=Zardi EM, Dobrina A, Amoroso A, Afeltra A |title=Prostacyclin in liver disease: a potential therapeutic option |journal=Expert Opin Biol Ther |volume=7 |issue=6 |pages=785–90 |year=2007 |pmid=17555364 |doi=10.1517/14712598.7.6.785 |url=}}</ref><ref name="pmid12500202">{{cite journal |vauthors=Graupera M, García-Pagán JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, Bosch J, Rodés J |title=Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers |journal=Hepatology |volume=37 |issue=1 |pages=172–81 |year=2003 |pmid=12500202 |doi=10.1053/jhep.2003.50004 |url=}}</ref> | * [[Prostacyclin]] is an endogenous [[vasodilator]]<ref name="pmid17555364">{{cite journal |vauthors=Zardi EM, Dobrina A, Amoroso A, Afeltra A |title=Prostacyclin in liver disease: a potential therapeutic option |journal=Expert Opin Biol Ther |volume=7 |issue=6 |pages=785–90 |year=2007 |pmid=17555364 |doi=10.1517/14712598.7.6.785 |url=}}</ref><ref name="pmid12500202">{{cite journal |vauthors=Graupera M, García-Pagán JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, Bosch J, Rodés J |title=Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers |journal=Hepatology |volume=37 |issue=1 |pages=172–81 |year=2003 |pmid=12500202 |doi=10.1053/jhep.2003.50004 |url=}}</ref> | ||
* Prostacyclin levels are inversely related to the size of varices<ref name="pmid22899584">{{cite journal |vauthors=Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S |title=Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver |journal=Hepatology |volume=57 |issue=1 |pages=320–30 |year=2013 |pmid=22899584 |pmc=3524382 |doi=10.1002/hep.26005 |url=}}</ref><ref name="pmid12181171">{{cite journal |vauthors=Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D |title=Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats |journal=Am. J. Physiol. Gastrointest. Liver Physiol. |volume=283 |issue=3 |pages=G587–94 |year=2002 |pmid=12181171 |doi=10.1152/ajpgi.00391.2001 |url=}}</ref> | * [[Prostacyclin]] levels are inversely related to the size of varices<ref name="pmid22899584">{{cite journal |vauthors=Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S |title=Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver |journal=Hepatology |volume=57 |issue=1 |pages=320–30 |year=2013 |pmid=22899584 |pmc=3524382 |doi=10.1002/hep.26005 |url=}}</ref><ref name="pmid12181171">{{cite journal |vauthors=Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D |title=Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats |journal=Am. J. Physiol. Gastrointest. Liver Physiol. |volume=283 |issue=3 |pages=G587–94 |year=2002 |pmid=12181171 |doi=10.1152/ajpgi.00391.2001 |url=}}</ref> | ||
* Decreased prostacyclin levels are found in cirrhotic patients | * Decreased [[prostacyclin]] levels are found in [[Cirrhosis|cirrhotic]] patients | ||
'''Role of hepatic vasoconstrictors''' | '''Role of hepatic vasoconstrictors''' | ||
'''(a) Endothelin''' | '''(a) Endothelin''' | ||
* Endothelin is involved in changes in the vascular tone in cirrhotic patients<ref name="pmid7551971">{{cite journal |vauthors=García-Pagán JC, Bosch J, Rodés J |title=The role of vasoactive mediators in portal hypertension |journal=Semin. Gastrointest. Dis. |volume=6 |issue=3 |pages=140–7 |year=1995 |pmid=7551971 |doi= |url=}}</ref> | * [[Endothelin]] is involved in changes in the [[Vascular|vascular tone]] in [[Cirrhosis|cirrhotic]] patients<ref name="pmid7551971">{{cite journal |vauthors=García-Pagán JC, Bosch J, Rodés J |title=The role of vasoactive mediators in portal hypertension |journal=Semin. Gastrointest. Dis. |volume=6 |issue=3 |pages=140–7 |year=1995 |pmid=7551971 |doi= |url=}}</ref> | ||
* Endothelin leads to increased vascular tone ( | * [[Endothelin]] leads to increased [[vascular]] tone ([[vasoconstriction]]) | ||
* Endothelin 1 and endothelin 3 are increased in cirrhosis<ref name="pmid7499784">{{cite journal |vauthors=Møller S, Gülberg V, Henriksen JH, Gerbes AL |title=Endothelin-1 and endothelin-3 in cirrhosis: relations to systemic and splanchnic haemodynamics |journal=J. Hepatol. |volume=23 |issue=2 |pages=135–44 |year=1995 |pmid=7499784 |doi= |url=}}</ref><ref name="pmid9647333">{{cite journal |vauthors=Leivas A, Jiménez W, Bruix J, Boix L, Bosch J, Arroyo V, Rivera F, Rodés J |title=Gene expression of endothelin-1 and ET(A) and ET(B) receptors in human cirrhosis: relationship with hepatic hemodynamics |journal=J. Vasc. Res. |volume=35 |issue=3 |pages=186–93 |year=1998 |pmid=9647333 |doi= |url=}}</ref> | * [[Endothelin 1]] and [[endothelin 3]] are increased in [[cirrhosis]]<ref name="pmid7499784">{{cite journal |vauthors=Møller S, Gülberg V, Henriksen JH, Gerbes AL |title=Endothelin-1 and endothelin-3 in cirrhosis: relations to systemic and splanchnic haemodynamics |journal=J. Hepatol. |volume=23 |issue=2 |pages=135–44 |year=1995 |pmid=7499784 |doi= |url=}}</ref><ref name="pmid9647333">{{cite journal |vauthors=Leivas A, Jiménez W, Bruix J, Boix L, Bosch J, Arroyo V, Rivera F, Rodés J |title=Gene expression of endothelin-1 and ET(A) and ET(B) receptors in human cirrhosis: relationship with hepatic hemodynamics |journal=J. Vasc. Res. |volume=35 |issue=3 |pages=186–93 |year=1998 |pmid=9647333 |doi= |url=}}</ref> | ||
'''(b) Angiotensin II''' | '''(b) Angiotensin II''' | ||
* Angiotensin II leads to increased | * [[Angiotensin II]] leads to increased intra-[[portal]] resistance via [[vasoconstriction]]<ref name="pmid20570385">{{cite journal |vauthors=Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J |title=Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis |journal=J. Hepatol. |volume=53 |issue=2 |pages=273–82 |year=2010 |pmid=20570385 |doi=10.1016/j.jhep.2010.03.013 |url=}}</ref> | ||
'''(c) Norepinephrine''' | '''(c) Norepinephrine''' | ||
* Norepinephrine is also a vasoconstrictor, which controls the | * [[Norepinephrine]] is also a [[vasoconstrictor]], which controls the intra-[[hepatic]] [[vascular]] tone, including [[portal]] [[Blood vessel|vessels]]<ref name="pmid3336005">{{cite journal |vauthors=Ballet F, Chretien Y, Rey C, Poupon R |title=Differential response of normal and cirrhotic liver to vasoactive agents. A study in the isolated perfused rat liver |journal=J. Pharmacol. Exp. Ther. |volume=244 |issue=1 |pages=283–9 |year=1988 |pmid=3336005 |doi= |url=}}</ref><ref name="pmid3561267">{{cite journal |vauthors=Lautt WW, Greenway CV, Legare DJ |title=Effect of hepatic nerves, norepinephrine, angiotensin, and elevated central venous pressure on postsinusoidal resistance sites and intrahepatic pressures in cats |journal=Microvasc. Res. |volume=33 |issue=1 |pages=50–61 |year=1987 |pmid=3561267 |doi= |url=}}</ref> | ||
'''Role of endothelial dysfunction''' | '''Role of endothelial dysfunction''' | ||
* Vascular endothelium harbors a number of vasocontrictive substance such as, prostaglandin H2(PGH2), thromboxane A2 (TXA2) and anion superoxide,which contribute to portal hypertension<ref name="pmid106436262">{{cite journal |vauthors=Wiest R, Groszmann RJ |title=Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=411–26 |year=1999 |pmid=10643626 |doi=10.1055/s-2007-1007129 |url=}}</ref> | * [[Vascular]] [[endothelium]] harbors a number of [[Vasoconstriction|vasocontrictive]] substance such as, [[prostaglandin H2]]([[Prostaglandin H2|PGH2]]), [[thromboxane A2]] ([[Thromboxane A2|TXA2]]) and [[anion]] [[superoxide]], which contribute to [[portal hypertension]]<ref name="pmid106436262">{{cite journal |vauthors=Wiest R, Groszmann RJ |title=Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance |journal=Semin. Liver Dis. |volume=19 |issue=4 |pages=411–26 |year=1999 |pmid=10643626 |doi=10.1055/s-2007-1007129 |url=}}</ref> | ||
* Cirrhosis leads to endothelial dysfunction<ref name="pmid9755227">{{cite journal |vauthors=Gupta TK, Toruner M, Chung MK, Groszmann RJ |title=Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats |journal=Hepatology |volume=28 |issue=4 |pages=926–31 |year=1998 |pmid=9755227 |doi=10.1002/hep.510280405 |url=}}</ref> | * [[Cirrhosis]] leads to [[endothelial]] dysfunction<ref name="pmid9755227">{{cite journal |vauthors=Gupta TK, Toruner M, Chung MK, Groszmann RJ |title=Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats |journal=Hepatology |volume=28 |issue=4 |pages=926–31 |year=1998 |pmid=9755227 |doi=10.1002/hep.510280405 |url=}}</ref> | ||
* Increased production of prostanoids, most likely thromboxane A2 (TXA2) has been known to be associated with endothelial dysfunction<ref name="pmid12971960">{{cite journal |vauthors=Graupera M, García-Pagán JC, Parés M, Abraldes JG, Roselló J, Bosch J, Rodés J |title=Cyclooxygenase-1 inhibition corrects endothelial dysfunction in cirrhotic rat livers |journal=J. Hepatol. |volume=39 |issue=4 |pages=515–21 |year=2003 |pmid=12971960 |doi= |url=}}</ref> | * Increased production of [[Prostanoid|prostanoids]], most likely [[thromboxane A2]] ([[Thromboxane A2|TXA2]]) has been known to be associated with [[endothelial]] dysfunction<ref name="pmid12971960">{{cite journal |vauthors=Graupera M, García-Pagán JC, Parés M, Abraldes JG, Roselló J, Bosch J, Rodés J |title=Cyclooxygenase-1 inhibition corrects endothelial dysfunction in cirrhotic rat livers |journal=J. Hepatol. |volume=39 |issue=4 |pages=515–21 |year=2003 |pmid=12971960 |doi= |url=}}</ref> | ||
'''Mechanism leading to variceal hemorrhage''' | '''Mechanism leading to variceal hemorrhage''' | ||
* The wall tension of the vessel determines if there will be rupture of the varices<ref name="pmid10425421">{{cite journal |vauthors=Jackson FW, Adrain AL, Black M, Miller LS |title=Calculation of esophageal variceal wall tension by direct sonographic and manometric measurements |journal=Gastrointest. Endosc. |volume=50 |issue=2 |pages=247–51 |year=1999 |pmid=10425421 |doi= |url=}}</ref> | * The wall tension of the [[Blood vessel|vessel]] determines if there will be rupture of the varices<ref name="pmid10425421">{{cite journal |vauthors=Jackson FW, Adrain AL, Black M, Miller LS |title=Calculation of esophageal variceal wall tension by direct sonographic and manometric measurements |journal=Gastrointest. Endosc. |volume=50 |issue=2 |pages=247–51 |year=1999 |pmid=10425421 |doi= |url=}}</ref> | ||
* The wall tension depends upon the variceal pressure, luminal pressure and radius of the vessel<ref name="pmid10425421">{{cite journal |vauthors=Jackson FW, Adrain AL, Black M, Miller LS |title=Calculation of esophageal variceal wall tension by direct sonographic and manometric measurements |journal=Gastrointest. Endosc. |volume=50 |issue=2 |pages=247–51 |year=1999 |pmid=10425421 |doi= |url=}}</ref> | * The wall tension depends upon the variceal pressure, luminal pressure and radius of the [[Blood vessel|vessel]]<ref name="pmid10425421">{{cite journal |vauthors=Jackson FW, Adrain AL, Black M, Miller LS |title=Calculation of esophageal variceal wall tension by direct sonographic and manometric measurements |journal=Gastrointest. Endosc. |volume=50 |issue=2 |pages=247–51 |year=1999 |pmid=10425421 |doi= |url=}}</ref> | ||
* The wall tension is calculated by using the | * The wall tension is calculated by using the “[[Laplace's law]]”: | ||
** Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall. | ** Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall. | ||
** The result is the force which is generated by the variceal wall opposing further dilation | ** The result is the force which is generated by the variceal wall opposing further dilation | ||
* When the wall tension | * When the wall tension overcomes the elastic limit of the varices, rupture occurs<ref name="pmid23193482">{{cite journal |vauthors=Hilzenrat N, Sherker AH |title=Esophageal varices: pathophysiology, approach, and clinical dilemmas |journal=Int J Hepatol |volume=2012 |issue= |pages=795063 |year=2012 |pmid=23193482 |pmc=3501997 |doi=10.1155/2012/795063 |url=}}</ref> | ||
=== Gastric varices === | === Gastric varices === | ||
Gastric varices may form secondary to chronic liver disease or splenic vein obstruction; splenic vein obstruction may be caused by pancreatitis, pancreatic pseudocysts, pancreatic carcinoma, other retroperitoneal tumors, or intrinsic thrombosis of the splenic vein | Gastric varices may form secondary to [[chronic liver disease]] or [[splenic vein]] obstruction; [[splenic vein]] obstruction may be caused by [[pancreatitis]], [[Pancreatic pseudocyst|pancreatic pseudocysts]], [[pancreatic carcinoma]], other [[retroperitoneal]] [[tumors]], or intrinsic [[thrombosis]] of the [[splenic vein]] | ||
* '''Vascular architecture and venous drainage of stomach''' | * '''Vascular architecture and venous drainage of stomach''' | ||
* Gastric varices consist of dilated veins present in the submucosa of the stomach in areas of port-caval anastomosis (fundus and cardia) | * Gastric varices consist of dilated [[veins]] present in the [[submucosa]] of the [[stomach]] in areas of [[Portocaval anastomoses|port-caval anastomosis]] ([[Fundus (stomach)|fundus]] and cardia) | ||
* The splenic vein and superior mesenteric vein join together to form the portal vein. The anastomosis contributing to gastric varices consists of short gastric vein, left gastric vein and esophageal branches | * The [[splenic vein]] and [[superior mesenteric vein]] join together to form the [[portal vein]]. The [[anastomosis]] contributing to gastric varices consists of [[Short gastric veins|short gastric vein]], [[left gastric vein]] and [[esophageal]] branches | ||
* Cardiac varices are supplied majorly by left gastric vein | * Cardiac varices are supplied majorly by [[left gastric vein]] | ||
* Fundic varices are supplied by short gastric or posterior gastric veins<ref name="pmid2210674">{{cite journal |vauthors=Kimura K, Ohto M, Matsutani S, Furuse J, Hoshino K, Okuda K |title=Relative frequencies of portosystemic pathways and renal shunt formation through the "posterior" gastric vein: portographic study in 460 patients |journal=Hepatology |volume=12 |issue=4 Pt 1 |pages=725–8 |year=1990 |pmid=2210674 |doi= |url=}}</ref> | * Fundic varices are supplied by [[Short gastric veins|short gastric]] or posterior gastric veins<ref name="pmid2210674">{{cite journal |vauthors=Kimura K, Ohto M, Matsutani S, Furuse J, Hoshino K, Okuda K |title=Relative frequencies of portosystemic pathways and renal shunt formation through the "posterior" gastric vein: portographic study in 460 patients |journal=Hepatology |volume=12 |issue=4 Pt 1 |pages=725–8 |year=1990 |pmid=2210674 |doi= |url=}}</ref> | ||
* Cardiac and fundic varices differ in the degree of anastomosis (more common in cardiac varices) and the caliber of the varicose veins | * Cardiac and fundic varices differ in the degree of [[anastomosis]] (more common in cardiac varices) and the caliber of the varicose veins | ||
'''Mechanism of development of gastric varices''' | '''Mechanism of development of gastric varices''' | ||
* Increased pressure in two main venous pathways are responsible development of gastric varices | * Increased pressure in two main venous pathways are responsible development of gastric varices | ||
'''(a) Cardiac varices''' | '''(a) Cardiac varices''' | ||
* First, through the right and left gastric veins, which drain varices around the distal esophagus and cardia (EV and GOV1) into the portal vein, or when flow is reversed the blood flows backwards into the azygous system | * First, through the right and [[Left gastric vein|left gastric veins]], which drain varices around the distal [[esophagus]] and [[cardia]] (EV and GOV1) into the portal vein, or when flow is reversed the [[blood]] flows backwards into the [[Azygos vein|azygous system]] | ||
'''(b) Fundic varices''' | '''(b) Fundic varices''' | ||
* Blood from the fundus of the stomach is drained via the short gastric and posterior gastric veins, these veins give rise to the fundic varices | * [[Blood]] from the [[Fundus (stomach)|fundus of the stomach]] is drained via the [[Short gastric veins|short gastric]] and posterior gastric veins, these [[veins]] give rise to the fundic varices | ||
* In portal hypertension, the flow often is reversed and blood drains from the spleen toward the stomach into fundal varices (GOV2 and IGV1) | * In [[portal hypertension]], the flow often is reversed and [[blood]] drains from the [[spleen]] toward the [[stomach]] into fundal varices (GOV2 and IGV1) | ||
* IGV2 often are caused by dilation of branches of the gastroepiploic veins | * IGV2 often are caused by dilation of branches of the gastroepiploic veins | ||
'''Mechanism leading to variceal hemorrhage''' | '''Mechanism leading to variceal hemorrhage''' | ||
* The wall tension of the vessel determines if there will be rupture of the varices<ref name="pmid10425421" /> | * The wall tension of the vessel determines if there will be rupture of the varices<ref name="pmid10425421" /> | ||
* The wall tension depends upon the variceal pressure, luminal pressure and radius of the vessel<ref name="pmid10425421" /> | * The wall tension depends upon the variceal pressure, [[luminal]] pressure and radius of the [[Blood vessel|vessel]]<ref name="pmid10425421" /> | ||
* The wall tension is calculated by using the | * The wall tension is calculated by using the “[[Laplace's law]]”: | ||
** Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall. | ** Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall. | ||
** The result is the force which is generated by the variceal wall opposing further dilation | ** The result is the force which is generated by the variceal wall opposing further dilation | ||
* When the wall tension over comes the elastic limit of the varices, rupture occurs<ref name="pmid23193482" /> | * When the wall tension over comes the elastic limit of the varices, rupture occurs<ref name="pmid23193482" /> | ||
* The vessel wall of the varix is covered by a thinned out serosa and mucosa, and the varix comes to be seen through from the serosa as well as from the mucosa. When such a large varix ruptures, bleeding is profuse and difficult to manage, and the mortality rate is high | * The [[Blood vessel|vessel]] wall of the varix is covered by a thinned out [[serosa]] and [[Mucosal|mucosa]], and the varix comes to be seen through from the [[serosa]] as well as from the [[mucosa]]. When such a large varix ruptures, bleeding is profuse and difficult to manage, and the [[mortality rate]] is high | ||
==Associated Conditions== | ==Associated Conditions== | ||
==Genetics== | ==Genetics== | ||
Congenital syndromes leading to gastrointestinal varices may involve genetic mutations in the following genes:<ref name="pmid18026579">{{cite journal |vauthors=Ling SC |title=Congenital cholestatic syndromes: what happens when children grow up? |journal=Can. J. Gastroenterol. |volume=21 |issue=11 |pages=743–51 |year=2007 |pmid=18026579 |pmc=2658590 |doi= |url=}}</ref><ref name="pmid23328456">{{cite journal |vauthors=Malloy L, Jensen M, Bishop W, Divekar A |title="Downhill" esophageal varices in congenital heart disease |journal=J. Pediatr. Gastroenterol. Nutr. |volume=56 |issue=2 |pages=e9–11 |year=2013 |pmid=23328456 |doi=10.1097/MPG.0b013e31824b5fff |url=}}</ref><ref name="urlCongenital Hepatic Fibrosis Overview - GeneReviews® - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK2701/# |title=Congenital Hepatic Fibrosis Overview - GeneReviews® - NCBI Bookshelf |format= |work= |accessdate=}}</ref> | |||
{| class="wikitable" | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Pattern of inheritance''' | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Disease''' | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Locus''' | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Gene''' | |||
|- | |||
| colspan="1" rowspan="68" |'''Autosomal''' | |||
'''recessive''' | |||
| colspan="1" rowspan="1" |[[Autosomal recessive polycystic kidney disease]] (ARPKD) | |||
| colspan="1" rowspan="1" |PKHD1 | |||
| colspan="1" rowspan="1" |''[[PKHD1]]'' | |||
|- | |||
| colspan="1" rowspan="15" |[[Nephronophthisis]] 1 | |||
| colspan="1" rowspan="1" |NPHP1 2 | |||
| colspan="1" rowspan="1" |''[[NPHP1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP2 | |||
| colspan="1" rowspan="1" |''[[INVS]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP3 | |||
| colspan="1" rowspan="1" |''[[NPHP3]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP4 | |||
| colspan="1" rowspan="1" |''[[NPHP4]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP5 | |||
(SLSN5) | |||
| colspan="1" rowspan="1" |''[[IQCB1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP6 3 | |||
(SLSN6) | |||
| colspan="1" rowspan="1" |''[[CEP290]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP7 | |||
| colspan="1" rowspan="1" |''[[GLIS2]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP8 4 | |||
| colspan="1" rowspan="1" |''[[RPGRIP1L]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP9 | |||
| colspan="1" rowspan="1" |''[[NEK8]]'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP11 | |||
| colspan="1" rowspan="1" |''TMEM67'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP12 | |||
| colspan="1" rowspan="1" |''TTC21B'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP13 | |||
| colspan="1" rowspan="1" |''WDR19'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP14 | |||
| colspan="1" rowspan="1" |''ZNF423'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP15 | |||
| colspan="1" rowspan="1" |''CEP164'' | |||
|- | |||
| colspan="1" rowspan="1" |NPHP16 | |||
| colspan="1" rowspan="1" |''ANKS6'' | |||
|- | |||
| colspan="1" rowspan="21" |[[Joubert syndrome]] and related disorders 5 | |||
| colspan="1" rowspan="1" |JBTS1 | |||
| colspan="1" rowspan="1" |''INPP5E'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS2 | |||
| colspan="1" rowspan="1" |''TMEM216'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS3 | |||
| colspan="1" rowspan="1" |''[[AHI1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS4 2 | |||
| colspan="1" rowspan="1" |''[[NPHP1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS5 3 | |||
| colspan="1" rowspan="1" |''[[CEP290]]'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS6 6 | |||
| colspan="1" rowspan="1" |''TMEM67'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS7 4 | |||
| colspan="1" rowspan="1" |''[[RPGRIP1L]]'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS8 | |||
| colspan="1" rowspan="1" |''ARL13B'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS9 | |||
| colspan="1" rowspan="1" |''CC2D2A'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS10 | |||
| colspan="1" rowspan="1" |''[[OFD1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS11 | |||
| colspan="1" rowspan="1" |''TTC21B'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS12 | |||
| colspan="1" rowspan="1" |''KIF7'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS13 | |||
| colspan="1" rowspan="1" |''TCTN1'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS14 | |||
| colspan="1" rowspan="1" |''TMEM237'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS15 | |||
| colspan="1" rowspan="1" |''CEP41'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS16 | |||
| colspan="1" rowspan="1" |''TMEM138'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS17 | |||
| colspan="1" rowspan="1" |''C5orf42'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS18 | |||
| colspan="1" rowspan="1" |''TCTN3'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS19 | |||
| colspan="1" rowspan="1" |''ZNF423'' | |||
|- | |||
| colspan="1" rowspan="1" |JBTS20 | |||
| colspan="1" rowspan="1" |''TMEM231'' | |||
|- | |||
| colspan="1" rowspan="1" | | |||
| colspan="1" rowspan="1" |''TCTN2'' | |||
|- | |||
| colspan="1" rowspan="14" |[[Bardet-Biedl syndrome]] 7 | |||
| colspan="1" rowspan="1" |BBS1 | |||
| colspan="1" rowspan="1" |''[[BBS1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS2 | |||
| colspan="1" rowspan="1" |''[[BBS2]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS3 | |||
| colspan="1" rowspan="1" |''[[ARL6]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS4 | |||
| colspan="1" rowspan="1" |''[[BBS4]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS5 | |||
| colspan="1" rowspan="1" |''[[BBS5]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS6 | |||
| colspan="1" rowspan="1" |''[[MKKS]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS7 | |||
| colspan="1" rowspan="1" |''BBS7'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS8 | |||
| colspan="1" rowspan="1" |''TTC8'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS9 | |||
| colspan="1" rowspan="1" |''BBS9'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS10 | |||
| colspan="1" rowspan="1" |''BBS10'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS11 | |||
| colspan="1" rowspan="1" |''[[TRIM32]]'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS12 | |||
| colspan="1" rowspan="1" |''BBS12'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS13 8 | |||
| colspan="1" rowspan="1" |''MKS1'' | |||
|- | |||
| colspan="1" rowspan="1" |BBS14 3 | |||
| colspan="1" rowspan="1" |''[[CEP290]]'' | |||
|- | |||
| colspan="1" rowspan="11" |[[Meckel syndrome]] 9 | |||
| colspan="1" rowspan="1" |MKS1 8 | |||
| colspan="1" rowspan="1" |''MKS1'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS2 | |||
| colspan="1" rowspan="1" |''TMEM216'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS3 5 | |||
| colspan="1" rowspan="1" |''TMEM67'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS4 3 | |||
| colspan="1" rowspan="1" |''[[CEP290]]'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS5 4 | |||
| colspan="1" rowspan="1" |''[[RPGRIP1L]]'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS6 | |||
| colspan="1" rowspan="1" |''CC2D2A'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS7 | |||
| colspan="1" rowspan="1" |''[[NPHP3]]'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS8 | |||
| colspan="1" rowspan="1" |''TCTN2'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS9 | |||
| colspan="1" rowspan="1" |''B9D1'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS10 | |||
| colspan="1" rowspan="1" |''B9D2'' | |||
|- | |||
| colspan="1" rowspan="1" |MKS11 | |||
| colspan="1" rowspan="1" |''TMEM231'' | |||
|- | |||
| colspan="1" rowspan="1" |Cranioectodermal dysplasia | |||
| colspan="1" rowspan="1" | | |||
| colspan="1" rowspan="1" |''IFT122'' | |||
|- | |||
| colspan="1" rowspan="2" |[[Ellis-van Creveld syndrome]] | |||
| colspan="1" rowspan="2" |EVC 10 | |||
| colspan="1" rowspan="1" |''[[EVC (gene)|EVC]]'' | |||
|- | |||
| colspan="1" rowspan="1" |''[[EVC2]]'' | |||
|- | |||
| colspan="1" rowspan="2" |Jeune asphyxiating thoracic dystrophy | |||
| colspan="1" rowspan="1" |JATD1 | |||
| colspan="1" rowspan="1" |Unknown | |||
|- | |||
| colspan="1" rowspan="1" |JATD2 | |||
| colspan="1" rowspan="1" |''IFT80'' | |||
|- | |||
| colspan="1" rowspan="1" |[[Renal]]-[[hepatic]]-[[pancreatic]] dysplasia | |||
| colspan="1" rowspan="1" | | |||
| colspan="1" rowspan="1" |''[[NPHP3]]'' | |||
|- | |||
| colspan="1" rowspan="1" |'''X-linked''' | |||
| colspan="1" rowspan="1" |OFD1 | |||
| colspan="1" rowspan="1" | | |||
| colspan="1" rowspan="1" |''[[OFD1]]'' | |||
|- | |||
| colspan="1" rowspan="2" |'''Autosomaldominant''' | |||
| colspan="1" rowspan="2" |[[Autosomal dominant polycystic kidney disease]] (ADPKD) | |||
| colspan="1" rowspan="1" |PKD1 | |||
| colspan="1" rowspan="1" |''[[PKD1]]'' | |||
|- | |||
| colspan="1" rowspan="1" |PKD2 | |||
| colspan="1" rowspan="1" |''[[PKD2]]'' | |||
|} | |||
==Gross Pathology== | ==Gross Pathology== | ||
On gross examination, the following findings may be observed in gastrointestinal varices: | On gross examination, the following findings may be observed in gastrointestinal varices: | ||
* Linear dark blue | * Linear dark blue [[submucosal]] dilated [[veins]] | ||
* In case of bleeding the varix may be colored dark red | * In case of [[bleeding]] the varix may be colored dark red | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
On microscopic examination the following findings may be observed:<ref name="pmid11928080">{{cite journal |vauthors=Arakawa M, Masuzaki T, Okuda K |title=Pathomorphology of esophageal and gastric varices |journal=Semin. Liver Dis. |volume=22 |issue=1 |pages=73–82 |year=2002 |pmid=11928080 |doi=10.1055/s-2002-23208 |url=}}</ref><ref name="pmid12865886">{{cite journal |vauthors=Wali MA, Dewan M, Eid RA |title=Histopathological changes in the wall of varicose veins |journal=Int Angiol |volume=22 |issue=2 |pages=188–93 |year=2003 |pmid=12865886 |doi= |url=}}</ref> | On microscopic examination the following findings may be observed:<ref name="pmid11928080">{{cite journal |vauthors=Arakawa M, Masuzaki T, Okuda K |title=Pathomorphology of esophageal and gastric varices |journal=Semin. Liver Dis. |volume=22 |issue=1 |pages=73–82 |year=2002 |pmid=11928080 |doi=10.1055/s-2002-23208 |url=}}</ref><ref name="pmid12865886">{{cite journal |vauthors=Wali MA, Dewan M, Eid RA |title=Histopathological changes in the wall of varicose veins |journal=Int Angiol |volume=22 |issue=2 |pages=188–93 |year=2003 |pmid=12865886 |doi= |url=}}</ref> | ||
* Very thin parallel veins in the lamina propria mucosae in the palisade zone become enlarged in portal hypertension and join the few larger submucosal veins to form esophageal varices | * Very thin parallel [[veins]] in the [[lamina propria]] [[Mucosa|mucosae]] in the palisade zone become enlarged in [[portal hypertension]] and join the few larger [[submucosal]] [[veins]] to form esophageal varices | ||
* The location of esophageal and gastric varices within the wall of gastrointestinal tract is different: | * The location of esophageal and gastric varices within the wall of [[gastrointestinal tract]] is different: | ||
** Esophageal varices: Lamina propria mucosae and submucosa | ** Esophageal varices: [[Lamina propria]] [[Mucosa|mucosae]] and [[submucosa]] | ||
** Gastric varices: Submucosa | ** Gastric varices: [[Submucosa]] | ||
*The wall of the varix (vein) may show deficiency in smooth muscle cells and elastic fibers and disproportionate increase in fibrous tissue | *The wall of the varix ([[vein]]) may show deficiency in [[smooth muscle cells]] and elastic fibers and disproportionate increase in [[fibrous tissue]] | ||
[[Image:15419.jpg|300px|left|Histological findings in esophageal varices, source:Pathguy.com]] | [[Image:15419.jpg|300px|left|Histological findings in esophageal varices, source:Pathguy.com]] | ||
<br style="clear:left"> | |||
== References == | == References == | ||
<references /> | <references /> | ||
Latest revision as of 14:23, 24 January 2018
|
Gastrointestinal varices Microchapters |
|
Differentiating Gastrointestinal varices from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Gastrointestinal varices pathophysiology On the Web |
|
American Roentgen Ray Society Images of Gastrointestinal varices pathophysiology |
|
Risk calculators and risk factors for Gastrointestinal varices pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Overview
Varices arise from hemodynamic disturbance between the systemic and portal venous system. The majority of venous drainage of the gastrointestinal system occurs via the portal venous system. Whenever there is an interruption of drainage through the portal system (for example due to cirrhosis), the vessels contributing to the porto-caval shunts become more prominent due to increased pressure gradient. The interruption in blood flow leads to the creation collateral vessels that involve veins of the esophagus, stomach, pelvis (hemorrhoids), retroperitoneum, liver, abdominal wall, and other areas.
Pathophysiology
Varices arise from hemodynamic disturbance between the systemic and portal venous system. The majority of venous drainage of the gastrointestinal system occurs via the portal venous system. Whenever there is an interruption of drainage through the portal system (for example due to cirrhosis), the vessels contributing to the porto-caval shunts become more prominent due to increased pressure gradient. The interruption in blood flow leads to the creation collateral vessels that involve veins of the esophagus, stomach, pelvis (hemorrhoids), retroperitoneum, liver, abdominal wall, and other areas.[1][2]
Esophageal varices
Esophageal varices are a major complication of portal hypertension (increased blood pressure in the portal venous system). In order to understand the mechanism leading to the development of esophageal varices, it is important to understand the normal vascular architecture and venous drainage of the esophagus.[3]
Vascular architecture and venous drainage of esophagus
- Vascular resistance increases against portal blood flow in cirrhosis, non-cirrhotic portal fibrosis, idiopathic portal hypertension, extra-hepatic portal vein obstruction, Budd-Chiari syndrome, and other portal hypertensive disorders, inducing congestion of blood in the splenic and mesenteric veins that lie upstream of the portal trunk[4][5][6]
- The major vessels draining blood from the esophagus include, the left gastric and less frequently short gastric veins[7][8]
Porto-caval collaterals in esophagus
- Portal hypertension develops due to the formation of porto-collateral circulation[9]
- Dilatation and hypertrophy of preexisting vascular channels lead to the formation of these collateral channels[10]
- Collaterals develop according to the increased portal pressure, and minimum threshold level of hepatic-venous portal gradient may be 10 mmHg for the development of porto-systemic collaterals and esophageal varices[11]
Role of hepatic vasodilators
(a) Nitric Oxide (NO)
- Nitric oxide (NO) acts as an intra-hepatic vasodilator[12][13]
- The levels of NO are decreased in patients suffering from chronic liver disease[14]
- This leads to an imbalance between the endogenous vasodilators and vasoconstrictors inside the hepatic vascular tree
- Reduced levels of hepatic NO production may contribute to the increased intra-hepatic vascular resistance in cirrhosis, thereby worsening portal hypertension[15]
- NO-dependent apoptosis maintains the hepatic sinusoidal homeostasis
- NO also leads to apoptosis of hepatic stellate cells through a signaling mechanism that involves mitochondria, and a decreased level of NO may lead to a disturbance of the intra-hepatic homeostasis[16]
(b) Glucagon
- Glucagon is a hormonal vasodilator which is associated with increased blood flow in the splanchnic bed and portal hypertension[17]
- Plasma glucagon levels are increased in cirrhotic patients due to decreased hepatic clearance of glucagon as well as an increased secretion of glucagon by pancreatic alpha cells[17][18]
- Hyperglucagonemia may play a part in splanchnic vasodilatation of chronic portal hypertension[19][20][21]
(c) Prostacyclin
- Prostacyclin is an endogenous vasodilator[22][23]
- Prostacyclin levels are inversely related to the size of varices[24][25]
- Decreased prostacyclin levels are found in cirrhotic patients
Role of hepatic vasoconstrictors
(a) Endothelin
- Endothelin is involved in changes in the vascular tone in cirrhotic patients[26]
- Endothelin leads to increased vascular tone (vasoconstriction)
- Endothelin 1 and endothelin 3 are increased in cirrhosis[27][28]
(b) Angiotensin II
- Angiotensin II leads to increased intra-portal resistance via vasoconstriction[29]
(c) Norepinephrine
- Norepinephrine is also a vasoconstrictor, which controls the intra-hepatic vascular tone, including portal vessels[30][31]
Role of endothelial dysfunction
- Vascular endothelium harbors a number of vasocontrictive substance such as, prostaglandin H2(PGH2), thromboxane A2 (TXA2) and anion superoxide, which contribute to portal hypertension[32]
- Cirrhosis leads to endothelial dysfunction[33]
- Increased production of prostanoids, most likely thromboxane A2 (TXA2) has been known to be associated with endothelial dysfunction[34]
Mechanism leading to variceal hemorrhage
- The wall tension of the vessel determines if there will be rupture of the varices[35]
- The wall tension depends upon the variceal pressure, luminal pressure and radius of the vessel[35]
- The wall tension is calculated by using the “Laplace's law”:
- Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall.
- The result is the force which is generated by the variceal wall opposing further dilation
- When the wall tension overcomes the elastic limit of the varices, rupture occurs[36]
Gastric varices
Gastric varices may form secondary to chronic liver disease or splenic vein obstruction; splenic vein obstruction may be caused by pancreatitis, pancreatic pseudocysts, pancreatic carcinoma, other retroperitoneal tumors, or intrinsic thrombosis of the splenic vein
- Vascular architecture and venous drainage of stomach
- Gastric varices consist of dilated veins present in the submucosa of the stomach in areas of port-caval anastomosis (fundus and cardia)
- The splenic vein and superior mesenteric vein join together to form the portal vein. The anastomosis contributing to gastric varices consists of short gastric vein, left gastric vein and esophageal branches
- Cardiac varices are supplied majorly by left gastric vein
- Fundic varices are supplied by short gastric or posterior gastric veins[37]
- Cardiac and fundic varices differ in the degree of anastomosis (more common in cardiac varices) and the caliber of the varicose veins
Mechanism of development of gastric varices
- Increased pressure in two main venous pathways are responsible development of gastric varices
(a) Cardiac varices
- First, through the right and left gastric veins, which drain varices around the distal esophagus and cardia (EV and GOV1) into the portal vein, or when flow is reversed the blood flows backwards into the azygous system
(b) Fundic varices
- Blood from the fundus of the stomach is drained via the short gastric and posterior gastric veins, these veins give rise to the fundic varices
- In portal hypertension, the flow often is reversed and blood drains from the spleen toward the stomach into fundal varices (GOV2 and IGV1)
- IGV2 often are caused by dilation of branches of the gastroepiploic veins
Mechanism leading to variceal hemorrhage
- The wall tension of the vessel determines if there will be rupture of the varices[35]
- The wall tension depends upon the variceal pressure, luminal pressure and radius of the vessel[35]
- The wall tension is calculated by using the “Laplace's law”:
- Wall tension = (variceal pressure – luminal pressure) × radius/thickening of variceal wall.
- The result is the force which is generated by the variceal wall opposing further dilation
- When the wall tension over comes the elastic limit of the varices, rupture occurs[36]
- The vessel wall of the varix is covered by a thinned out serosa and mucosa, and the varix comes to be seen through from the serosa as well as from the mucosa. When such a large varix ruptures, bleeding is profuse and difficult to manage, and the mortality rate is high
Associated Conditions
Genetics
Congenital syndromes leading to gastrointestinal varices may involve genetic mutations in the following genes:[38][39][40]
| Pattern of inheritance | Disease | Locus | Gene |
|---|---|---|---|
| Autosomal
recessive |
Autosomal recessive polycystic kidney disease (ARPKD) | PKHD1 | PKHD1 |
| Nephronophthisis 1 | NPHP1 2 | NPHP1 | |
| NPHP2 | INVS | ||
| NPHP3 | NPHP3 | ||
| NPHP4 | NPHP4 | ||
| NPHP5
(SLSN5) |
IQCB1 | ||
| NPHP6 3
(SLSN6) |
CEP290 | ||
| NPHP7 | GLIS2 | ||
| NPHP8 4 | RPGRIP1L | ||
| NPHP9 | NEK8 | ||
| NPHP11 | TMEM67 | ||
| NPHP12 | TTC21B | ||
| NPHP13 | WDR19 | ||
| NPHP14 | ZNF423 | ||
| NPHP15 | CEP164 | ||
| NPHP16 | ANKS6 | ||
| Joubert syndrome and related disorders 5 | JBTS1 | INPP5E | |
| JBTS2 | TMEM216 | ||
| JBTS3 | AHI1 | ||
| JBTS4 2 | NPHP1 | ||
| JBTS5 3 | CEP290 | ||
| JBTS6 6 | TMEM67 | ||
| JBTS7 4 | RPGRIP1L | ||
| JBTS8 | ARL13B | ||
| JBTS9 | CC2D2A | ||
| JBTS10 | OFD1 | ||
| JBTS11 | TTC21B | ||
| JBTS12 | KIF7 | ||
| JBTS13 | TCTN1 | ||
| JBTS14 | TMEM237 | ||
| JBTS15 | CEP41 | ||
| JBTS16 | TMEM138 | ||
| JBTS17 | C5orf42 | ||
| JBTS18 | TCTN3 | ||
| JBTS19 | ZNF423 | ||
| JBTS20 | TMEM231 | ||
| TCTN2 | |||
| Bardet-Biedl syndrome 7 | BBS1 | BBS1 | |
| BBS2 | BBS2 | ||
| BBS3 | ARL6 | ||
| BBS4 | BBS4 | ||
| BBS5 | BBS5 | ||
| BBS6 | MKKS | ||
| BBS7 | BBS7 | ||
| BBS8 | TTC8 | ||
| BBS9 | BBS9 | ||
| BBS10 | BBS10 | ||
| BBS11 | TRIM32 | ||
| BBS12 | BBS12 | ||
| BBS13 8 | MKS1 | ||
| BBS14 3 | CEP290 | ||
| Meckel syndrome 9 | MKS1 8 | MKS1 | |
| MKS2 | TMEM216 | ||
| MKS3 5 | TMEM67 | ||
| MKS4 3 | CEP290 | ||
| MKS5 4 | RPGRIP1L | ||
| MKS6 | CC2D2A | ||
| MKS7 | NPHP3 | ||
| MKS8 | TCTN2 | ||
| MKS9 | B9D1 | ||
| MKS10 | B9D2 | ||
| MKS11 | TMEM231 | ||
| Cranioectodermal dysplasia | IFT122 | ||
| Ellis-van Creveld syndrome | EVC 10 | EVC | |
| EVC2 | |||
| Jeune asphyxiating thoracic dystrophy | JATD1 | Unknown | |
| JATD2 | IFT80 | ||
| Renal-hepatic-pancreatic dysplasia | NPHP3 | ||
| X-linked | OFD1 | OFD1 | |
| Autosomaldominant | Autosomal dominant polycystic kidney disease (ADPKD) | PKD1 | PKD1 |
| PKD2 | PKD2 |
Gross Pathology
On gross examination, the following findings may be observed in gastrointestinal varices:
- Linear dark blue submucosal dilated veins
- In case of bleeding the varix may be colored dark red
Microscopic Pathology
On microscopic examination the following findings may be observed:[41][42]
- Very thin parallel veins in the lamina propria mucosae in the palisade zone become enlarged in portal hypertension and join the few larger submucosal veins to form esophageal varices
- The location of esophageal and gastric varices within the wall of gastrointestinal tract is different:
- Esophageal varices: Lamina propria mucosae and submucosa
- Gastric varices: Submucosa
- The wall of the varix (vein) may show deficiency in smooth muscle cells and elastic fibers and disproportionate increase in fibrous tissue
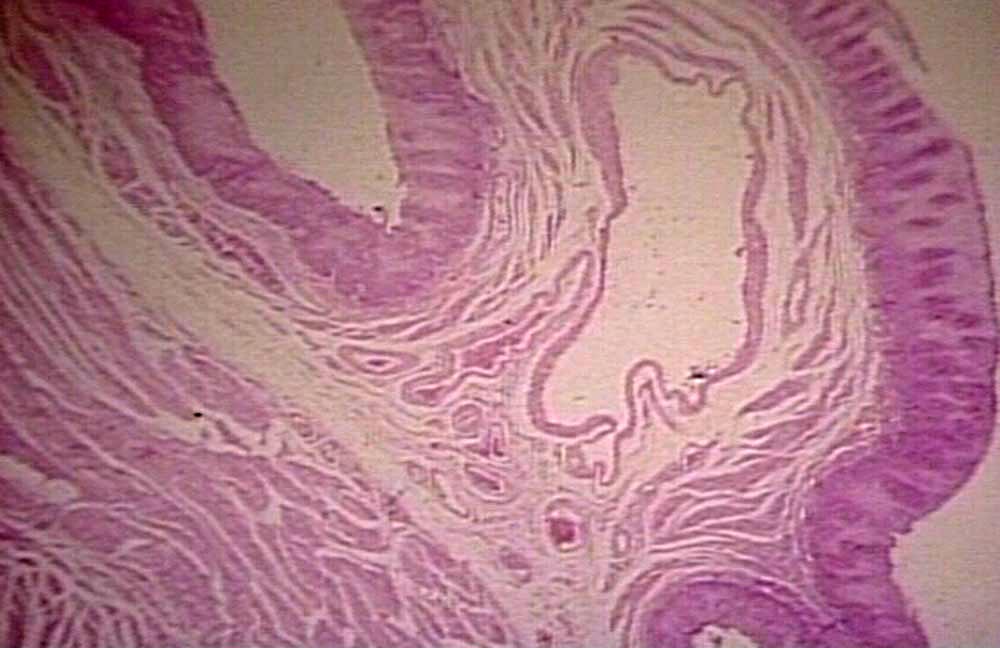
References
- ↑ "Anatomy - The Gastrointestinal Circulation - NCBI Bookshelf".
- ↑ Mahl TC, Groszmann RJ (1990). "Pathophysiology of portal hypertension and variceal bleeding". Surg. Clin. North Am. 70 (2): 251–66. PMID 2181704.
- ↑ Maruyama H, Yokosuka O (2012). "Pathophysiology of portal hypertension and esophageal varices". Int J Hepatol. 2012: 895787. doi:10.1155/2012/895787. PMC 3362051. PMID 22666604.
- ↑ Moreno AH, Burchell AR, Rousselot LM, Panke WF, Slafsky F, Burke JH (1967). "Portal blood flow in cirrhosis of the liver". J. Clin. Invest. 46 (3): 436–45. doi:10.1172/JCI105545. PMC 297064. PMID 6023778.
- ↑ Ponziani FR, Zocco MA, Campanale C, Rinninella E, Tortora A, Di Maurizio L, Bombardieri G, De Cristofaro R, De Gaetano AM, Landolfi R, Gasbarrini A (2010). "Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment". World J. Gastroenterol. 16 (2): 143–55. PMC 2806552. PMID 20066733.
- ↑ Tanaka M, Wanless IR (1998). "Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules". Hepatology. 27 (2): 488–96. doi:10.1002/hep.510270224. PMID 9462648.
- ↑ Adithan S, Venkatesan B, Sundarajan E, Kate V, Kalayarasan R (2010). "Color Doppler evaluation of left gastric vein hemodynamics in cirrhosis with portal hypertension and its correlation with esophageal varices and variceal bleed". Indian J Radiol Imaging. 20 (4): 289–93. doi:10.4103/0971-3026.73541. PMC 3056627. PMID 21423905.
- ↑ Rebibo L, Chivot C, Fuks D, Sabbagh C, Yzet T, Regimbeau JM (2012). "Three-dimensional computed tomography analysis of the left gastric vein in a pancreatectomy". HPB (Oxford). 14 (6): 414–21. doi:10.1111/j.1477-2574.2012.00468.x. PMC 3384867. PMID 22568419.
- ↑ Sikuler E, Groszmann RJ (1986). "Interaction of flow and resistance in maintenance of portal hypertension in a rat model". Am. J. Physiol. 250 (2 Pt 1): G205–12. PMID 3953799.
- ↑ Sharma M, Rameshbabu CS (2012). "Collateral pathways in portal hypertension". J Clin Exp Hepatol. 2 (4): 338–52. doi:10.1016/j.jceh.2012.08.001. PMC 3940321. PMID 25755456.
- ↑ Kumar A, Sharma P, Sarin SK (2008). "Hepatic venous pressure gradient measurement: time to learn!". Indian J Gastroenterol. 27 (2): 74–80. PMID 18695309.
- ↑ Simmonds MJ, Detterich JA, Connes P (2014). "Nitric oxide, vasodilation and the red blood cell". Biorheology. 51 (2–3): 121–34. doi:10.3233/BIR-140653. PMID 24819865.
- ↑ González-Abraldes J, García-Pagán JC, Bosch J (2002). "Nitric oxide and portal hypertension". Metab Brain Dis. 17 (4): 311–24. PMID 12602508.
- ↑ Wiest R, Groszmann RJ (1999). "Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance". Semin. Liver Dis. 19 (4): 411–26. doi:10.1055/s-2007-1007129. PMID 10643626.
- ↑ "Nitric Oxide - Hepatic Circulation - NCBI Bookshelf".
- ↑ Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH (2008). "Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species". Hepatology. 47 (6): 1983–93. doi:10.1002/hep.22285. PMC 2562502. PMID 18459124.
- ↑ 17.0 17.1 Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J (2010). "Physiopathology of splanchnic vasodilation in portal hypertension". World J Hepatol. 2 (6): 208–20. doi:10.4254/wjh.v2.i6.208. PMC 2999290. PMID 21160999.
- ↑ Gomis R, Fernández-Alvarez J, Pizcueta P, Fernández M, Casamitjana R, Bosch J, Rodés J (1994). "Impaired function of pancreatic islets from rats with portal hypertension resulting from cirrhosis and partial portal vein ligation". Hepatology. 19 (5): 1257–61. PMID 8175150.
- ↑ Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P (2015). "Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans". Mol Metab. 4 (8): 551–60. doi:10.1016/j.molmet.2015.06.001. PMC 4529499. PMID 26266087.
- ↑ Tibblin, Sten (1970). "Splanchnic Hemodynamic Responses to Glucagon". Archives of Surgery. 100 (1): 84. doi:10.1001/archsurg.1970.01340190086020. ISSN 0004-0010.
- ↑ García-Pagán JC, Escorsell A, Moitinho E, Bosch J (1999). "Influence of pharmacological agents on portal hemodynamics: basis for its use in the treatment of portal hypertension". Semin. Liver Dis. 19 (4): 427–38. PMID 10643627.
- ↑ Zardi EM, Dobrina A, Amoroso A, Afeltra A (2007). "Prostacyclin in liver disease: a potential therapeutic option". Expert Opin Biol Ther. 7 (6): 785–90. doi:10.1517/14712598.7.6.785. PMID 17555364.
- ↑ Graupera M, García-Pagán JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, Bosch J, Rodés J (2003). "Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers". Hepatology. 37 (1): 172–81. doi:10.1053/jhep.2003.50004. PMID 12500202.
- ↑ Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S (2013). "Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver". Hepatology. 57 (1): 320–30. doi:10.1002/hep.26005. PMC 3524382. PMID 22899584.
- ↑ Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D (2002). "Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats". Am. J. Physiol. Gastrointest. Liver Physiol. 283 (3): G587–94. doi:10.1152/ajpgi.00391.2001. PMID 12181171.
- ↑ García-Pagán JC, Bosch J, Rodés J (1995). "The role of vasoactive mediators in portal hypertension". Semin. Gastrointest. Dis. 6 (3): 140–7. PMID 7551971.
- ↑ Møller S, Gülberg V, Henriksen JH, Gerbes AL (1995). "Endothelin-1 and endothelin-3 in cirrhosis: relations to systemic and splanchnic haemodynamics". J. Hepatol. 23 (2): 135–44. PMID 7499784.
- ↑ Leivas A, Jiménez W, Bruix J, Boix L, Bosch J, Arroyo V, Rivera F, Rodés J (1998). "Gene expression of endothelin-1 and ET(A) and ET(B) receptors in human cirrhosis: relationship with hepatic hemodynamics". J. Vasc. Res. 35 (3): 186–93. PMID 9647333.
- ↑ Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J (2010). "Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis". J. Hepatol. 53 (2): 273–82. doi:10.1016/j.jhep.2010.03.013. PMID 20570385.
- ↑ Ballet F, Chretien Y, Rey C, Poupon R (1988). "Differential response of normal and cirrhotic liver to vasoactive agents. A study in the isolated perfused rat liver". J. Pharmacol. Exp. Ther. 244 (1): 283–9. PMID 3336005.
- ↑ Lautt WW, Greenway CV, Legare DJ (1987). "Effect of hepatic nerves, norepinephrine, angiotensin, and elevated central venous pressure on postsinusoidal resistance sites and intrahepatic pressures in cats". Microvasc. Res. 33 (1): 50–61. PMID 3561267.
- ↑ Wiest R, Groszmann RJ (1999). "Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance". Semin. Liver Dis. 19 (4): 411–26. doi:10.1055/s-2007-1007129. PMID 10643626.
- ↑ Gupta TK, Toruner M, Chung MK, Groszmann RJ (1998). "Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats". Hepatology. 28 (4): 926–31. doi:10.1002/hep.510280405. PMID 9755227.
- ↑ Graupera M, García-Pagán JC, Parés M, Abraldes JG, Roselló J, Bosch J, Rodés J (2003). "Cyclooxygenase-1 inhibition corrects endothelial dysfunction in cirrhotic rat livers". J. Hepatol. 39 (4): 515–21. PMID 12971960.
- ↑ 35.0 35.1 35.2 35.3 Jackson FW, Adrain AL, Black M, Miller LS (1999). "Calculation of esophageal variceal wall tension by direct sonographic and manometric measurements". Gastrointest. Endosc. 50 (2): 247–51. PMID 10425421.
- ↑ 36.0 36.1 Hilzenrat N, Sherker AH (2012). "Esophageal varices: pathophysiology, approach, and clinical dilemmas". Int J Hepatol. 2012: 795063. doi:10.1155/2012/795063. PMC 3501997. PMID 23193482.
- ↑ Kimura K, Ohto M, Matsutani S, Furuse J, Hoshino K, Okuda K (1990). "Relative frequencies of portosystemic pathways and renal shunt formation through the "posterior" gastric vein: portographic study in 460 patients". Hepatology. 12 (4 Pt 1): 725–8. PMID 2210674.
- ↑ Ling SC (2007). "Congenital cholestatic syndromes: what happens when children grow up?". Can. J. Gastroenterol. 21 (11): 743–51. PMC 2658590. PMID 18026579.
- ↑ Malloy L, Jensen M, Bishop W, Divekar A (2013). ""Downhill" esophageal varices in congenital heart disease". J. Pediatr. Gastroenterol. Nutr. 56 (2): e9–11. doi:10.1097/MPG.0b013e31824b5fff. PMID 23328456.
- ↑ "Congenital Hepatic Fibrosis Overview - GeneReviews® - NCBI Bookshelf".
- ↑ Arakawa M, Masuzaki T, Okuda K (2002). "Pathomorphology of esophageal and gastric varices". Semin. Liver Dis. 22 (1): 73–82. doi:10.1055/s-2002-23208. PMID 11928080.
- ↑ Wali MA, Dewan M, Eid RA (2003). "Histopathological changes in the wall of varicose veins". Int Angiol. 22 (2): 188–93. PMID 12865886.